Abstract
Background
Prader–Willi syndrome (PWS) is a rare and multisystemic genetic disorder that is characterized by severe hypotonia, hyperphagia, short stature, and global developmental delay. Although early recombinant human growth hormone (rhGH) treatment has been proven to rescue some symptoms and bring additional benefits to PWS patients, studies in patients under 2 years old are scarce. Thus, this study aims to investigate the effectiveness and safety of rhGH treatment for young children.
Methods
A total of 96 genetically confirmed Chinese PWS infants or toddlers (47 males) followed between 2013 and 2022 were retrospectively analyzed. Sixty-five infants (early treatment group) started rhGH treatment during their first year, and 31 toddlers (later treatment group) started at the age of 1–2 years. Auxological parameters, carbohydrate metabolism parameters, thyroid function, liver function, insulin-like growth factor-1 (IGF-1), and radiographs were acquired before the initiation of the treatment and every 3–6 months thereafter. Height/length, weight, and weight for height were expressed as standard deviation scores (SDSs) according to WHO child growth standards.
Results
The mean SDS of length/height in the early treatment group was significantly higher than that in the later treatment group throughout the observation period (all P < 0.001). The change in the length SDS between the two groups at 1 year old and 4 years old was 1.50 (95% CI, 0.88–2.13) and 0.63 (95% CI, 0.16–1.10), respectively. Compared to the later treatment group, the weight SDS in the early treatment group increased by 0.94 (95% CI, 0.37–1.52) at 1 year old and 0.84 (95% CI, 0.28–1.39) at 2 years old. No statistical significance was found after 2.5 years of age. No significant differences were observed in IGF-1, incidence of liver dysfunction, hypothyroidism or spinal deformity between the two groups.
Conclusions
rhGH treatment improved growth and body composition in infants and toddlers. Furthermore, an early start of rhGH treatment is expected to have more efficacy than the later treatment group without an increase in adverse effects.
Similar content being viewed by others
Introduction
Prader–Willi syndrome (PWS) is a rare genetic disorder resulting from the underexpression of imprinted genes within the chromosome 15q11-q13 region [1, 2]. The prevalence of PWS is approximately 1 in 10,000–30,000 newborns [3, 4]. PWS has three main genetic types, including deletion type with deletion of paternal copy of key region that accounts for 65%-70%, maternal uniparental disomy (mUPD) for chromosome 15 that accounts for 20%-30%, and the imprinting center defect (ID) that includes the imprinting center deletion and epimutation, accounting for less than 5%. Translocation in the region of chromosome 15q11-q13 or small deletions in the key gene of PWS were also reported in very rare cases [5,6,7,8,9,10,11]. The clinical presentations vary with age, impacting multiple systems, with the endocrine system being the most affected in PWS [6]. Growth hormone (GH) deficiency (GHD) is present in most cases and may be associated with hypotonia after birth, short stature, increased fat mass, and decreased movement and energy expenditure [12, 13].
Recombinant human GH (rhGH) treatment has been suggested for adults and children with PWS. GH treatment will improve growth body composition, muscle strength, respiratory function, and even psychomotor development. Early rhGH treatment has been found to improve feeding difficulty of infants and cognition of young children with PWS [14,15,16,17,18,19,20] which suggests it is better to start treatment before 2 years of age. The timing of starting GH treatment is still controversial (e.g., less than 12 months or 24 months) [21]. Several authors recommended that starting rhGH therapy as early as possible (approximately 3–6 months of age) resulted in additional benefits [22,23,24]. However, large-sample studies in patients treated at an early age and comparisons of the efficacy of rhGH treatment at different ages are still lacking. Therefore, this study aimed to investigate the effect and safety in Chinese PWS patients aged less than 2 years, especially starting from infancy.
Material and methods
Subjects and grouping
This study is part of a project started by the PWS Research Group from the Children’s Hospital of Zhejiang University School of Medicine. In the PWS Research Group and PWS Care & Support Center (an association of PWS patients and parents) register system, 1491 Chinese patients with genetically confirmed PWS were registered until Dec. 2021. A total of 84 subjects were excluded due to a lack of detailed data. Among 1407 PWS patients, 688 (48.90%) had accepted rhGH treatment.
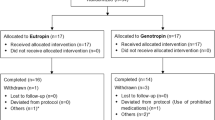
Only 170 patients had correct initiation time of rhGH treatment. Fifteen patients had interrupted rhGH treatment. Among the 170 patients, 86 (50.59%) PWS infants accepted rhGH therapy early in infancy. A total of 65 patients were followed up to 4 years old as the early treatment group, while 21 patients dropped out for unknown reasons. Another 36 patients (approximately 21.17%) started rhGH treatment between 1 and 2 years as the later treatment group. Among them, 31 were followed up to 4 years old. Approximately 19.41% of subjects (33/170) initiated rhGH therapy after 2 years of age.
Finally, 96 PWS pediatric patients were included in this study (Fig. 1), including 65 patients in the early treatment group and 31 patients in the later treatment group. All patients were genetically confirmed to have PWS, including 63 (65.62%) deletion types, 22 (22.92%) nondeletion types, and 11 (11.46%) unknown diagnosed with methylation-sensitive polymerase chain reaction (MS-PCR). rhGH treatment in these patients started at the age of 2 months to 2 years and was not interrupted to 4 years old. The differences in auxological parameters, carbohydrate metabolism, and safety between these two groups of PWS pediatric patients were compared.
The study protocol was approved by the Ethics Committee of the Children's Hospital of Zhejiang University School of Medicine. Informed consent was obtained from all parents or the patients registered in the PWS Registry.
Follow-up
The dosage of rhGH administered to PWS pediatric patients ranged from 0.46–1.04 mg/m2/d with a median of 0.70 (0.20) mg/m2/d and was slightly adjusted by professional pediatric endocrinologists according to height, weight and insulin-like growth factor 1 (IGF-1), which kept IGF-1 levels within a 2 standard deviation score (SDS) [21, 25, 26]. No significant difference in rhGH dose was noted between these two groups (P > 0.05). Physical examination, fasting blood samples, standing spinal coronal and radiographs were collected before rhGH therapy and at the ages of 1, 1.5, 2, 2.5, 3, 3.5, and 4 years. Only data within 2 months before and after these time points were considered valid data. The follow-up rates of body composition at 1, 1.5, 2, 2.5, 3, 3.5, and 4 years old were 87.69%, 87.69%, 84.62%, 80.00%, 72.31%, 58.46%, and 47.69% for the early treatment group and 74.19%, 83.87%, 96.77%, 90.32%, 83.87%, 70.97%, and 64.52% for the later treatment group, respectively, without a significant difference.
Anthropometry
Height/length was measured in a standing/supine position to the nearest 1 mm. Weight was assessed under fasting conditions to the nearest 0.1 kg. SDS for height/length, weight, and weight for height were adjusted for sex and age according to WHO child growth standards (www.who.int).
Laboratory assessments
Fasting blood samples were collected approximately every 3–6 months for the measurement of fasting glucose (FG) and fasting insulin (FI), IGF-1, thyroid function including thyroid stimulating hormone (TSH), thyroxine (T4), free thyroxine (fT4), triiodothyronine (T3), free triiodothyronine (fT3), and liver function including aspartate transaminase (AST), alanine aminotransferase (ALT). Fasting blood samples were collected at least 3 h after feeding in infancy and overnight after 1 year of age. Insulin resistance (IR) was assessed by the homeostasis model assessment of IR (HOMA-IR), which was defined as [FI (μIU/mL) × FG (mg/dL)]/405 [27]. Due to different kinds of assays in measuring IGF-1, the IGF-1 level was evaluated by age-matched reference values supplied by corresponding manufacturers. The diagnosis of hypothyroidism was made with decreased fT4 (fT4 < 9.01 pmol/L) or increased TSH (> 4.94 mIU/L) 2 times. Liver dysfunction was diagnosed as ALT > 50 U/L and/or AST > 60 U/L without hemolysis in blood samples.
Spinal radiographs
Standing anteroposterior and later spinal radiographs were evaluated by professional pediatric orthopedic surgeons during the observation period. Spinal deformities, including scoliosis, kyphosis, and kyphoscoliosis, were diagnosed by professional pediatric orthopedic surgeons. The Cobb angle was the curve between the most tilted cephalad and the inferior end plate of the most caudad vertebra. Scoliosis was defined as Cobb angle > 10 degrees.
Statistical analysis
Statistical analysis was performed by using the Statistical Package for Social Sciences (SPSS Version 21.0, Chicago, IL). Normality was tested by the Shapiro–Wilk test. Normally distributed measurement data are presented as the mean ± SD, while nonnormally distributed measurement data are presented as the median (interquartile range, IQR) depending on the distribution of the data. The homogeneity of the baseline data was assessed by an independent sample t test. Nonparametric tests were performed if needed. Considering multiple testing, the generalized estimated equation (GEE) model followed by Sidak’s post hoc test was performed to evaluate differences in growth and IGF-1 between the early and later treatment groups. According to the QIC value, the auto regression structure was used in this study. In the GEE model, auxological and metabolism parameters were presented as the outcome, age and group were considered the main effect, and the interaction effect of the group by age was also assessed. A significant interaction effect of the group by age suggests differential rates of change in the parameters between the two groups. In addition, the differences in sex and gene subtype were evaluated. The chi-square test was employed to analyze the enumeration data (e.g., sex, genotype, incidence of spinal deformity, and hypothyroidism) between the two groups. A P value less than 0.05 was considered statistically significant.
Results
Sample description and baseline characteristics
The early treatment group included 29 males and 36 females. Their age of rhGH treatment ranged from 2 to 11 months, with a median age of 6 months. The later treatment group consisted of 18 males and 13 females. Their age of rhGH treatment ranged from 12 to 24 months, with a median of 17 months. The difference in sex was not significant between the two groups (χ2 = 1.52, P = 0.22).
There were 42 patients (64.62%) with the deletion type, 15 (23.08%) with the nondeletion type, and 8 (12.31%) with undefined types in the early treatment group. There were 21 patients (67.74%) with the deletion type, 7 (22.58%) with the nondeletion type, and 3 (9.68%) with undefined types in the later treatment group. There was no significant difference in genotypes between these two groups (χ2 = 0.16, P = 0.92).
The mean baseline height/length SDS in the early and later treatment groups were −1.10 ± 1.36 and −1.42 ± 1.54, respectively, without statistical significance (t = 1.04, P = 0.30). The baseline weight SDS in the early treatment group was less than that in the later treatment group (−1.81 ± 1.02 vs. −1.24 ± 1.12, t = 2.46, P = 0.02). The baseline weight-for-length SDS was -1.58 (2.26) in the early treatment group and −0.94 (1.36) in the later treatment group, without statistical significance (Z = 1.02, P = 0.31).
FG and FI were obtained from 32 patients (15 males, 17 females), including 19 in the early treatment group and 13 in the later treatment group. Sex (P = 0.28) and genotype (χ2 = 0.96, P = 0.81) were balanced between these two groups. At baseline, no differences in FG (Z = 0.39, P = 0.71), FI (Z = 0.82, P = 0.43), or HOMA-IR (Z = 0.68, P = 0.52) were found between these two groups, as shown in Table 1.
Height/length SDS
The generalized estimated equation showed a significant increase in the height/length SDS in both groups over time, as shown in Fig. 2A (time effect χ2 = 67.78, P < 0.001). The early treatment group was significantly higher than the later treatment group throughout the whole observation period (group effect χ2 = 15.54, P < 0.001). The height/length SDS at the age of 1 year was significantly higher than that at baseline in the early treatment group (t = 5.38, P < 0.001). Nevertheless, in the later treatment group, the height/length SDS was similar to the baseline at 1 year old (t = 1.23, P = 0.23). The difference in height/length SDS between the two groups gradually decreased with the development of time. The difference in height/length SDS between the two groups at 1 year old was 1.50 (95% CI, 0.88–2.13), while it was 0.63 (95% CI, 0.16–1.10) and 4 years old (Table 2).
Changes in height SDS (A), weight SDS (B), and weight for height SDS (C) between the early treatment group (shaded) and later treatment group (blank) from baseline to 4 years old. Height-SDS, weight-SDS, and weight for height-SDS were adjusted for sex and age according to WHO child growth standards. Error bars represent means with 95% CI. #, P < 0.05, compared with the later treatment group
Weight and weight for height/length SDS
The GEE model showed a significant time effect in weight SDS (χ2 = 94.64, P < 0.001). However, there were no significant main effects of group (χ2 = 3.16, P = 0.08) or group by age interaction (χ2 = 11.50, P = 0.07). The difference in weight SDS between the two groups at 1 year old was 0.94 (95% CI, 0.37–1.52) and 0.84 (95% CI, 0.28–1.39) at 2 years old. However, at the age of 2.5 years old, the difference in weight SDS was 0.46 (95% CI, −0.14 to 1.06), without statistical significance. From 2.5 years old, the weight SDS did not show significant differences between the early and later treatment groups (P = 0.14), as shown in Fig. 2B.
No significant differences in group effect (χ2 = 0.09, P = 0.77) or group by age interaction effect of weight and height SDS (χ2 = 10.23, P = 0.12) were observed throughout the whole observation. Only at the age of 4 years old was the weight for height slightly increased by 0.83 (95% CI, −0.01 to 1.68, P = 0.052) in the later treatment group compared to the early treatment group (Fig. 2C and Table 2).
Serum IGF-1
Serum IGF-1 levels were obtained from 37 children, including 25 in the early treatment group and 12 in the later treatment group. The kinds of assays that measure serum IGF-1 were different in different laboratories in this study. Thus, compared with age-matched reference intervals supplied by corresponding manufacturers, the rank of IGF-1 level was divided into high (> mean + 1SD), normal (mean ± 1SD), and low (< mean-1SD). No significant effects of group (χ2 = 0.47, P = 0.49), gender (χ2 = 0.44, P = 0.51), or genotype (χ2 = 0.96, P = 0.62) were observed by the GEE model. The time effect was strong (χ2 = 22.66, P < 0.001). At 1 year old, 9 individuals had lower levels of IGF-1 than the normal range, while only one was 4 years old, with a significant difference (P < 0.001).
Carbohydrate metabolism
Fasting blood samples were obtained from 32 patients (15 males, 17 females), including 19 in the early treatment group and 13 in the later treatment group. At the age of 1 year, the FIs in the early treatment group and later treatment group were 4.47 (5.90) and 2.00 (3.50), respectively, with a significant difference (Z = 2.37, P = 0.016). Moreover, HOMA-IR in the early treatment group was significantly higher than that in the later treatment group at 1 year old (Z = 2.33, P = 0.018). However, the effect was reserved when the later treatment group started rhGH treatment, with no statistical significance during the course of the study. No significant difference in FG was observed between the two groups throughout the observation period (Fig. 3). In the first or second year of treatment, 3 patients (15.79%) had FG > 5.6 mmol/L in the early treatment group, while 2 patients (15.38%) had FG > 5.6 mmol/L in the later treatment group. The highest FG in our study was 6.32 mmol/L. However, their FG eventually returned to the normal range in the following time. No cases of diabetes were observed during the whole observation period.
Changes in fasting insulin and HOMA-IR between the early treatment group (shaded) and later treatment group (blank) from baseline to 4 years old. The lower and upper bounds are the 25th percentile and the 75th percentile, respectively. The horizontal line in the box shows the median. #, P < 0.05, compared with the later treatment group
Thyroid and liver function
Hypothyroidism was reported in 13 patients (13.54%), and 3 patients were diagnosed before rhGH treatment. Among the 10 patients diagnosed with hypothyroidism during rhGH treatment, 9 (13.85%) were in the early treatment group, while 1 (3.23%) was found in the later treatment group. No significant difference (χ2 = 1.53, P = 0.22) was found, as shown in Table 3. Of them, 9 patients had accepted thyroxine treatment during the observation period. Hyperthyroidism was not noted throughout the observation period.
Liver dysfunction was noted in seven patients, and ALT ranged from 54 U/L to 190 U/L. Of them, 5 patients were found before accepting rhGH treatment. During rhGH treatment, 2 patients (3.08%) were diagnosed in the early treatment group, and no patient was found in the later treatment group, without a significant difference (P = 1.00), as shown in Table 3. Three patients had accepted liver-protected drugs, and ALT in all patients returned to the normal range in the following time.
Spinal deformity and others
In this series, spinal deformities were noted in 21 (21.88%) patients by spine radiography. Of them, 15 (23.08%) patients were in the early treatment group, and 6 (19.35%) were in the later treatment group without statistical significance (χ2 = 0.17, P = 0.68), as shown in Table 3. Approximately 27.66% (13/47) of patients with spinal deformities were male, and 16.33% (8/49) were female, without a significant sex difference (χ2 = 1.80, P = 0.18). No allergic reaction or injection site infection was reported during the rhGH treatment.
Discussion
Among the registered PWS patients in China, 48.9% (688/1407) accepted rhGH treatment, which was comparable to the Italian National survey for PWS patients from 1986 to 2006 [28]. The relatively “higher” ratio of rhGH treatment may be associated with the fact that over 70% registered PWS younger than 5 years and diagnosed in the past 5 years (unpublished data). In fact, the ratio of rhGH treatment may be lower than 48.9%, as rhGH treatment may be lower in unregistered PWS patients. In Hong Kong, only 1.79% of PWS patients (1/56) had accepted rhGH treatment in the Chinese PWS cohort from 1995 to 2010 [29]. A considerable cost and incurability might result in a low ratio of accepting rhGH therapy [30].
Moreover, the median age at rhGH treatment start in our project ranged from 1 to 201 months with a median of 10.5 months, which was significantly younger than that in the United States and Europe (approximately 4 to 5 years) [31]. This may also be associated with the fact that most patients in our cohort were less than 10 years old. These results implied significant improvements in the diagnosis and management of PWS in recent years. However, misdiagnosis, misdiagnosis of PWS, and less rhGH treatment might have been common in China 10 years ago.
To our knowledge, this is the first large sample study of rhGH treatment in younger children in China. In our longitudinal follow-up, rhGH treatment significantly improved the height/length SDS from baseline in both early (from −1.10 ± 1.36 SDS at baseline to 0.42 ± 1.10 SDS at 4 years old) and later treatment groups (from −1.42 ± 1.54 SDS at baseline to −0.14 ± 0.85 SDS at 4 years old), which was consistent with previous reports [32, 33]. At the age of 1 year, the height/length SDS in the later treatment group was similar to the baseline height/length SDS, which was comparable with an untreated Chinese PWS population[34]. Notably, the height/length in the early treatment group was normalized to an SDS of 0.06 ± 1.53 at 1 year old, which was significantly greater than that in the untreated later treatment group. Although the height velocity increased dramatically in the first rhGH treatment year in both the early and later treatment groups, the height/length SDSs in the early treatment group were all different from those in the later treatment group at various age points. At 4 years of age, the mean height (0.42 ± 1.10 SDS) in the early treatment group was slightly higher than normal but shorter in the later treatment group (-0.14 ± 0.85 SDS). Several studies reported that initiation of rhGH treatment in prepuberty could completely normalize stature after long-term therapy[35,36,37]. Our results showed that early rhGH treatment may reach the normalization height earlier than later treatment. Whether early rhGH treatment significantly improved the final height required further longer follow-up, as this cohort was only followed up to 4 years old.
It was notable that the later treatment group was heavier than the early treatment group at baseline, which may be associated with parents being more willing to use rhGH early in patients with poorer suck and feeding difficulty in infancy. The weight for height in the early treatment group was slightly lower than that in the later treatment group at 4 years old, which suggested that rhGH treatment could increase lean muscle mass and decrease body fat in PWS patients [37, 38]. However, it is worth noting that weight in the early treatment group was higher than that in the later treatment group before the age of 2.5 years, especially the weight almost reaching the normal range in the early treatment at the age of 1 year. These results suggested that rhGH treatment had a “two-way regulation” function for body weight. It increases muscle mass, improves feeding difficulties, and then increases energy intake [39]. Both muscle mass and energy intake increase may improve the weight and weight for height in infancy. Conversely, rhGH may reduce fat mass by increasing the metabolic rate to reduce obesity after 2–2.5 years of age. Although the differences in weight and weight for height were not significant, we noted that the weight and weight for height were closer to the normal range in the early treatment group. In addition, it may also be associated with the fact that parents in the early treatment group have better compliance and stronger aspiration to control their children’s body weight. Weight intervention programs, including diet control and physical activity, may cause body composition improvement [40]. Thus, early diagnosis and treatment, as well as education for parents, are important for PWS management.
During the follow-up, allergic reactions and injection site infections were not reported. It was notable that FI in the early treatment group was higher than that in the later treatment group during the first treatment year. This implied that IR may be present during rhGH treatment. Fortunately, FI and HOMA-IR were stable, and no differences were observed between the two groups in the following years. Moreover, FG was not different between the two groups, and no cases of diabetes were observed, although transient hyperglycemia was noted in 5 patients. This was similar to most previous studies about adult or older children [41,42,43]. However, different from those studies in which rhGH treatment had no effects on glucose homeostasis in PWS patients [37, 44,45,46]. Thus, although early rhGH treatment did not increase the IR, glucose homeostasis should be evaluated regularly for PWS patients treated with rhGH.
Although no hyperthyroidism was found, hypothyroidism was reported in 13 patients (13.5%) during rhGH treatment, which was similar to the prevalence in the Italian multicenter study [47]. Moreover, liver dysfunction was found only in 2 patients (3.08%), and ALT returned to the normal range in the following time. No significant differences in the prevalence of hypothyroidism or liver dysfunction were observed between the two groups. Oto et al. also reported that thyroid function did not significantly change after 2 years of rhGH treatment [48]. However, the prevalence was statistically higher in the early treatment group. Therefore, thyroid and liver function should be routinely monitored during rhGH treatment, especially in infancy.
The incidence of spinal deformities was as high as 21.88%, which was similar to previous studies [49, 50]. Whether it is associated with rhGH treatment is unknown, as nonrhGH treatment data were not included in this study. However, there were no significant differences in the incidence of spinal deformities between the two groups, which was similar to a previous study of older children [51, 52]. It was reported that older and higher BMI PWS patients presented a higher incidence of scoliosis [53]. Hence, scoliosis was still periodically monitored during rhGH treatment [21]. Nevertheless, scoliosis should not be considered a contraindication of rhGH therapy in PWS patients.
There were some limitations in our study. First, some follow-up data were lacking at some time points and were lost to follow-up, as most patients are from all over the country and have relatively poor compliance in China. Second, as most untreated PWS patients in the PWS Research Group missed follow-up, we could not compare auxological parameters with those of untreated patients. Third, rhGH treatment was also reported to improve psychomotor development in some literature, which was not documented in our study. Fourth, lean body mass and percent body fat were not measured.
In summary, this longitudinal study showed that younger children with PWS may benefit greatly from rhGH treatment and that early rhGH treatment had a more favorable outcome in height/length and body composition. Thyroid dysfunction, liver dysfunction, and scoliosis should be monitored during rhGH treatment, and the risk of adverse effects will not increase for PWS patients starting rhGH therapy in infancy.
Availability of data and materials
Unpublished data in this manuscript will be shared by request from any qualified investigator.
Abbreviations
- PWS:
-
Prader–Willi syndrome
- rhGH:
-
Recombinant human growth hormone
- IGF-1:
-
Insulin-like growth factor-1
- SDS:
-
Standard deviation score
- FG:
-
Fasting glucose
- FI:
-
Fasting insulin
- TSH:
-
Thyroid stimulating hormone
- T4:
-
Thyroxine
- fT4:
-
Free thyroxine
- T3:
-
Triiodothyronine
- fT3:
-
Free triiodothyronine
- AST:
-
Aspartate transaminase
- ALT:
-
Alanine aminotransferase
References
Cassidy SB, Schwartz S, Miller JL, Driscoll DJ. Prader–Willi syndrome. Genet Med Offl J Am College Med Genet. 2012;14(1):10–26.
Nicholls RD, Knepper JL. Genome organization, function, and imprinting in Prader–Willi and Angelman syndromes. Annu Rev Genomics Hum Genet. 2001;2:153–75.
Vogels A, Van Den Ende J, Keymolen K, Mortier G, Devriendt K, Legius E, Fryns JP. Minimum prevalence, birth incidence and cause of death for Prader–Willi syndrome in Flanders. Eur J Hum Genet. 2004;12(3):238–40.
Yakoreva M, Kahre T, Žordania R, Reinson K, Teek R, Tillmann V, Peet A, Õiglane-Shlik E, Pajusalu S, Murumets Ü, et al. A retrospective analysis of the prevalence of imprinting disorders in Estonia from 1998 to 2016. Eur J Hum Genet. 2019;27(11):1649–58.
de Smith AJ, Purmann C, Walters RG, Ellis RJ, Holder SE, Van Haelst MM, Brady AF, Fairbrother UL, Dattani M, Keogh JM, et al. A deletion of the HBII-85 class of small nucleolar RNAs (snoRNAs) is associated with hyperphagia, obesity and hypogonadism. Hum Mol Genet. 2009;18(17):3257–65.
Costa RA, Ferreira IR, Cintra HA, Gomes LHF, Guida LDC. Genotype-phenotype relationships and endocrine findings in Prader–Willi syndrome. Front Endocrinol. 2019;10:864.
Bieth E, Eddiry S, Gaston V, Lorenzini F, Buffet A, Conte Auriol F, Molinas C, Cailley D, Rooryck C, Arveiler B, et al. Highly restricted deletion of the SNORD116 region is implicated in Prader–Willi syndrome. Eur J Hum Genet. 2015;23(2):252–5.
Duker AL, Ballif BC, Bawle EV, Person RE, Mahadevan S, Alliman S, Thompson R, Traylor R, Bejjani BA, Shaffer LG, et al. Paternally inherited microdeletion at 15q11.2 confirms a significant role for the SNORD116 C/D box snoRNA cluster in Prader-Willi syndrome. Eur J Hum Gen. 2010;18(11):1196–201.
Sahoo T, del Gaudio D, German JR, Shinawi M, Peters SU, Person RE, Garnica A, Cheung SW, Beaudet AL. Prader–Willi phenotype caused by paternal deficiency for the HBII-85 C/D box small nucleolar RNA cluster. Nat Genet. 2008;40(6):719–21.
Fontana P, Grasso M, Acquaviva F, Gennaro E, Galli ML, Falco M, Scarano F, Scarano G, Lonardo F. SNORD116 deletions cause Prader–Willi syndrome with a mild phenotype and macrocephaly. Clin Genet. 2017;92(4):440–3.
Buiting K. Prader–Willi syndrome and Angelman syndrome. Am J Med Genetcs Part C Sem Med Genet. 2010;154(3):365–76.
Grugni G, Sartorio A, Crinò A. Growth hormone therapy for Prader–Willi syndrome: challenges and solutions. Ther Clin Risk Manag. 2016;12:873–81.
Tauber M, Cutfield W. KIGS highlights: growth hormone treatment in Prader–Willi Syndrome. Horm Res. 2007;68(Suppl 5):48–50.
Lecka-Ambroziak A, Wysocka-Mincewicz M, Doleżal-Ołtarzewska K, Zygmunt-Górska A, Wędrychowicz A, Żak T, Noczyńska A, Birkholz-Walerzak D, Stawerska R, Hilczer M, et al. Effects of recombinant human growth hormone treatment, depending on the therapy start in different nutritional phases in paediatric patients with Prader–Willi syndrome: a polish multicentre study. J Clin Med. 2021;10(14):3176.
Bakker NE, Siemensma EP, van Rijn M, Festen DA, Hokken-Koelega AC. Beneficial effect of growth hormone treatment on health-related quality of life in children with Prader–Willi syndrome: a randomized controlled trial and longitudinal study. Hormone Res Paediatr. 2015;84(4):231–9.
Nyberg F, Hallberg M. Growth hormone and cognitive function. Nat Rev Endocrinol. 2013;9(6):357–65.
Donze SH, Damen L, Mahabier EF, Hokken-Koelega ACS. Cognitive functioning in children with Prader–Willi syndrome during 8 years of growth hormone treatment. Eur J Endocrinol. 2020;182(4):405–11.
Deal CL, Rogol AD. Growth hormone treatments and cognitive functioning in children with Prader–Willi syndrome. Eur J Endocrinol. 2020;182(6):C21-c25.
Donze SH, Damen L, Mahabier EF, Hokken-Koelega ACS. Improved mental and motor development during 3 years of GH treatment in very young children with Prader–Willi syndrome. J Clin Endocrinol Metab. 2018;103(10):3714–9.
Luo Y, Zheng Z, Yang Y, Bai X, Yang H, Zhu H, Pan H, Chen S. Effects of growth hormone on cognitive, motor, and behavioral development in Prader–Willi syndrome children: a meta-analysis of randomized controlled trials. Endocrine. 2021;71(2):321–30.
Deal CL, Tony M, Höybye C, Allen DB, Tauber M, Christiansen JS. GrowthHormone Research Society workshop summary: consensus guidelines for recombinant human growth hormone therapy in Prader–Willi syndrome. J Clin Endocrinol Metab. 2013;98(6):E1072-1087.
Corripio R, Tubau C, Calvo L, Brun C, Capdevila N, Larramona H, Gabau E. Safety and effectiveness of growth hormone therapy in infants with Prader–Willi syndrome younger than 2 years: a prospective study. J Pediatr Endocrinol Metab. 2019;32(8):879–84.
Muscogiuri G, Formoso G, Pugliese G, Ruggeri RM, Scarano E, Colao A. Prader–Willi syndrome: an uptodate on endocrine and metabolic complications. Rev Endocr Metab Disord. 2019;20(2):239–50.
Yang A, Choi JH, Sohn YB, Eom Y, Lee J, Yoo HW, Jin DK. Effects of recombinant human growth hormone treatment on growth, body composition, and safety in infants or toddlers with Prader–Willi syndrome: a randomized, active-controlled trial. Orphanet J Rare Dis. 2019;14(1):216.
Carel JC, Ecosse E, Landier F, Meguellati-Hakkas D, Kaguelidou F, Rey G, Coste J. Long-term mortality after recombinant growth hormone treatment for isolated growth hormone deficiency or childhood short stature: preliminary report of the French SAGhE study. J Clin Endocrinol Metab. 2012;97(2):416–25.
Carrel AL, Myers SE, Whitman BY, Allen DB. Benefits of long-term GH therapy in Prader–Willi syndrome: a 4-year study. J Clin Endocrinol Metab. 2002;87(4):1581–5.
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–9.
Grugni G, Crinò A, Bosio L, Corrias A, Cuttini M, De Toni T, Di Battista E, Franzese A, Gargantini L, Greggio N, et al. The Italian national survey for Prader–Willi syndrome: an epidemiologic study. Am J Med Genet Part A. 2008;146(7):861–72.
Lo IF, Luk HM, Tong TM, Lai KK, Chan DH, Lam AC, Chan DK, Hau EW, Fung CO, Lam ST. Prader–Willi syndrome: 16-year experience in Hong Kong. J Genet Genom. 2012;39(4):191–4.
Orso M, Polistena B, Granato S, Novelli G, Di Virgilio R, La Torre D, d’Angela D, Spandonaro F. Pediatric growth hormone treatment in Italy: a systematic review of epidemiology, quality of life, treatment adherence, and economic impact. PLoS ONE. 2022;17(2):e0264403.
Sävendahl L, Polak M, Backeljauw P, Blair J, Miller BS, Rohrer TR, Pietropoli A, Ostrow V, Ross J. Treatment of children with GH in the United States and Europe: long-term follow-up from NordiNet® IOS and ANSWER program. J Clin Endocrinol Metab. 2019;104(10):4730–42.
Obata K, Sakazume S, Yoshino A, Murakami N, Sakuta R. Effects of 5 years growth hormone treatment in patients with Prader–Willi syndrome. J Pediatr Endocrinol Metab. 2003;16(2):155–62.
Carrel AL, Allen DB. Prader–Willi syndrome: How does growth hormone affect body composition and physical function? J Pediatr Endocrinol Metab. 2001;14(Suppl 6):1445–51.
Yang H, Zhang M, Song H, Zhu H, Pan H. Growth patterns of Chinese patients with Prader–Willi syndrome. Congenit Anom. 2015;55(4):173–7.
Eiholzer U, l’Allemand D. Growth hormone normalises height, prediction of final height and hand length in children with Prader-Willi syndrome after 4 years of therapy. Hormone Res Paediatr. 2000;53(4):185-92.
Butler MG, Lee J, Cox DM, Manzardo AM, Gold JA, Miller JL, Roof E, Dykens E, Kimonis V, Driscoll DJ. Growth charts for Prader–Willi syndrome during growth hormone treatment. Clin Pediatr. 2016;55(10):957–74.
de Lind van Wijngaarden RF, Siemensma EP , Festen DA, Otten BJ, van Mil EG, Rotteveel J, Odink RJ, Bindels de Heus GC, van Leeuwen M, Haring DA et al: Efficacy and safety of long term continuous growth hormone treatment in children with Prader Willi syndrome. J Clin Endocrinol Metab. 2009;94(11):4205–15.
Festen DA. de Lind van Wijngaarden R, van Eekelen M, Otten BJ, Wit JM, Duivenvoorden HJ, Hokken-Koelega AC. Randomized controlled GH trial: effects on anthropometry, body composition and body proportions in a large group of children with Prader-Willi syndrome. Clin Endocrinol. 2008;69(3):443–51.
Bakker NE, Siemensma EP, Koopman C, Hokken-Koelega AC. Dietary energy intake, body composition and resting energy expenditure in prepubertal children with Prader–Willi syndrome before and during growth hormone treatment: a randomized controlled trial. Hormone Res Paediatr. 2015;83(5):321–31.
Tan Q, Orsso CE, Deehan EC, Triador L, Field CJ, Tun HM, Han JC, Müller TD, Haqq AM. Current and emerging therapies for managing hyperphagia and obesity in Prader–Willi syndrome: a narrative review. Obesity Rev Offl J Int Assoc Study Obesity. 2020;21(5):e12992.
Magill L, Laemmer C, Woelfle J, Fimmers R, Gohlke B. Early start of growth hormone is associated with positive effects on auxology and metabolism in Prader–Willi-syndrome. Orphanet J Rare Dis. 2020;15(1):283.
Colmenares A, Pinto G, Taupin P, Giuseppe A, Odent T, Trivin C, Laborde K, Souberbielle JC, Polak M. Effects on growth and metabolism of growth hormone treatment for 3 years in 36 children with Prader–Willi syndrome. Hormone Res Paediatr. 2011;75(2):123–30.
Bakker NE, Kuppens RJ, Siemensma EP, Lind Tummers-de, van Wijngaarden RF, Festen DA, Bindels-de Heus GC, Bocca G, Haring DA, Hoorweg-Nijman JJ, Houdijk EC, et al. Eight years of growth hormone treatment in children with Prader–Willi syndrome: maintaining the positive effects. J Clin Endocrinol Metab. 2013;98(10):4013–22.
Carrel AL, Moerchen V, Myers SE, Bekx MT, Whitman BY, Allen DB. Growth hormone improves mobility and body composition in infants and toddlers with Prader–Willi syndrome. J Pediatr. 2004;145(6):744–9.
Carrel AL, Myers SE, Whitman BY, Eickhoff J, Allen DB. Long-term growth hormone therapy changes the natural history of body composition and motor function in children with Prader–Willi syndrome. J Clin Endocrinol Metab. 2010;95(3):1131–6.
Crinò A, Grugni G. Update on diabetes mellitus and glucose metabolism alterations in Prader–Willi syndrome. Curr DiabRep. 2020;20(2):7.
Iughetti L, Vivi G, Balsamo A, Corrias A, Crinò A, Delvecchio M, Gargantini L, Greggio NA, Grugni G, Hladnik U, et al. Thyroid function in patients with Prader–Willi syndrome: an Italian multicenter study of 339 patients. J Pediatr Endocrinol Metab. 2019;32(2):159–65.
Oto Y, Murakami N, Matsubara K, Saima S, Ogata H, Ihara H, Nagai T, Matsubara T. Effects of growth hormone treatment on thyroid function in pediatric patients with Prader–Willi syndrome. Am J Med Genet A. 2020;182(4):659–63.
Dağdeviren Çakır A, Baş F, Akın O, Şıklar Z, Özcabı B, Berberoğlu M, Kardelen AD, Bayramoğlu E, Poyrazoğlu Ş, Aydın M, et al. Clinical characteristics and growth hormone treatment in patients with Prader–Willi syndrome. J Clin Res Pediatr Endocrinol. 2021;13(3):308–19.
van Bosse HJ, Butler MG. Clinical observations and treatment approaches for scoliosis in Prader–Willi syndrome. Genes. 2020;11(3):260.
Grootjen LN, Rutges J, Damen L, Donze SH, Juriaans AF, Kerkhof GF, Hokken-Koelega ACS. Effects of 8 years of growth hormone treatment on scoliosis in children with Prader–Willi syndrome. Eur J Endocrinol. 2021;185(1):47–55.
de Lind van Wijngaarden RF, de Klerk LW, Festen DA, Duivenvoorden HJ, Otten BJ, Hokken-Koelega AC. Randomized controlled trial to investigate the effects of growth hormone treatment on scoliosis in children with Prader–Willi syndrome. J Clin Endocrinol Metab. 2009;94(4):1274–80.
de Lind van Wijngaarden RF, de Klerk LW, Festen DA, Hokken Koelega AC: Scoliosis in Prader Willi syndrome: prevalence, effects of age, gender, body mass index, lean body mass and genotype. Archiv Dis Childhood. 2008;93(12):1012–6.
Acknowledgements
We extend our gratitude to all of the children and families for their enthusiasm to participate in this study.
Funding
This work was supported by the National Natural Science Foundation (81371215 and 81670786), Key R & D Projects of Zhejiang Provincial Department of Science and Technology (2021C03094) and Medical and Health Science and Technology Project of Zhejiang Province (2020KY230).
Author information
Authors and Affiliations
Contributions
CCZ and QZ conceptualized and designed the study and reviewed and revised the manuscript. YG and LLY drafted the initial manuscript and collected the data. DYL and SZ conducted the statistical analyses. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study protocol was approved by the Ethics Committee of the Children's Hospital of Zhejiang University School of Medicine (No. 2019-IRB-025). Informed consent was obtained from all parents or the patients registered in the PWS Registry.
Consent for publication
Not applicable.
Competing interests
There are no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Gao, Y., Yang, LL., Dai, YL. et al. Effects of early recombinant human growth hormone treatment in young Chinese children with Prader–Willi syndrome. Orphanet J Rare Dis 18, 25 (2023). https://doi.org/10.1186/s13023-023-02615-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13023-023-02615-7