Abstract
Background
Transthyretin amyloidosis (ATTR amyloidosis) is a progressive, multisystemic, life-threatening disease resulting from the deposition of variant or wild-type (ATTRwt amyloidosis) transthyretin amyloid fibrils in various tissues and organs.
Methods
Established in 2007, the Transthyretin Amyloidosis Outcomes Survey (THAOS) is the largest ongoing, global, longitudinal, observational study of patients with ATTR amyloidosis, including both hereditary and wild-type disease, and asymptomatic carriers of pathogenic TTR mutations. This analysis describes the baseline characteristics of symptomatic patients and asymptomatic gene carriers enrolled in THAOS since its inception in 2007 (data cutoff: August 1, 2022), providing a consolidated overview of 15-year data from the THAOS registry.
Results
This analysis included 4428 symptomatic patients and 1707 asymptomatic gene carriers. The majority of symptomatic patients were male (70.8%) with a mean (standard deviation [SD]) age at symptom onset of 56.6 (17.9) years. Compared with the 14-year analysis, V30M remained the most prevalent genotype in Europe (62.2%), South America (78.6%), and Japan (74.2%) and ATTRwt remained most common in North America (56.2%). Relative to the 14-year analysis, there was an increase of mixed phenotype (from 16.6 to 24.5%) and a reduction of predominantly cardiac phenotype (from 40.7 to 31.9%). The proportion of patients with predominantly neurologic phenotype remained stable (from 40.1 to 38.7%). Asymptomatic gene carriers were 58.5% female with a mean age at enrollment of 41.9 years (SD 15.5).
Conclusions
This overview of > 6000 patients enrolled over 15 years in THAOS represents the largest registry analysis of ATTR amyloidosis to date and continues to emphasize the genotypic and phenotypic heterogeneity of the disease. Nearly a quarter of the symptomatic population within THAOS was mixed phenotype, underscoring the need for multidisciplinary management of ATTR amyloidosis.
Trial registration
ClinicalTrials.gov Identifier: NCT00628745.
Similar content being viewed by others
Introduction
Transthyretin amyloidosis (ATTR amyloidosis) is a progressive, multisystemic, life-threatening disease characterized by deposits of amyloid fibrils in the peripheral nerves, heart, and other tissues and organs, resulting in polyneuropathy, cardiomyopathy, or a mix of both neurologic and cardiac manifestations [1,2,3]. ATTR amyloidosis may be caused by one of over 130 pathogenic mutations that destabilize the TTR protein (hereditary ATTR amyloidosis [ATTRv amyloidosis]) or the accumulation of non-mutated TTR protein (wild-type ATTR amyloidosis [ATTRwt amyloidosis]) [3, 4]. The phenotypic presentation of ATTRv amyloidosis is clinically heterogeneous and can be predominantly neurologic, predominantly cardiac, or mixed phenotype, depending on the particular TTR variant and other factors [2, 5]. ATTRwt amyloidosis most often presents as cardiomyopathy [1]. If left untreated, median survival estimates in patients with ATTR amyloidosis range from 2 to 10 years, depending on genotype and other factors [1, 2, 6]. Diagnosing ATTR amyloidosis can be difficult due to low disease awareness, indeterminate family history, and the heterogeneity of clinical presentation that can overlap with more common diseases [2, 6, 7]. Better clinical characterization of the disease may lead to earlier identification and intervention, which is associated with improved outcomes [8].
Established in 2007, the Transthyretin Amyloidosis Outcomes Survey (THAOS) is an ongoing, global, longitudinal, observational survey of patients with ATTR amyloidosis, including both hereditary and wild-type disease, and asymptomatic carriers with TTR mutations [9]. THAOS collects multinational, longitudinal data on the natural history of the disease from a large, diverse patient population to help inform the characterization of ATTR amyloidosis and improve disease diagnosis and patient management. This analysis provides an annual update [10] of the characteristics of patients with ATTR amyloidosis (both ATTRv and ATTRwt) and asymptomatic gene carriers at the time of their enrollment into THAOS, providing a global overview of THAOS data since its inception 15 years ago.
Methods
Study design and patient population
The study design and eligibility criteria of THAOS have been described [11]. All study sites received ethical or institutional review board approval prior to patient enrollment, and all patients provided written informed consent. The Good Pharmacoepidemiology Practice guidelines and the principles of the Declaration of Helsinki were duly followed for this study.
The analysis population comprised all patients enrolled in THAOS (data cutoff date: August 1, 2022). The definitions of symptomatic status, asymptomatic status, and those with missing symptomatic status have been described previously [10]. Demographics, clinical characteristics, and patient-reported outcomes collected at enrollment were analyzed in the overall cohort of symptomatic patients and by the following genotype subgroups: ATTRwt amyloidosis, V30M (p.V50M) with early-onset disease (age ≤ 50 years, based on age at diagnosis), V30M with late-onset disease (age > 50 years), and non-V30M.
Phenotype at enrollment was analyzed in symptomatic patients by region as previously described [10]. Patients were classified by phenotype based on the following definitions: Predominantly cardiac phenotype included patients with abnormal electrocardiogram (ECG) due to rhythm disturbance, heart failure, or dyspnea and no more than mild neurologic or gastrointestinal (GI) symptoms (excluding erectile dysfunction, constipation, and carpal tunnel); cardiac symptoms did not need to be ongoing at a given visit to be included for phenotyping, but symptoms had to be definitely ATTR amyloidosis related. Predominantly neurologic phenotype included patients with neurologic or GI symptoms of any severity and without abnormal ECG due to rhythm disturbance, heart failure, or dyspnea; neurologic and GI symptoms had to be ongoing and definitely ATTR amyloidosis related. A modified Polyneuropathy Disability (mPND) score ≥ I was considered a neurologic symptom in this analysis, whereas it was not in prior analyses [10]. Mixed phenotype included patients with abnormal ECG due to rhythm disturbance, heart failure, or dyspnea, and neurologic or GI symptoms of any severity, but who did not satisfy criteria for a predominantly cardiac or predominantly neurologic phenotype. Unknown phenotype included all other symptomatic patients who did not meet any of the above criteria for either predominantly cardiac, predominantly neurologic, or mixed phenotypes. All patients with ATTRwt amyloidosis were classified as predominantly cardiac at enrollment unless they had any neurologic symptoms definitely related to ATTR amyloidosis, in which case they were classified as having a mixed phenotype.
The categorization of symptoms and their manifestations have been described [10]. Demographics collected at enrollment were also summarized for the asymptomatic carriers overall and by genotype category (V30M and non-V30M).
Assessments
Assessments have been described in detail [10]. Briefly, a patient’s ability to perform normal life activities and need for assistance was assessed in symptomatic patients using the Karnofsky Performance Status Scale Score. Neurologic impairment was measured in symptomatic patients using the derived Neuropathy Impairment Score in the Lower Limbs (NIS-LL) and mPND scores. Cardiac measures included select echocardiographic measures, N-terminal pro-B-type natriuretic peptide (NT-proBNP) concentration, and New York Heart Association (NYHA) class. Quality of Life (QoL) was assessed using the EQ-5D-3L and the Norfolk Quality of Life – Diabetic Neuropathy questionnaire.
Statistical analyses
This was a descriptive analysis. Continuous data are presented as mean (standard deviation [SD]) or median (10th, 90th percentile), and categorical data are presented as count (percentage).
Results
Demographics and genotype
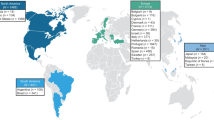
There were 6368 patients from 85 study sites and 23 countries enrolled in THAOS at the data cutoff date (Fig. 1). This included 4428 symptomatic patients and 1707 asymptomatic gene carriers. There were 233 wild-type patients who, despite having all symptoms assessed, did not meet the definition for the symptomatic set [10]. V30M remained the most prevalent genotype enrolled in THAOS patients (48.0%) as was reported in the previous year’s report [10], followed by ATTRwt amyloidosis (25.2%) and V122l (p.V142I) (6.0%) (Additional file 1: Table S1). Among the symptomatic patients, V30M (early or late onset) was most commonly enrolled in Europe (54.2%), South America (79.5%), and Japan (75.4%), and wild-type disease was most commonly enrolled in North America (59.5%) (Fig. 2a; Additional file 2: Table S2). The non-V30M variant was more common (even more prevalent than the wild type) in some individual countries (Mexico, Bulgaria, Denmark, Israel, Italy, Romania, Turkey, Malaysia and Taiwan), although the overall trend showed that V30M was the predominant genotype. Among the symptomatic patients with a predominantly cardiac phenotype, the non-Val30Met subgroup accounted for more than half the patients in Asia (55.3%) and South America (52.6%) (Fig. 2b). The non-Val30Met subgroup also accounted for 90.2% of the patients with a predominantly neurologic phenotype in North America, whereas, for other regions (South America, Europe and Asia), the V30M genotype (early or late onset) was more prevalent (Fig. 2c). The ATTRwt genotype accounted for almost half (49.4%) of the symptomatic patients with a mixed phenotype in North America (Fig. 2d).
Regional distribution of genotype subgroups in symptomatic patients. The proportion of patients with each genotype shown by region in a the overall population of symptomatic patients and in patients with b predominantly cardiac, c predominantly neurologic, and d mixed phenotypes. ATTRwt amyloidosis = wild-type transthyretin amyloidosis
Symptomatic patients were predominantly male across all genotype subgroups (Table 1). Overall, the mean age at symptom onset was 56.6 years and was higher in patients with ATTRwt amyloidosis compared with the other genotype subgroups. Mean time from symptom onset to diagnosis was 4.0 years in all symptomatic patients and ranged from 2.8 years in the early-onset V30M subgroup to 4.6 years in the ATTRwt amyloidosis subgroup. V30M remained the most prevalent genotype among the symptomatic patients in South America (79.5%), Europe (54.2%), and Asia (including Japan; 47.8%). The majority of these patients had early-onset disease in each of these regions (Fig. 2a; Additional file 2: Table S2).
In contrast to symptomatic patients, the asymptomatic carriers had a higher proportion of females overall (58.5%), as did the V30M subgroup (61.4%) (Table 2). The mean age at enrollment was 41.9 years overall and was higher in the non-V30M subgroup compared with V30M asymptomatic gene carriers.
Distribution of phenotypes at enrollment in symptomatic patients
The overall phenotype distribution for symptomatic patients at enrollment showed, in respect to last-year’s analysis, an increase in the proportion of mixed phenotype from 16.6% [10] to 24.5%. The predominantly cardiac (31.9%), predominantly neurologic (38.7%), and unknown (4.9%) phenotypes accounted for the remaining symptomatic patients (Additional file 2: Table S2). In North America, the majority of symptomatic patients had a predominantly cardiac phenotype overall (63.9%) as well as in the ATTRwt amyloidosis (84.5%) and non-V30M (34.4.%) subgroups (Fig. 3a; Additional file 2: Table S2). In South America, predominantly neurologic was the most prevalent phenotype at enrollment overall (65.6%) as well as in the V30M (early and late onset) subgroup (76.7%). The proportion of symptomatic patients with predominantly neurologic phenotype in Europe was 48.7% (Fig. 3a; Additional file 2: Table S2). In symptomatic patients with ATTRwt amyloidosis, 84.5% had a predominantly cardiac phenotype in North America, which was higher than in the other regions (Europe, South America and Asia; Fig. 3b). In Europe, the predominantly neurologic phenotype was found in 72.2% of patients in the V30M (early or late onset) subgroup, and in 40.6% of patients in the non-V30M subgroup (Fig. 3c, d, e; Additional file 2: Table S2). The mixed phenotype was more prevalent in Asian countries (excluding Japan) with an overall 40.6% of symptomatic patients (Additional file 2: Table S2).
Regional distribution of phenotype at enrollment in symptomatic patients. The proportion of patients with each phenotype shown by region in a the overall population of symptomatic patients and by genotype category, b ATTRwt amyloidosis, c V30M early onset, d V30M late onset, e non-V30M. ATTRwt amyloidosis = wild-type transthyretin amyloidosis
The V30M late-onset subgroup had a greater proportion of mixed phenotypes versus the early-onset subgroup (30.6% vs. 13.8%) (Additional file 2: Table S2). The V30M late-onset predominantly cardiac phenotype showed a decrease in proportion among the symptomatic patients as compared to last year (4.3% vs. 1.4% [10]). The proportion of symptomatic patients with ATTRwt amyloidosis with a mixed phenotype at enrollment also showed a jump from 9.8% as observed in the previous year [10] to 23.9% (Additional file 2: table S2), but as discussed further below this result is due to a change in the definition of mixed phenotype in THAOS.
The non-Val30Met subgroup was prevalent among symptomatic patients with a mixed phenotype (36.3%; Fig. 4). The V30M early onset group accounted for half of the symptomatic patients with a predominantly neurologic phenotype (Fig. 4).
Clinical characteristics at enrollment in symptomatic patients
The neurologic impairment was more prominent in the V30M (including early and late onset) and non-V30M subgroups as indicated by the derived NIS-LL score (Additional file 3: Table S3). Patients with late-onset V30M had the highest neurologic impairment. Greater cardiac involvement in patients with ATTRwt amyloidosis compared with V30M ATTRv amyloidosis was suggested by greater left ventricular septum thickness and decreased left ventricular ejection fraction (Additional file 3: Table S3). Quality of life, measured by EQ-5D-3L index score and visual analog scale, was comparable between the ATTRwt, V30M, and non-V30M subgroups; however, higher corresponding Norfolk Quality of Life – Diabetic Neuropathy questionnaire scores were observed for the V30M late-onset and non-V30M subgroups.
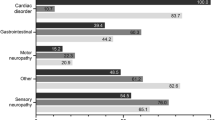
As observed in last year’s data cutoff [10], sensory neuropathy (54.6%), cardiac disorder (55.4%), and autonomic neuropathy (45.1%) were the most common symptoms corresponding to the symptomatic patients overall. GI manifestations were more common in the V30M (early or late onset) subgroup (59.9%) as compared to the ATTRwt amyloidosis subgroup (3.5%; Fig. 5a, b, c). Similar trends were also observed for autonomic neuropathy and sensory neuropathy with more patients in the V30M (early or late onset) subgroup having these symptom categories. Conversely, cardiac disorders were more frequent among patients with ATTRwt amyloidosis as compared to those with V30M (86.0% vs. 28.4%; Fig. 5a, b, c). Among the non-Val30Met subgroup, sensory neuropathy and cardiac disorders were more frequent (60.1% and 58.8% respectively) as compared with the other symptoms (Fig. 5d).
Cardiac characteristics at enrollment in symptomatic patients with a predominantly cardiac or mixed phenotype
Heart failure was present in 87.0% of the ATTRwt amyloidosis subgroup as compared to 72.3% of the ATTRv amyloidosis subgroup in the subset of patients with a predominantly cardiac or mixed phenotype (Table 3). A similar greater proportion of patients with abnormal ECG, atrial fibrillation/flutter, pacemaker/implantable cardioverter-defibrillator, and higher values of the cardiac biomarkers (Troponin T, NT-proBNP) were observed in the ATTRwt amyloidosis subgroup as compared to the ATTRv amyloidosis subgroup.
Neurologic characteristics at enrollment in symptomatic patients with a predominantly neurologic or mixed phenotype
Most patients with a predominantly neurologic or mixed phenotype had a score of I (56.9%) or II (19.1%) on the mPND (Additional file 4: Table S4). Patients with V30M late-onset disease had greater walking impairment than those with V30M early-onset disease (mPND > II, 24.7% vs. 9.1%).
Discussion
This 15-year global overview of > 6000 patients with ATTR amyloidosis and asymptomatic gene carriers is the largest analysis of ATTR amyloidosis to date. The findings presented in this study are largely consistent with the data from the last annual assessment [10].
In this study, V30M and the associated predominantly neurologic phenotype continued to be more prevalent in Europe, South America, and Japan, whereas wild-type disease and the predominantly cardiologic phenotype were most common in North America. Notably, for all genotype subgroups (ATTRwt amyloidosis, early- and late-onset V30M, non-V30M), the highest rate of predominantly cardiologic patients was always found in North America. The reasons for the differences observed are unknown: it has been suggested that they could be related to differences in the age of the populations, the use of scintigraphy imaging techniques in clinical practice, reliance on and/or expertise in the performance and interpretation of endomyocardial biopsy specimens, and specialty of THAOS investigators (e.g., neurologist vs. cardiologist) [12]. However, they might also reflect the true differences in the prevalence of the condition, suggesting a possible role of ambient or socioeconomic factors in increasing the predisposition to cardiac disease. Finally, non-V30M genotypes were highly represented in some individual European, American, and Asian countries (e.g., Mexico, Bulgaria, Denmark, Israel, Italy, Romania, Turkey, Malaysia, and Taiwan).
Male predominance among symptomatic patients was observed in this analysis for all subgroups. However, in the early-onset V30M subgroup, this difference was less pronounced (males, 52.2%; females, 47.8%), consistent with the findings of a Japanese nationwide survey [13]. In contrast, a higher proportion of asymptomatic carriers were female than male, as suggested in previous studies that recorded lower disease penetrance in females, especially those associated with specific genotypes or cardiac manifestation of ATTR amyloidosis [14,15,16,17,18].
As expected, patients with ATTRwt amyloidosis were the oldest at symptom onset (mean age, 72.3 years), which occurred almost 10 years after the late-onset V30M subgroup and > 15 years after the non-V30M subgroup. Patients with early-onset V30M were the youngest at disease onset (mean age, 33.8 years) and received an ATTR amyloidosis diagnosis earlier than the other genotype subgroups (0.9–1.7 years before). This last finding may contribute to the fact that these patients presented with a significant minor grade of walking impairment (9.1% of patients with mPND score ≥ 2) as compared to the other genotypes (20.4% in ATTRwt, 22.7% in late-onset V30M, 18.4% in non-V30M). It should also be noted that, despite everything, patients with early-onset V30M received a diagnosis after a significant amount of time (a mean of 2.8 years after symptom onset). This delay could result from the rarity of ATTR amyloidosis, the wide spectrum of possible symptoms, and the likely presence of confounding comorbidity (especially for patients with ATTRwt amyloidosis).
Compared with last year’s analysis, the proportion of predominantly neurologic patients remained stable (from 40.1 to 38.7%). However, we observed a considerable overall reduction of predominantly cardiac phenotype (from 40.7 to 31.9%) and an increase in mixed phenotype (from 16.6 to 24.5%). This difference was mainly influenced by a change in the definition of the mixed phenotype in THAOS, namely that an mPND score ≥ I is now considered a definitely ATTR amyloidosis–related neurologic symptom, whereas before it was not. As a result of this modification, among the four subgroups of patients (ATTRwt, early- and late-onset V30M, non-V30M), phenotype distribution varied primarily in the ATTRwt subgroup. In these patients, mixed phenotype went from 9.8% as observed in the last year [10] to 23.9%, with a decrease of predominantly cardiac phenotype (from 89 to 76.1%). The global geographic distribution of the mixed phenotype illustrated it was more prevalent in Asia for the ATTRwt and V30M subgroups, and in South America for the non-V30M subgroup.
THAOS has notable strengths in that the registry represents a large, geographically diverse group of countries and study sites worldwide, and provides a more accurate global picture of the current state of ATTR amyloidosis. The large study population of > 6000 patients is a further strength of this analysis. Study limitations include potential differences among study sites in data collection methods, and that the inclusion criteria for THAOS have evolved over time. In addition, temporal bias may have played a role in these findings, as the number and specialties of study sites increased and changed over time, which may have been the greatest influential factor in the patterns observed. Referral bias may have impacted phenotype categorization since cardiac centers may not conduct comprehensive neurologic assessments and vice versa. This could have resulted in an under-representation of the mixed phenotype. In addition, recruitment rates with study sites may vary over time, and registry data, by nature, are not always complete and are limited by the data imputed. Lastly, management of ATTR amyloidosis has evolved over the last few decades and clinical awareness has increased given the emergence of disease-modifying treatments; therefore, patients enrolled in the later years of THAOS likely received more comprehensive assessments than those enrolled in the earlier era.
Conclusion
This analysis of > 6000 patients and asymptomatic TTR gene carriers from THAOS continues to underscore the heterogeneity and increasing awareness of ATTR amyloidosis. The mixed phenotype and multisystemic involvement are increasingly recognized, highlighting the need for a consistent, multidisciplinary approach to the management of ATTR amyloidosis.
Availability of data and materials
Upon request, and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions and exceptions, Pfizer may also provide access to the related individual de-identified participant data. See https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information.
References
Ruberg FL, Grogan M, Hanna M, Kelly JW, Maurer MS. Transthyretin amyloid cardiomyopathy: JACC state-of-the-art review. J Am Coll Cardiol. 2019;73:2872–91.
Ando Y, Coelho T, Berk JL, Cruz MW, Ericzon BG, Ikeda S, et al. Guideline of transthyretin-related hereditary amyloidosis for clinicians. Orphanet J Rare Dis. 2013;8:31.
Gertz MA, Benson MD, Dyck PJ, Grogan M, Coelho T, Cruz M, et al. Diagnosis, prognosis, and therapy of transthyretin amyloidosis. J Am Coll Cardiol. 2015;66:2451–66.
Plante-Bordeneuve V, Said G. Familial amyloid polyneuropathy. Lancet Neurol. 2011;10:1086–97.
Adams D, Ando Y, Beirao JM, Coelho T, Gertz MA, Gillmore JD, et al. Expert consensus recommendations to improve diagnosis of ATTR amyloidosis with polyneuropathy. J Neurol. 2021;268:2109–22.
Witteles RM, Bokhari S, Damy T, Elliott PM, Falk RH, Fine NM, et al. Screening for transthyretin amyloid cardiomyopathy in everyday practice. JACC Heart Fail. 2019;7:709–16.
Conceicao I, Coelho T, Rapezzi C, Parman Y, Obici L, Galan L, et al. Assessment of patients with hereditary transthyretin amyloidosis - understanding the impact of management and Disease progression. Amyloid. 2019;26:103–11.
Elliott P, Drachman BM, Gottlieb SS, Hoffman JE, Hummel SL, Lenihan DJ, et al. Long-term survival with tafamidis in patients with transthyretin amyloid cardiomyopathy. Circ Heart Fail. 2022;15:e008193.
Coelho T, Vinik A, Vinik EJ, Tripp T, Packman J, Grogan DR. Clinical measures in transthyretin familial amyloid polyneuropathy. Muscle Nerve. 2017;55:323–32.
Dispenzieri A, Coelho T, Conceicao I, Waddington-Cruz M, Wixner J, Kristen AV, et al. Clinical and genetic profile of patients enrolled in the Transthyretin Amyloidosis outcomes Survey (THAOS): 14-year update. Orphanet J Rare Dis. 2022;17:236.
Plante-Bordeneuve V, Suhr OB, Maurer MS, White B, Grogan DR, Coelho T. The Transthyretin Amyloidosis outcomes Survey (THAOS) registry: design and methodology. Curr Med Res Opin. 2013;29:77–84.
Maurer MS, Hanna M, Grogan M, Dispenzieri A, Witteles R, Drachman B, et al. Genotype and phenotype of transthyretin cardiac amyloidosis: THAOS (transthyretin amyloid outcome survey). J Am Coll Cardiol. 2016;68:161–72.
Koike H, Misu K, Ikeda S, Ando Y, Nakazato M, Ando E, et al. Type I (transthyretin Met30) familial amyloid polyneuropathy in Japan: early- vs late-onset form. Arch Neurol. 2002;59:1771–6.
Caponetti AG, Rapezzi C, Gagliardi C, Milandri A, Dispenzieri A, Kristen AV, et al. Sex-related risk of cardiac involvement in hereditary transthyretin amyloidosis: insights from THAOS. JACC Heart Fail. 2021;9:736–46.
Batra J, Rosenblum H, Defilippis EM, Griffin JM, Saith SE, Gamino D, et al. Sex differences in the phenotype of transthyretin cardiac amyloidosis due to Val122Ile mutation: insights from noninvasive pressure-volume analysis. J Card Fail. 2021;27:67–74.
Russo M, Mazzeo A, Stancanelli C, Di Leo R, Gentile L, Di Bella G, et al. Transthyretin-related familial amyloidotic polyneuropathy: description of a cohort of patients with Leu64 mutation and late onset. J Peripher Nerv Syst. 2012;17:385–90.
Campbell CM, LoRusso S, Dispenzieri A, Kristen AV, Maurer MS, Rapezzi C, et al. Sex differences in wild-type transthyretin amyloidosis: an analysis from the Transthyretin Amyloidosis outcomes Survey (THAOS). Cardiol Ther. 2022;11:393–405.
Patel RK, Ioannou A, Razvi Y, Chacko L, Venneri L, Bandera F, et al. Sex differences among patients with transthyretin amyloid cardiomyopathy - from diagnosis to prognosis. Eur J Heart Fail. 2022;24:2355–63.
Acknowledgements
Additional THAOS investigators contributing to this analysis: Anna Hüsing-Kabar, Universitatsklinikum Muenster, Transplant Hepatology, Muenster, Germany; Brian Drachman, University of Pennsylvania, Perelman Center for Advanced Medicine, Philadelphia, PA, USA; Fabio Adrian Barroso, FLENI, Ciudad Autonoma de Buenos Aires, Argentina; Mitsuharu Ueda, Kumamoto University, Kumamoto, Japan; Eun-Seok Jeon, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Republic of Korea; Marco Luigetti, Fondazione Policlinico Gemelli, Universita Cattolica del Sacro Cuore, Roma, Italy; Yoshiki Sekijima, Shinshu University School of Medicine, Matsumoto, Japan; Anna Mazzeo, AOU Policlinico G. Martino, Messina, Italy; Francisco Munoz Beamud, Hospital Juan Ramon Jimenez, Huelva, Spain; David Slosky, Vanderbilt University School of Medicine, Nashville, TN, USA; Mazen Hanna, Cleveland Clinic Foundation, Cleveland, OH, USA; Miriam Freimer, The Ohio University College of Medicine, Columbus, OH, USA; Hans Nienhuis, University Medical Center Groningen, Groningen, The Netherlands; Henning Moelgaard, Aarhus University Hospital, Skejby, Aarhus, Denmark; David Adams, CHU de Bicetre, Cedex, France; Edward Miller, Smilow Cancer Hospital, New Haven, CT, USA; Amir Dori, Sheba Medical Center, Ramat Gan, Israel; Rayomand Press, Karolinska University Hospital, Huddinge, Stockholm, Sweden; Jocelyn Inamo, Chu De Fort De France, Fort de France, France; Calogero Lino Cirami, Azienda Ospedaliero - Universitaria di Careggi, Firenze, Italy; Josep Maria Campistol Plana, Institut Clinic de Nefrologia i Urologia, ICNU, Hospital Clinic i Provincial de Barcelona, Barcelona, Spain; Michele Emdin, Fondazione Toscana Gabriele Monasterio Per La Ricerca Medica E Di Sanita Pubblica, Pisa, Italy; Dianna Quan, UC Denver, Aurora, CO, USA; Scott Hummel, University of Michigan, Ann Arbor, MI, USA; Edileide de Barros Correia, Instituto Dante Pazzanese De Cardiologia, Sao Paulo, Brazil; Ronald Witteles, Stanford University School of Medicine, Stanford, CA, USA; Cheng Yin Tan, University Malaya Medical Centre, Kuala Lumpur, Malaysia; Olga Azevedo, Centro Hospitalar Do Alto Ave, Epe, Guimaraes, Portugal; Sanjiv Shah, Northwestern University, Chicago, IL, USA; Daniel Lenihan, Washington University School of Medicine, St. Louis, MO, USA; Sorina Badelita, Institutul De Cardiologie, Bucuresti, Romania; Srinivas Murali, Wexford Health and Wellness Pavillion, Pittsburgh, PA, USA; Sasa Zivkovic, University of Pittsburgh Medical Center, Pittsburgh, PA, USA; Jose Nativi Nicolau, The University of Utah Health Sciences Center, Salt Lake City, UT, USA; Jose Tallaj, University of Alabama, Birmingham, AL, USA; Nowell Fine, University of Alberta Foothills Medical Centre, Calgary, Canada; Carsten Tschoepe, Charite Campus Rudolf-Virchow-Klinikum, Berlin, Germany; Roberto Fernandéz Torrón, Hospital Universitario Donostia, Gipuzkoa, San Sebastian, Spain; Laura Obici, Centro per lo Studio e la Cura delle Amiloidosi Sistemiche, Pavia, Italy; Michael Polydefkis, Johns Hopkins Hospital, Baltimore, MD, USA; Stephen Gottlieb, University of Maryland, Baltimore, MD, USA; James Tauras, Montefiore Medical Center, Jack D. Weiler Hospital, Bronx, NY, USA; Hector Ventura, John Ochsner Heart & Vascular Institute, New Orleans, LA, USA; Christopher Mueller, Medical College of Wisconsin, Milwaukee, WI, USA; Robert Brunkhorst, University Hospital of RWTH Aachen, Aachen, Germany; Felix Darstein, Johann-Gutenberg-Universität, Mainz, Germany; Jeeyoung Oh, Konkuk University Medical Center, Seoul, Republic of Korea; Tessa Marburger, Oregon Health and Science University, Portland, OR, USA; Alberta Warner, VA Greater Los Angeles Healthcare System, Los Angeles, CA, USA; Johan Van Cleemput, Afdeling Klinische Cardiologie, O&N I, Leuven, Belgium; Diego Delgado, Toronto General Hospital, Toronto, Canada; Valeria Lujan Salutto, Instituto De Investigaciones Medicas, Ciudad Autonoma De Buenos Aires, Argentina; Yesim Parman, Istanbul University, Istanbul Faculty of Medicine, Department of Neurology, Istanbul, Turkey; Chi-Chao Chao, National Taiwan University Hospital, Taipei, Taiwan; Nitasha Sarswat, University of Chicago Medical Center, Chicago, IL, USA; David Steidley, Mayo Clinic Arizona, Phoenix, AZ, USA; Jeffrey Ralph, University of California, Department of Neurology, San Francisco, LA, USA; William Cotts, Advocate Christ Medical Centre, Oak Lawn, IL, USA; James Hoffman, University of Miami Hospital & Clinics, Miami, FL, USA; Marcelo Rugiero, Hospital Italiano de Buenos Aires, Buenos Aires, Argentina; Sonoko Misawa, Chiba University Hospital, Chiba-shi, Japan; Jose Luis Munoz Blanco, Hospital Gregorio Marañón, Madrid, Spain; Lucia Galan Davila, Hospital Clinico San Carlos, Madrid, Spain; Menachem Sadeh, Wolfson Medical Center, Holon, Israel; Jin Luo, Temple University School of Medicine, Philadelphia, PA, USA; Theodoros Kyriakides, Cyprus Institute of Neurology and Genetics, NICOSIA, Cyprus; Annabel Wang, University of California, Irvine, Orange, CA, USA; Horacio Kaufmann, NYU Medical Center, New York, NY, USA.We thank all THAOS patients and investigators for their important contributions to this study. Manuscript formatting support was provided by Emily Balevich, PhD, of Engage Scientific Solutions and was funded by Pfizer; no contribution was made to editorial content.
Funding
The THAOS registry and this analysis were sponsored by Pfizer. Pfizer contributed to the study design and management and collection of data. In their role as authors, employees of Pfizer were involved in the interpretation of data, preparation, review, and approval of the manuscript and the decision to submit for publication, along with their co-authors. The study sponsors approved the manuscript from an intellectual property perspective but had no right to veto the publication.
Author information
Authors and Affiliations
Consortia
Contributions
LG, TC, AD, IC, MW-C, AK, JW, ID, JG-M, EC, MSM, VP-B, PG-P, IT, JG-C, AGD, MG, and AM contributed data to the analysis as a participating study site in the clinical study. LG, DC, PG, OG, and LA contributed to the analysis of the data. All authors contributed to the study design, interpretation of data, and preparation, review, and approval of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All THAOS sites received ethical or institutional review board approval prior to patient enrollment, and each patient provided written informed consent. The study followed the Good Pharmacoepidemiology Practice guidelines and the principles of the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
Luca Gentile reports travel grants from Kedrion and CSL Behring to attend scientific meetings. Teresa Coelho reports serving as a medical advisor for Pfizer and receiving funding for scientific meeting expenses (travel, accommodation and registration) from Pfizer. Angela Dispenzieri reports research grants from Celgene, Millennium, Pfizer, Janssen and Alnylam; funding from Pfizer for meeting expenses (travel); and attending advisory boards for Akcea and Intellia. Isabel Conceição reports consulting fees and funding for scientific meeting expenses (travel, accommodation and registration) from Pfizer. Márcia Waddington-Cruz reports research funding, consulting fees and travel support for advisory boards and meetings from FoldRx Pharmaceuticals and Pfizer. Arnt V. Kristen reports reimbursement for study visits from Pfizer during the conduct of the study. Jonas Wixner reports consulting fees and travel support for lectures and advisory boards from Pfizer and Alnylam; and consulting fees from Akcea and Intellia. Igor Diemberger received speaker fees from Biotronik, Boston Scientific, Daiichi Sankyo, Medtronic, Pfizer and Philips outside the submitted work. Juan Gonzalez-Moreno reports speaker fees from Pfizer, Alnylam and Akcea. Eve Cariou has no conflicts to report. Mathew S. Maurer reports grants from Pfizer during the conduct of the study; grants and personal fees from Pfizer, Eidos, Prothena and Ionis; grants from Alnylam; and personal fees from GSK and Akcea outside the submitted work. Violaine Planté-Bordeneuve reports serving as a medical advisor for Pfizer, Alnylam, Eidos and Ionis. Pablo Garcia-Pavia reports speaking fees from Pfizer, Eidos, Alnylam and Akcea; consulting fees from Pfizer, Eidos, Neurimmune, Alnylam, Prothena and Akcea; and research/educational support to his institution from Pfizer, Eidos and Alynylam. Ivailo Tournev reports speakers bureau/lecturer fees from Ewopharma, Genesis Pharma, Pfizer, Roche and Sanofi. Jose Gonzalez-Costello reports attending advisory boards for Pfizer and Alnylam. Alejandra González-Duarte reports financial support from Alnylam and Pfizer for her role as a principal investigator in studies sponsored by these entities, and as a consultant and speaker. Martha Grogan reports grants and advisory board and consultancy fees paid to her institution from Alnylam, Eidos, Prothena and Pfizer. Anna Mazzeo reports financial grants (honoraria and speaking) from Ackea, Alnylam, and Pfizer. Doug Chapman, Pritam Gupta, Oliver Glass, and Leslie Amass are current or former employees of Pfizer and hold stock and/or stock options in Pfizer.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1
Most frequent genotypes recorded at enrollment in the overall population
Additional file 2: Table S2
Distribution of phenotype at enrollment in symptomatic patients according to genotype category
Additional file 3: Table S3
Clinical characteristics and patient-reported outcomes at enrollment in symptomatic patients according to genotype category
Additional file 4: Table S4
Neurologic characteristics at enrollment in symptomatic patients with a predominantly neurologic or mixed phenotype
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Gentile, L., Coelho, T., Dispenzieri, A. et al. A 15-year consolidated overview of data in over 6000 patients from the Transthyretin Amyloidosis Outcomes Survey (THAOS). Orphanet J Rare Dis 18, 350 (2023). https://doi.org/10.1186/s13023-023-02962-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13023-023-02962-5