Abstract
Background
Female carriers of dystrophin gene mutations (DMD-FC) were previously considered non-manifesting, but in recent decades, cardiomyopathy associated with muscular dystrophy and myocardial fibrosis has been described. Our study aimed to assess prospectively myocardial fibrosis in asymptomatic DMD-FC compared to a sex-matched control group (CG) with similar age distribution using native T1 mapping and extracellular volume (ECV) quantification by cardiovascular magnetic resonance (CMR) imaging.
Materials and methods
38 DMD-FC with verified genetic mutation and 22 healthy volunteers were included. Using CMR, native T1 relaxation time and ECV quantification were determined in each group. Late gadolinium enhancement (LGE) was assessed in all cases.
Results
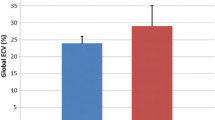
There were 38 DMD-FC (mean age 39.1 ± 8.8 years) and 22 healthy volunteers (mean age 39.9 ± 12.6 years) imagined by CMR. The mean global native T1 relaxation time was similar for DMD-FC and CG (1005.1 ± 26.3 ms vs. 1003.5 ± 25.0 ms; p-value = 0.81). Likewise, the mean global ECV value was also similar between the groups (27.92 ± 2.02% vs. 27.10 ± 2.89%; p-value = 0.20). The segmental analysis of mean ECV values according to the American Heart Association classification did not show any differences between DMD-FC and CG. There was a non-significant trend towards higher mean ECV values of DMD-FC in the inferior and inferolateral segments of the myocardium (p-value = 0.075 and 0.070 respectively).
Conclusion
There were no statistically significant differences in the mean global and segmental native T1 relaxation times and the mean global or segmental ECV values. There was a trend towards higher segmental mean ECV values of DMD-FC in the inferior and inferolateral walls of the myocardium.
Similar content being viewed by others

Introduction
Duchenne (DMD) and Becker (BMD) muscular dystrophies are X-linked recessive disorders caused by mutations in the dystrophin gene. Progressive muscular wasting, weakness, respiratory failure, and cardiovascular diseases are caused by pathogenic variants in DMD patients [1, 2]. It has been found that cardiac complications play a relevant role in muscular dystrophies [3].
The most common form of cardiac involvement in muscular dystrophy is dilated cardiomyopathy [4, 5], presenting as an age-related progression of left ventricular (LV) dysfunction and myocardial fibrosis, which can be detected by late gadolinium enhancement (LGE) cardiovascular magnetic resonance (CMR) imaging [6].
Female carriers of Duchenne muscular dystrophy gene mutations (DMD- healthy patients) were previously considered non-manifesting [5]. However, in recent decades, it has become evident that DMD-FC can be affected similarly, albeit more mildly, than DMD patients (affected males) [7,8,9,10]. DMD-FC can present cardiac involvement, such as cardiomyopathy, associated with muscular dystrophy [11, 12]. Although DMD-FC are usually asymptomatic, they also can be affected, similarly to DMD patients, by myocardial fibrosis. A well-established technique for assessing myocardial fibrosis is late gadolinium enhancement (LGE). However, it is limited for identifying interstitial diffuse fibrotic changes in the myocardium [13]. Since it may precede the development of LV dysfunction [11], its assessment is crucial in DMD-FC.
Therefore, other CMR-based methods, such as native T1 mapping and extracellular volume (ECV) quantification, might be more suitable for detecting diffuse myocardial fibrosis, as already-published studies in DMD/BMD patients have shown [14,15,16,17,18]. Also, there have been a few published reports about mildly elevated native T1 relaxation time or elevated ECV values in DMD/BMD-FC [11, 19, 20].
This study aims to assess prospectively myocardial fibrosis in asymptomatic DMD-FC compared to a sex-matched control group (CG) with similar age distribution using native T1 mapping and ECV quantification by CMR. Prior to now, there had been no direct comparison to CG.
Materials and methods
The demographically similar study population included 38 DMD-FC with verified genetic mutation and 22 healthy volunteers. The most common mutation was the deletion of 45–52 exons. The asymptomatic DMD-FC were defined based on clinical examination, and all completed the prepared questionnaire.
All eligible subjects who fulfilled the inclusion criteria (age over 18 years, signed informed consent, absence of CMR contraindications, and cardiovascular pathology besides dystrophic cardiomyopathies) were enrolled. The volunteers had no pathological findings on CMR, no anamnesis of cardiac disease, and no other pathological tests. Exclusion criteria for both groups included renal insufficiency (estimated glomerular filtration rate < 30 mL/min/1.73 m2), CMR contraindications, or limited life expectancy.
The hematocrit was obtained on the same day that CMR was performed. Following the Declaration of Helsinki (2000) of the World Medical Association, the Faculty Hospital St. Anne’s Ethics Committee (reference number 55 V/2016) approved the study.
CMR acquisition
CMR was performed using a 1.5T scanner (Ingenia, Philips Medical Systems, Best, The Netherlands) according to our standard protocol. It was equipped with 5-element posterior and 32-element anterior phased-array receiver coils allowing for parallel acquisition techniques in the supine position with repeated breath-hold. Functional imaging using balanced turbo field echo (b-TFE) cine sequences included four-chamber, two-chamber, and LV outflow tract long-axis views and a short-axis (SAX) stack. Typical acquisition parameters were: field of view 320 × 280 mm, reconstruction matrix 256, slice thickness 8 mm, acquisition voxel size 1.7 × 1.7 × 8.0 mm, repetition time ≈ 3.2 ms, echo time ≈ 1.6 ms, and SENSE factor 1.7.
LGE images in all long-axis views and the SAX view were acquired 10 min after an intravenous bolus of 0.2 mmol/kg of the gadolinium-based contrast agent gadobutrol (Gadovist, Bayer-Schering Pharma, Germany) using an inversion recovery (IR-TFE) sequence, and, if uncertain, by phase-sensitive inversion recovery TFE (PSIR-TFE). Both two- and three-dimensional acquisitions were performed in mid-diastole. Typical parameters for the IR-TFE sequence were field of view 320 × 320 mm, reconstruction matrix 288, voxel size 1.6 × 1.7 × 10 mm, repetition time ≈ 4.1 ms, echo time ≈ 1.2 ms, and SENSE factor 2.5.
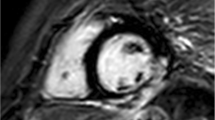
T1 mapping was performed as described previously [17] using a Modified Look-Locker Inversion recovery sequence (MOLLI) with a 5(3)3 scheme to measure native T1 (pre-contrast) and a 4(1)3(1)2 scheme for T1 post-gadolinium (15 min after contrast agent administration). MOLLI sequences were acquired at the mid-ventricular level in the SAX plane using typical imaging parameters (field of view 300 × 300 mm, reconstruction matrix 256, slice thickness 10 mm, acquisition voxel size 2.00 × 2.00 × 10.00 mm, time to repetition ≈ 2.2 ms, echo time ≈ 1.1 ms, flip angle 35°, SENSE factor 2).
MR data analysis
Two experienced readers (MLMP, TH) used cvi42 (release 5.13.9, Circle Cardiovascular Imaging, Calgary, Canada) to construct the native T1 and post-gadolinium T1 maps. They manually contoured the epi- and endocardial walls in the mid-ventricular slice of SAX using 10% of the myocardial wall as border cutting in both the native and the post-gadolinium images. Then, a motion correction algorithm (integrated into cvi42) was applied to register the images, and the software calculated pixel-by-pixel maps from these images. The ECV quantification was calculated according to (1-hematocrit) (1/T1myo,post – 1/T1myo,native)/(1/T1blood,post – 1/T1blood,native) for each segment, and the mean global ECV value was the average of the values in those segments [21]. The acronyms of the mentioned formula represent: the native and post-gadolinium T1 values of the myocardium/blood before and after the application of the contrast agent.
Wall motion abnormalities were assessed qualitatively (visually) by an experienced cardiologist (RP) with 29 years of experience and a radiologist (VF) with 38 years of experience with CMR. LV functional and morphological parameters were calculated from the SAX stack using the summation-of-disc method following the recommendations on post-processing evaluation from the Society for Cardiovascular Magnetic Resonance (SCMR) [22].
The radiologist (VF) and the cardiologist (RP) employed a semi-quantitative approach to determine the presence of LGE according to the American Heart Association (AHA) 17-segment model [23] in the IntelliSpace Portal workspace (version 11, Philips Healthcare). LGE was defined as positive if the visual enhancement was higher than the mean signal intensity of the reference myocardium, a remote or unaffected myocardial region within the same patient. A DMD-FC with LGE in at least one myocardial segment was considered LGE-positive. If no enhancement was observed, the DMD-FC was identified as LGE-negative.
Statistics
Variables in both groups were compared using the Student’s t-test for unpaired data. The normal distribution was checked by the Kolmogorov-Smirnov test and a visual inspection of histograms; in a case when the Student’s t-test revealed no difference, this meant the Kolmogorov-Smirnov test was also used to check the identity of distributions of quantitative variables in both groups. The data are presented as mean ± standard deviation (SD).
The power of the tests was considered for native T1 relaxation time and ECV values to determine the magnitude of the effect that could be identified as statistically significant by the Student’s t-test. The following assumptions were used: α = 0.05; power = 0.8 (0.6, respectively); normal distribution; standard deviation corresponding with the actual distribution in the CG. As the nature of our study was explorative, we neither assumed nor performed any multiple testing corrections, which would increase the risk of type II error and decrease the power of the test.
The interobserver agreement of both native and post-gadolinium global T1 relaxation time was assessed with the intraclass correlation coefficient (ICC, two-way mixed-effects model), which was determined from eight randomly selected cases analysed by two readers (MLMP, TH).
All analyses were performed using Statistica (version 14.0. TIBCO Software Inc., 2020) and in R (v4.2.1) with RStudio IDE (v2022.7.1.554, RStudio, PBC) software. The value of α = 0.05 was used as a threshold for statistical significance throughout the analyses.
Results
38 DMD-FC (mean age 39.1 ± 8.8 years) and 22 healthy volunteers (mean age 39.9 ± 12.6 years) were included. There was no statistically significant difference between the LVEF of DMD-FC (65.7 ± 5.5%) and CG (68.4 ± 6.57%; p = 0.09). 5% of asymptomatic DMD-FC of our cohort had small hypokinesia of the apex and the inferolateral wall of LV. Also, 20% of DMD-FC had LGE that was detected on the inferolateral wall (4 DMD-FC) and inferior wall (3 DMD-FC) of LV, while LGE was absent in the CG.
The mean global native T1 relaxation time was similar for DMD-FC and CG (1005.1 ± 26.3 ms vs. 1003.5 ± 25 ms; p-value = 0.81) (Fig. 1), as well as the mean global ECV value (27.92 ± 2.02% vs. 27.09 ± 2.89%; p-value = 0.20) (Fig. 2). The representative native and post-gadolinium T1 maps are presented in Fig. 3 for DMD-FC and Fig. 4 for healthy volunteer. No statistically significant differences were detected between DMD-FC with LGE positive and LGE negative in the mean ECV segmental values. The ECV segmental quantification analysis, according to the AHA classification, did not show any differences for DMD-FC vs. CG. Although statistically non-significant, there was a trend towards higher mean ECV values in the inferior (p = 0.075) and inferolateral (p = 0.070) segments of the myocardium in DMD-FC. The detailed results are shown in Table 1. When the identical distribution of measured variables in DMD-FC and in CG was checked by Kolmogorov-Smirnov test, it yielded a p-value > 0.10 in all cases, including mean global and segmental T1 relaxation times and ECV values, age and LVEF.
Regarding the global native T1 relaxation time, the sample size was sufficiently large to statistically prove a difference of 19 ms with a power of 80% and a difference of 15 ms with a power of 60%. In the case of ECV values, the sample size enabled the discrimination of values differing by 2.3% with a power of 80% and a difference of 1.8% with a power of 60%.
The values of ICC were 0.980 (95% CI: 0.921 to 0.995) for mean global native T1 and 0.976 (95% CI: 0.906 to 0.994) for mean global T1 post-gadolinium relaxation times, showing excellent interobserver reliability.
Discussion
There were no statistically significant differences in the mean global and segmental native T1 relaxation times or the mean global and segmental ECV values in asymptomatic DMD-FC with normal LVEF compared to sex -matched CG with similar age distribution. Regarding the segmental values, although statistically non-significant, there was a trend towards higher mean ECV values in the inferior and inferolateral segments of the myocardium in DMD-FC in our study. This may be potentially of concern, as similar segments are affected as in DMD patients; however it should be noted that the magnitude of those differences is much smaller than of those observed between DMD patients and CG, as is evident in various cited studies [15, 16] including ours [17], which correspond to the typically affected regions in the muscular dystrophies.
Cardiac involvement, such as cardiomyopathy and the presence of LGE in typical localization, was recently described in DMD-FC [11, 12]. To date, there have been only 3 published works focused on native T1 mapping and ECV quantification in DMD/BMD-FC [11, 19, 20], of which only one used a case-control design.
The first of the studies describes diffuse myocardial fibrosis assessed by ECV quantification in DMD/BMD-FC of gene mutations for muscular dystrophies [20]. Analysed were 5 DMD/BMD-FC ranging from 43.0 to 51.7 years. It showed elevated global ECV values of 37.4% in DMD/BMD-FC and BMD/DMD/myotonic dystrophic patients regardless of the presence of LGE. All 5 screened DMD/BMD-FC were older than DMD-FC in our study. Moreover, one of them had reduced LVEF, 2 of them were LGE positive, and there was also a combination of DMD and BMD-FC.
The second one [11] also reported mildly abnormal ECV values in DMD-FC.
The last study compared the DMD-FC to non-carrier female relatives and CG and found higher native T1 relaxation time in DMD/BMD-FC compared to non-carriers and CG [19]; however this study did not assess the ECV quantification or regional differences in native T1 relaxation time. While our study had sufficient power to reveal the differences in global native T1 relaxation time with a size comparable to [19] and employed study subjects of a comparable age, it did not confirm the increase in native T1 relaxation time found in this study.
The main contribution of our study compared to the mentioned studies [11, 12, 19, 20] is the evaluation of the diffuse fibrotic changes of the myocardium using native T1 mapping and ECV quantification in a much larger cohort of DMD-FC, and with regards to CG. Moreover, we focused on the assessment of more than just the presence of LGE in the typical location or the described mildly elevated value of ECV or global native T1 relaxation time. This is the first study that compares global and segmental native T1 and ECV values between DMD-FC and CG in a case/control design.
LGE can aid in detecting the extent and the location of myocardial fibrosis. Additionally LGE can uncover incipient heart disease by its presence [14, 24]. Whereas native T1 mapping and ECV quantification allow for the measurement of diffuse fibrosis that foregoes the mentioned LGE. These methods are also useful in detecting myocardial inflammation or any alteration of the extracellular space [14, 25].
Contrary to DMD-FC, many more studies have assessed native T1 mapping and quantified ECV in DMD patients [16, 26, 27]. Although LGE positive DMD patients exhibited an increased global native T1 relaxation time compared to CG, there was no difference between LGE negative DMD patients and CG [27]. Moreover, DMD patients had a significantly increased lateral myocardial native T1 relaxation time compared to CG [28].
These results align with other authors who detected increased native T1 relaxation time and ECV values in DMD patients. ECV quantification was regardless the ability to differentiate LGE negative DMD patients and CG [29].
Both native T1 mapping and ECV quantification can identify the differences and regional abnormalities on the inferolateral wall of the LV between DMD patients and CG with or without LGE [21, 30,31,32,33]. Additionally, increased native T1 relaxation time and reduced LVEF was reported in LGE positive DMD patients, evidencing a relationship between native T1 relaxation time and LV function [28].
Based on our previous studies, all global LV strains and tissue Doppler parameters in asymptomatic DMD-FC were decreased compared to CG [34, 35]. Despite a preserved LVEF, subclinical changes in the LV systolic function were discovered in DMD-FC, including decreased global LV strains. However, their mean global and segmental native T1 relaxation times and mean global and segmental ECV values were similar to sex- matched CG with similar age distribution. Furthermore, 20% of our DMD-FC cohort had LGE on the inferolateral wall of LV, which is typical localization for the extracellular volume expansion described in DMD patients [36, 37].
While the differences in native T1 relaxation time and ECV quantification in symptomatic DMD patients are already known, our DMD-FC cohort did not have any cardiac symptoms.
Limitations
This work has some limitations. Some of them are related to the general limitations of native T1 mapping [4, 38]. It is also limited by its sample size due to the rare occurrence of DMD disease. It is a single centre study. All the subjects involved in this study, were analysed under the same conditions: the images were acquired with the same equipment and protocol, and the analyses were assessed similarly. The healthy volunteers were not tightly selected, as they were considered healthy because of their lack of CMR-based evidence of cardiac problems. Any possible bias affected all the subjects equally. Although the interobserver agreement was excellent, it was determined only for the mean global native T1 relaxation time and mean global ECV values. Future research is essential to validate the whole process’s reproducibility.
Conclusion
The mean global and segmental native T1 relaxation times and mean global and segmental ECV values in asymptomatic DMD-FC with normal LVEF were similar to CG, as there was no statistically significant difference in any of the CMR-assessed parameters. Although non-significant, there was a trend towards higher mean ECV values of DMD-FC in inferior and inferolateral segments of the myocardium, which should be subjected to prospective assessment.
Data Availability
The datasets analysed during the current study are available from the corresponding author upon reasonable request. Data are located in controlled access data storage REDCap at St. Anne’s University Hospital (https://redcap.fnusa.cz/redcap/).
Change history
19 October 2023
A Correction to this paper has been published: https://doi.org/10.1186/s13023-023-02922-z
Abbreviations
- AHA:
-
American heart association
- BMD:
-
Becker muscular dystrophy
- b-TFE:
-
balanced - turbo field echo
- CI:
-
Confidence interval
- CG:
-
Control group
- CMR:
-
Cardiac magnetic resonance
- DMD:
-
Duchenne muscular dystrophy
- DMD-FC:
-
Female carriers of Duchenne muscular dystrophy gene mutations
- ECV:
-
Extracellular volume
- EDV:
-
End-diastolic volume
- ESV:
-
End-systolic volume
- ICC:
-
Intraclass correlation
- IR-TFE:
-
Inversion-recovery turbo field echo
- LGE:
-
Late gadolinium enhancement
- LV:
-
Left ventricle
- LVEF:
-
Ejection fraction of left ventricle
- PSIR:
-
Phase-sensitive inversion recovery
- SAX:
-
Short axis
- SCMR:
-
Society for Cardiovascular Magnetic Resonance
- SD:
-
Standard deviation
- T1myo,post:
-
post-gadolinium T1 values of myocardium
- T1myo,native:
-
native T1 values of myocardium
- T1blood,post:
-
post-gadolinium T1 values of blood
- T1blood,native:
-
native T1 values of blood
References
Kamdar F, Garry DJ. Dystrophin-Deficient Cardiomyopathy. J Am Coll Cardiol. 2016;67(21):2533–46.
Hermans MCE, Pinto YM, Merkies ISJ, de Die-Smulders CEM, Crijns HJGM, Faber CG. Hereditary muscular dystrophies and the heart. Neuromuscul Disord NMD. 2010;20(8):479–92.
Feingold B, Mahle WT, Auerbach S, Clemens P, Domenighetti AA, Jefferies JL, et al. Management of Cardiac involvement Associated with Neuromuscular Diseases: A Scientific Statement from the American Heart Association. Circulation. 2017;136(13):e200–31.
McNally EM, Kaltman JR, Benson DW, Canter CE, Cripe LH, Duan D, et al. Contemporary cardiac issues in Duchenne muscular dystrophy. Working Group of the National Heart, Lung, and Blood Institute in collaboration with parent project muscular dystrophy. Circulation. 2015;131(18):1590–8.
Demos J, Dreyfus JC, Schapira F, Schapira G. [Biological anomalies in the apparently healthy transmitters of muscular dystrophy]. Rev Can Biol. 1962;21:587–97.
Aikawa T, Takeda A, Oyama-Manabe N, Naya M, Yamazawa H, Koyanagawa K, et al. Progressive left ventricular dysfunction and myocardial fibrosis in Duchenne and Becker muscular dystrophy: a longitudinal cardiovascular magnetic resonance study. Pediatr Cardiol. 2019;40(2):384–92.
Mccaffrey T, Guglieri M, Murphy AP, Bushby K, Johnson A, Bourke JP. Cardiac involvement in female carriers of duchenne or becker muscular dystrophy. Muscle Nerve. 2017;55(6):810–8.
Lang SM, Shugh S, Mazur W, Sticka JJ, Rattan MS, Jefferies JL, et al. Myocardial fibrosis and left ventricular dysfunction in Duchenne muscular dystrophy carriers using Cardiac magnetic resonance imaging. Pediatr Cardiol. 2015;36(7):1495–501.
Solheim TA, Fornander F, Raja AA, Møgelvang R, Poulsen NS, Dunø M, et al. Cardiac involvement in women with pathogenic dystrophin gene variants. Front Neurol. 2021;12:707838. Available from: https://www.frontiersin.org/articles/. https://doi.org/10.3389/fneur.2021.707838.
Soltanzadeh P, Friez MJ, Dunn D, von Niederhausern A, Gurvich OL, Swoboda KJ, et al. Clinical and genetic characterization of manifesting carriers of DMD mutations. Neuromuscul Disord NMD. 2010;20(8):499–504.
Wexberg P, Avanzini M, Mascherbauer J, Pfaffenberger S, Freudenthaler B, Bittner R, et al. Myocardial late gadolinium enhancement is associated with clinical presentation in Duchenne muscular dystrophy carriers. J Cardiovasc Magn Reson off J Soc Cardiovasc Magn Reson. 2016;18(1):61.
Florian A, Rösch S, Bietenbeck M, Engelen M, Stypmann J, Waltenberger J, et al. Cardiac involvement in female Duchenne and Becker muscular dystrophy carriers in comparison to their first-degree male relatives: a comparative cardiovascular magnetic resonance study. Eur Heart J Cardiovasc Imaging. 2016;17(3):326–33.
Mewton N, Liu CY, Croisille P, Bluemke D, Lima JAC. Assessment of myocardial fibrosis with cardiovascular magnetic resonance. J Am Coll Cardiol. 2011;57(8):891–903.
Florian A, Ludwig A, Rösch S, Yildiz H, Sechtem U, Yilmaz A. Myocardial fibrosis imaging based on T1-mapping and extracellular volume fraction (ECV) measurement in muscular dystrophy patients: diagnostic value compared with conventional late gadolinium enhancement (LGE) imaging. Eur Heart J Cardiovasc Imaging. 2014;15(9):1004–12.
Starc JJ, Moore RA, Rattan MS, Villa CR, Gao Z, Mazur W, et al. Elevated myocardial extracellular volume fraction in Duchenne muscular dystrophy. Pediatr Cardiol. 2017;38(7):1485–92.
Soslow J, Damon S, Crum K, Lawson M, Slaughter J, Xu M et al. Increased myocardial native T1 and extracellular volume in patients with Duchenne muscular dystrophy. J Cardiovasc Magn Reson. 2015;18.
Panovský R, Pešl M, Máchal J, Holeček T, Feitová V, Juříková L, et al. Quantitative assessment of left ventricular longitudinal function and myocardial deformation in Duchenne muscular dystrophy patients. Orphanet J Rare Dis. 2021;16(1):57.
Soslow JH, Damon SM, Crum K, Lawson MA, Slaughter JC, Xu M, et al. Increased myocardial native T1 and extracellular volume in patients with Duchenne muscular dystrophy. J Cardiovasc Magn Reson. 2016;18(1):5.
Mah ML, Cripe L, Slawinski MK, Al-Zaidy SA, Camino E, Lehman KJ, et al. Duchenne and Becker muscular dystrophy carriers: evidence of cardiomyopathy by exercise and cardiac MRI testing. Int J Cardiol. 2020;316:257–65.
Koyanagawa K, Kobayashi Y, Aikawa T, Takeda A, Shiraishi H, Tsuneta S, et al. Myocardial T(1)-mapping and extracellular volume quantification in patients and putative carriers of muscular dystrophy: early experience. Magn Reson Med Sci MRMS off J Jpn Soc Magn Reson Med. 2021;20(3):320–4.
Jerosch-Herold M, Kwong RY. Cardiac T(1) imaging. Top Magn Reson Imaging TMRI. 2014;23(1):3–11.
Schulz-Menger J, Bluemke DA, Bremerich J, Flamm SD, Fogel MA, Friedrich MG, et al. Standardized image interpretation and post-processing in cardiovascular magnetic resonance – 2020 update: Society for Cardiovascular magnetic resonance (SCMR): Board of Trustees Task Force on standardized post-processing. J Cardiovasc Magn Reson off J Soc Cardiovasc Magn Reson. 2020;22(1):19.
Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation. 2002;105(4):539–42.
Mavrogeni S, Papavasiliou A, Giannakopoulou K, Markousis-Mavrogenis G, Pons MR, Karanasios E et al. Oedema-fibrosis in Duchenne muscular dystrophy: role of cardiovascular magnetic resonance imaging. Eur J Clin Invest. 2017;47(12).
Hor KN, Taylor MD, Al-Khalidi HR, Cripe LH, Raman SV, Jefferies JL, et al. Prevalence and distribution of late gadolinium enhancement in a large population of patients with Duchenne muscular dystrophy: effect of age and left ventricular systolic function. J Cardiovasc Magn Reson off J Soc Cardiovasc Magn Reson. 2013;15(1):107.
van Woerden G, van Veldhuisen DJ, Gorter TM, Willems TP, van Empel VPM, Peters A, et al. The clinical and prognostic value of late Gadolinium enhancement imaging in heart failure with mid-range and preserved ejection fraction. Heart Vessels. 2022;37(2):273–81.
Xu K, Xu H, Xu R, Xie L, jun, Yang Z gang, Yu L et al. Global, segmental and layer specific analysis of myocardial involvement in Duchenne muscular dystrophy by cardiovascular magnetic resonance native T1 mapping. J Cardiovasc Magn Reson. 2021;23(1):110.
Maforo NG, Magrath P, Moulin K, Shao J, Kim GH, Prosper A, et al. T(1)-Mapping and extracellular volume estimates in pediatric subjects with Duchenne muscular dystrophy and healthy controls at 3T. J Cardiovasc Magn Reson off J Soc Cardiovasc Magn Reson. 2020;22(1):85.
Olivieri LJ, Kellman P, McCarter RJ, Cross RR, Hansen MS, Spurney CF. Native T1 values identify myocardial changes and stratify disease severity in patients with Duchenne muscular dystrophy. J Cardiovasc Magn Reson. 2016;18(1):72.
Frankel KA, Rosser RJ. The pathology of the heart in progressive muscular dystrophy: epimyocardial fibrosis. Hum Pathol. 1976;7(4):375–86.
Everett RJ, Stirrat CG, Semple SIR, Newby DE, Dweck MR, Mirsadraee S. Assessment of myocardial fibrosis with T1 mapping MRI. Clin Radiol. 2016;71(8):768–78.
Messroghli DR, Radjenovic A, Kozerke S, Higgins DM, Sivananthan MU, Ridgway JP. Modified Look-Locker inversion recovery (MOLLI) for high-resolution T1 mapping of the heart. Magn Reson Med. 2004;52(1):141–6.
Puntmann VO, Voigt T, Chen Z, Mayr M, Karim R, Rhode K, et al. Native T1 mapping in differentiation of normal myocardium from diffuse disease in hypertrophic and dilated cardiomyopathy. JACC Cardiovasc Imaging. 2013;6(4):475–84.
Kincl V, Panovský R, Pešl M, Máchal J, Juříková L, Haberlová J, et al. Echocardiographic signs of subclinical cardiac function impairment in Duchenne dystrophy gene carriers. Sci Rep. 2020;10(1):20794.
Masárová L, Mojica-Pisciotti ML, Panovský R, Kincl V, Pešl M, et al. Decreased global strains of LV in asymptomatic female duchenne muscular dystrophy gene carriers using CMR-FT. JACC Cardiovasc Imaging. 2021 May;14(5):1070–1072. https://doi.org/10.1016/j.jcmg.2020.09.016.
Silva MC, Meira ZMA, Gurgel Giannetti J, da Silva MM, Campos AFO, de Barbosa M. Myocardial delayed enhancement by magnetic resonance imaging in patients with muscular dystrophy. J Am Coll Cardiol. 2007;49(18):1874–9.
Puchalski MD, Williams RV, Askovich B, Sower CT, Hor KH, Su JT, et al. Late gadolinium enhancement: precursor to cardiomyopathy in Duchenne muscular dystrophy? Int J Cardiovasc Imaging. 2009;25(1):57–63.
von Knobelsdorff-Brenkenhoff F, Prothmann M, Dieringer MA, Wassmuth R, Greiser A, Schwenke C, et al. Myocardial T1 and T2 mapping at 3 T: reference values, influencing factors and implications. J Cardiovasc Magn Reson off J Soc Cardiovasc Magn Reson. 2013;15(1):53.
Acknowledgements
Not applicable.
Funding
This work was supported by the European Regional Development Fund - Project ENOCH (No. CZ.02.1.01/0.0/0.0/16_019/0000868). This publication was written at Masaryk University as part of the project entitled “Novel imaging, computing and analytical methods in cardiovascular diseases diagnostics and monitoring” number MUNI/A/1462/2021 with the support of the Specific University Research Grant, as provided by the Ministry of Education, Youth and Sports of the Czech Republic in the year 2022.
Author information
Authors and Affiliations
Contributions
RP, VK, and MP were the main contributors to the study; they also designed the study. The main role of LM was to write the manuscript. LJ and LO contributed to patient recruitment and inclusion, VF and TH supervised the CMR examinations, and VF and LO performed the clinical data analysis. MMP and TH performed the CMR data analysis, and JM performed the statistical analysis. All authors read, critically reviewed, and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was performed in accordance with the Declaration of Helsinki (2000) of the World Medical Association. Ethics Committee of the Faculty Hospital St. Anne’s (reference number 55 V/2016) approved the study. Written informed consent was obtained from the subjects and/or their legally authorised representative.
Consent for publication
no person´s personal data are published.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original version of this article was revised: authors’ first and last names were switched and incorrectly tagged. Also, there were incorrect style issues in body text. Figure 3 and Figure 4 and their captions had to be corrected as well.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Masárová, L., Panovský, R., Pešl, M. et al. Myocardial native T1 mapping and extracellular volume quantification in asymptomatic female carriers of Duchenne muscular dystrophy gene mutations. Orphanet J Rare Dis 18, 283 (2023). https://doi.org/10.1186/s13023-023-02899-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13023-023-02899-9