Abstract
Background
The development and approval of disease modifying treatments have dramatically changed disease progression in patients with spinal muscular atrophy (SMA). Nusinersen was approved in Europe in 2017 for the treatment of SMA patients irrespective of age and disease severity. Most data on therapeutic efficacy are available for the infantile-onset SMA. For patients with SMA type 2 and type 3, there is still a lack of sufficient evidence and long-term experience for nusinersen treatment. Here, we report data from the SMArtCARE registry of non-ambulant children with SMA type 2 and typen 3 under nusinersen treatment with a follow-up period of up to 38 months.
Methods
SMArtCARE is a disease-specific registry with data on patients with SMA irrespective of age, treatment regime or disease severity. Data are collected during routine patient visits as real-world outcome data. This analysis included all non-ambulant patients with SMA type 2 or 3 below 18 years of age before initiation of treatment. Primary outcomes were changes in motor function evaluated with the Hammersmith Functional Motor Scale Expanded (HFMSE) and the Revised Upper Limb Module (RULM).
Results
Data from 256 non-ambulant, pediatric patients with SMA were included in the data analysis. Improvements in motor function were more prominent in upper limb: 32.4% of patients experienced clinically meaningful improvements in RULM and 24.6% in HFMSE. 8.6% of patients gained a new motor milestone, whereas no motor milestones were lost. Only 4.3% of patients showed a clinically meaningful worsening in HFMSE and 1.2% in RULM score.
Conclusion
Our results demonstrate clinically meaningful improvements or stabilization of disease progression in non-ambulant, pediatric patients with SMA under nusinersen treatment. Changes were most evident in upper limb function and were observed continuously over the follow-up period. Our data confirm clinical trial data, while providing longer follow-up, an increased number of treated patients, and a wider range of age and disease severity.
Similar content being viewed by others
Background
Treatment and care of patients with spinal muscular atrophy (SMA) have changed dramatically over the past years due to the development and approval of different disease-specific drugs. SMA is a rare neuromuscular disorder with the leading symptom of a proximal and progressive muscle weakness. In most cases, SMA is caused by a homozygous deletion in the survival motor neuron 1 (SMN1) gene on chromosome 5 [1]. SMN2 is a centromeric copy of SMN1 that produces transcripts of SMN protein lacking exon 7. The result is an alternatively spliced truncated and non-functional SMN protein (SMNΔ7) but to small proportions also functional SMN protein [1, 2]. SMN2 is expressed in variable copy numbers in patients with SMA, with SMN2 copy number inversely correlating with disease severity [3]. SMA affects patients of all ages with a broad spectrum of disease severity. In patients with symptom onset later than 6 months of age, a differentiation is made between SMA type 2 (patients gaining the ability to sit unassisted but not to walk) and SMA type 3 (patients gaining the ability to walk unassisted) [4, 5].
For the treatment of SMA patients, three different drugs (nusinersen, onasemnogene abeparvovec, and risdiplam) have been approved with a positive influence on disease progression [6, 7]. While nusinersen was approved in Europe already in 2017, the approval of onasemnogene abeparvovec and risdiplam followed later. Nusinersen is available for the treatment of SMA patients independent of age, SMN2 copy number, or motor function. As antisense oligonucleotide, nusinersen acts as splicing modifier targeting the intronic splicing silencer N1 in SMN2 [8]. Sham-controlled, clinical trial data showed that nusinersen treatment significantly improved motor function in children with later-onset SMA aged between 2 and 12 years, with a follow-up period of 15 months [9]. Real-world data from different countries and disease registries could confirm these results in the short-term follow-up [10,11,12,13,14]. Despite data from a small cohort of 15 non-ambulant patients who participated in the phase I/II clinical trials [15], data on the long-term effect of nusinersen in pediatric later-onset SMA patients is still lacking.
The disease-specific SMArtCARE registry aims to collect real-world data on all available SMA patients in Germany, Austria and Switzerland [16]. Here, we report data on pediatric patients with SMA type 2 and 3 with focus on long-term effects on motor, respiratory, and bulbar function.
Methods
SMArtCARE registry
With currently 58 participating centers in Germany, Austria and Switzerland, SMArtCARE collects longitudinal data on all available SMA patients as a disease-specific registry. As of November 2021, the registry encompasses data on 1190 patients of any age, SMA type and treatment regime. The only inclusion criteria for patients to be enrolled in SMArtCARE are a genetically confirmed 5q-SMA, and written consent of patients or caregivers. Data are collected during routine patient visits as real-life outcome data. Data are documented using standardized case report forms and not extracted from medical records. Content of these case report forms is aligned with the international consensus for SMA registries [17]. Amongst others, these include information on motor function and motor milestones, respiratory, bulbar and orthopedic symptoms, in addition to adverse events. Genetic test results including SMN2 copy number are documented by the treating physicians according to the original genetic test results of the patients. SMN2 copy number is not reassessed centrally within the SMArtCARE registry. Thus, especially SMN2 copy number is not available for all patients. To evaluate motor function of patients, standardized physiotherapeutic assessments every 4 months are recommended, but are not mandatory within the SMArtCARE data collection und thus not available for all patients at all time-points. Central ethics approval was obtained by the ethics committee of the University of Freiburg (EK-Freiburg 56/18), and local ethics approvals were obtained from all participating centers.
Patient cohort
In this analysis, we included all non-ambulant patients below 18 years of age with SMA type 2 or type 3, who were treated with nusinersen (data cut 15th of November 2021). Only patients were included with documented baseline characteristics and motor function before start of treatment. Patients were stratified to the following subgroups: “Younger sitters” included all patients with SMA type 2 who were ≤ 5 years and able to sit, but never able to walk independently at start of treatment (n = 107), and “older sitters” included those > 5 years of age (n = 73). “Lost sitters” were all children with SMA type 2 who were never able to walk and lost the ability to sit independently before start of treatment (n = 37), and “lost walkers” were children with SMA type 3 who lost ambulation and partly also the ability to sit independently before start of treatment (n = 39). The first visit of each patient corresponded to treatment initiation with a follow-up of maximum 38 months. The follow-up period of maximum 38 months was chosen, because with the approval of nusinersen in 2017, a conclusive cohort size was still available in all subgroups after 38 months of treatment. According to the different timing of treatment initiation, follow-up times varied and thus not all patients had a follow-up time of 38 months. The last available visit of each patient was considered within the observation period (see Table 1).
Outcomes
Primary outcome of this data analysis were changes in motor function evaluated with the Hammersmith Functional Motor Scale Expanded (HFMSE), the Revised Upper Limb Module (RULM), and motor milestones following WHO criteria [18]. The HFMSE consists of 33 items with a total score of 66 points (higher scores indicating better motor function). A change of ≥ 3 points in the HFMSE score is considered clinically meaningful [19]. The RULM consists of 20 items focusing on changes in upper limb function. The total score is 37 points with a change of ≥ 2 points considered clinically meaningful [20]. Participating physiotherapists were regularly trained to ensure interrater reliability. Further, longitudinal data on the need for ventilator support, the need for tube feeding, and mortality were evaluated. Adverse events (AE) were recorded as AE with or without hospitalization and specified using the Medical Dictionary for Regulatory Activities (MedDRA) code [21]. For each AE, the treating physician was asked to assess whether the AE was related or possibly related to the treatment with nusinersen.
Statistical analysis
Primary and secondary outcomes and cohorts for subgroup analysis were defined in a statistical analysis plan before data were extracted from the database. Descriptive analysis was performed by calculating absolute frequencies and percentages. Continuous data were analyzed as mean ± standard deviation. Analyses of HFMSE and RULM were additionally based on comparisons of different time-periods: baseline to month 14 (m14), m14 to month 26 (m26), and m26 to month 38 (m38). The last available visit was set as the individual endpoint for each patient and was considered for the analysis of clinically meaningful changes in HFMSE and RULM. For patients who stopped treatment within the 38 months of follow-up, data were considered for analysis maximum 6 months after treatment discontinuation. If patients changed drug treatment, no further data were evaluated after discontinuation of nusinersen treatment. Inferential analyses were applied to evaluate the effect of age at diagnosis, age at start of treatment, SMN2 copy number, gender, baseline HFMSE or RULM score, and elapsed time from baseline on changes in HFMSE or RULM score. For time-to-event analysis, Kaplan–Meier curves were computed for the probabilities of gaining the ability to walk independently. All curves are presented as cumulative incidence. Statistical analysis was performed using R statistical software (version 4.0.4). A p-value of ≤ 0.01 was considered statistically significant.
Results
We included data from 256 patients in this analysis. Data were collected and documented at 34 neuropediatric or neurological departments. Table 2 summarizes baseline characteristics of all patients. Younger sitters were considerably younger at start of treatment than children in the other three cohorts. Further, HMFSE and RULM scores at baseline were highest in lost walkers. The need for ventilator support or tube feeding was higher in older and lost sitters, consistent with lower HMFSE scores. In all cohorts, the majority of children had three SMN2 copies. Before start of treatment, mean age at loss of independent sitting was 29.9 ± 35.7 months in lost sitters and mean age at loss of ambulation was 65 ± 51.1 months in lost walkers. Of all lost walkers, four patients (10.2%) additionally lost the ability to sit independently before start of treatment at a mean age of 63.0 ± 47.9 months.
During the observation period, only 13 patients (5.1%) had at least one interval greater than 6 months between nusinersen treatments. Further, of all 2416 documented nusinersen treatments, only 0.8% were given at intervals greater than 6 months. Consequently, despite the impact of COVID-19 pandemia almost all patients received nusinersen treatments at recommended intervals during their follow-up time. Thirteen patients (5.1%) stopped nusinersen treatment. Of these, seven patients (53.5%) changed treatment to risdiplam or onasemnogene abeparvovec. None of the patients received a combination therapy with nusinersen and risdiplam or onasemnogene abeparvovec. Fifteen patients (5.9%) were lost to follow-up with no data entered over more than 12 months.
Motor milestones
During the observation period, 16 younger sitters (14.9%) and one older sitter (1.3%) gained the ability to walk independently according to WHO criteria. Of these, 14 children (82.3%) gained the ability to walk between baseline and m14. None of the lost sitters or lost walkers gained the ability to walk unassisted. Figure 1 displays the probability to gain the ability to walk in younger and older sitters. Four lost sitters (10.8%) and one lost walker (2.6%) gained the ability to sit independently. In all cohorts, no motor milestones were lost under nusinersen treatment, in particular no child lost the ability to sit.
HFMSE
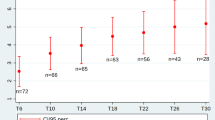
After 38 months of treatment, changes in HFMSE scores were mean + 7.0 points in younger sitters, + 0.1 points in older sitters and + 2.9 points in lost walkers. The number of lost sitters at m38 was too small to show changes. After 14 months of treatment, mean change in HFMSE was + 2.5 points in lost sitters. Figure 2 illustrates the longitudinal progression of all patients in the different cohorts.
Longitudinal progression of HFMSE score. HFMSE score for younger sitters (blue), older sitters (red), lost sitters (yellow), and lost walkers (green). Data are listed as mean and 99% confidence interval. Available patients at baseline, m14, m26 and m38 are added. For a group size fewer than 10 patients no data are depicted
Clinically meaningful changes in HFMSE score were observed in 63 children (24.6%): 37 younger sitters (34.6%), 11 older sitters (15.1%), five lost sitters (13.5%), and 10 lost walkers (25.6%). Eleven children (4.3%) lost ≥ 3 points in HFMSE score during the observation period (one younger sitters (1.0%), six older sitters (8.2%) and four lost walkers (10.3%)). Especially in younger sitters, main improvements were observed between baseline and m26: 23 children (21.5%) experienced an improvement ≥ 3 points within the first 14 months, and 16 children (14.9%) between m14 and m26. Only nine children (8.4%) gained ≥ 3 points between m26 and m 38. Figure 3 displays changes in HFMSE score in each cohort.
Inferential analysis revealed SMN2 copy number as the only covariate having a significant influence on changes in HFMSE score. Further, higher baseline score was associated with smaller improvements in HFMSE score (see Table 3).
RULM
RULM scores improved in all cohorts. At m38, mean changes in RULM score were + 9.1 points in younger sitters, + 2.2 points in older sitters, + 7.3 points in lost sitters, and + 3.3 points in lost walkers. Figure 4 depicts the difference of the longitudinal progression of patients in the different cohorts.
Longitudinal progression of RULM score. RULM score for younger sitters (blue), older sitters (red), lost sitters (yellow) and lost walkers (green). Data are listed as mean and 99% confidence interval. Available patients at baseline, m14, m26 and m38 are added. The difference in cohort size is explained by incomplete data availability for all patients at all time-points. Further, RULM can only be performed in children from an age of 2 years
Clinically meaningful changes in RULM score were observed in 83 children (32.4%): 30 younger sitters (28.0%), 30 older sitters (41.1%), eight lost sitters (21.6%) and 15 lost walkers (38.5%). Only three older sitters (4.1%) lost ≥ 2 points during the observation period. Improvements in RULM score were observed continuously during the observation period (see Fig. 5).
Inferential analysis revealed the following covariates to have a significant influence on changes in RULM score: Children with higher baseline score showed smaller improvements than children with lower RULM scores. Further, changes in RULM score were observed continuously during the 38 months follow-up (see Table 4).
Respiratory function and feeding
At baseline, 39 children (15.2%) used part-time (< 16 h per day), non-invasive ventilator support. During the observation period, one older sitter was able to discontinue part-time ventilator support. Twenty-three children (9.0%) additionally started to use occasional ventilator support: two younger sitters (1.9%), 12 older sitters (16.4%), six lost sitters (16.2%) and three lost walkers (7.7%). None of the children required permanent or invasive ventilator support.
At baseline, 14 children (5.5%) required tube feeding due to bulbar dysfunction. During the observation period, five children (2.0%) additionally required tube feeding despite nusinersen treatment: two younger sitters (1.9%), one older sitter (1.4%), and two lost sitters (5.4%). One younger sitter who started tube feeding could discontinue after 4 months.
Adverse events
In total, 144 AEs among 64 patients were reported during the observation period. Of all AEs, 122 (84.7%) were AEs with hospitalization and 22 (15.3%) without hospitalization. The most common type of AEs were respiratory tract infections (45.8%), followed by gastroenteritis (20.8%), post-lumbar puncture syndrome (9.0%), other type of infections (8.3%), fractures (4.1%), respiratory symptoms including respiratory distress and transient cyanosis (4.2%), pain (3.5%), abdominal symptoms including abdominal pain (1.4%), and others (2.8%). No children died during the observation period. None of the AEs were considered as related to drug treatment, but 31 (25.4%) were rated as possibly related to drug treatment by the treating physician. The latter included procedure-related symptoms as post-lumbar puncture syndrome but also acute infections or respiratory symptoms.
Discussion
The development and approval of disease modifying treatments have significantly changed the SMA landscape. The longest experience is available for nusinersen treatment, but data are still lacking on long-term benefit and response to treatment for the broad spectrum of SMA patients.
In particular, non-ambulant SMA type 2 and type 3 patients with childhood onset of symptoms have not been extensively studied in long-term, prospectively conducted clinical trials, or in real-world data collections. Phase III clinical trials were limited to patients aged between 2 and 12 years, not having severe contractures or scoliosis, and not using ventilator support more than 6 h per day. In the 84 patients who received nusinersen treatment, motor function improved in the short-term follow-up of 15 months [9]. Data from international disease registries encompass broader patient cohorts with a maximum follow-up period of 24 months and thus still do not provide evidence on the long-term effect of nusinersen treatment [11, 12, 14, 22, 23].
Here, we report real-world data of a broad spectrum of pediatric non-ambulant SMA patients with SMA type 2 or type 3 using a pre-specified analysis. Despite a wide age range, different functional ability at start of treatment reflected by baseline HFMSE and RULM scores in the different cohorts, and different comorbidities (e.g. need for ventilator support or tube feeding), the majority of children experienced a stabilization of disease progression and a great part of children derived clinically meaningful benefit from nusinersen treatment by either gaining a new motor milestone or improving upper limb function or both. In contrast, natural history data of SMA type 2 patients reflect the continuous progression of muscle weakness: within 12 months, a loss of sitting independently in 3.1% of patients and a mean decline in HFMSE score of − 0.54 points within 12 months, and a mean decline in RULM score of − 0.79 points within 24 months were described [24, 25]. Thus, a stabilization of disease status can already be considered as positive response to treatment. We observed main improvements in HFMSE score within the first 24 months of treatment, consistent with data of young SMA type 1 patients [26]. RULM score improved continuously and consistently in all cohorts during the observation period. Especially in older and more severely affected children the performance of HFMSE might be limited due to scoliosis or contractures. Thus, RULM score seems to be preferable for monitoring changes in motor function of these non-ambulant patients.
Despite improvements in motor function, we could not observe a positive effect of nusinersen treatment on the need for non-invasive ventilator support or tube feeding: Our results are in line with results of a previous natural history study, where 38% of sitters required ventilator support with a median age of start of ventilator support of 5.0 years (range 1.8–16.6 years) [27].
Reported AEs from the phase III clinical trial resulted mainly from known side effects of lumbar puncture or were related to the underlying disease with a comparable incidence of AEs in the nusinersen group and the control group. These included amongst others respiratory tract infections, pyrexia, respiratory distress, but also symptoms such as headache, vomiting or back pain [9]. In our pediatric cohort, we did not observe any new safety signals under long-term treatment with nusinersen.
The real-world data approach represents a major advantage, but also a limitation of the present study. Not all data are available for all patients at all time-points. Further, data quality within a registry is not comparable to clinical trials. To ensure high data quality within the SMArtCARE registry, we use standardized case report forms and outcome measures for data collection. Physiotherapists and raters are regularly trained to ensure interrater reliability. In addition, data is carefully reviewed for completeness, consistency and plausibility.
In conclusion, our results demonstrate improvements or stabilization of disease progression in most non-ambulant children with SMA type 2 or type 3 under nusinersen treatment. Changes were most evident in upper limb function while there was no impact on the need for ventilator support or tube feeding.
Availability of data and materials
All data included in this analysis are recorded in the SMArtCARE registry. Data can be obtained anonymized and aggregated upon request and approval by the SMArtCARE steering committee.
References
Lefebvre S, Bürglen L, Reboullet S, Clermont O, Burlet P, Viollet L, et al. Identification and characterization of a spinal muscular atrophy-determining gene. Cell. 1995;80:155–65. https://doi.org/10.1016/0092-8674(95)90460-3.
Lorson CL, Androphy EJ. An exonic enhancer is required for inclusion of an essential exon in the SMA-determining gene SMN. Hum Mol Genet. 2000;9:259–65. https://doi.org/10.1093/hmg/9.2.259.
Prior TW, Swoboda KJ, Scott HD, Hejmanowski AQ. Homozygous SMN1 deletions in unaffected family members and modification of the phenotype by SMN2. Am J Med Genet A. 2004;130A:307–10.
Zerres K, Rudnik-Schöneborn S. Natural history in proximal spinal muscular atrophy: clinical analysis of 445 patients and suggestions for a modification of existing classifications. Arch Neurol. 1995;52:518–23. https://doi.org/10.1001/archneur.1995.00540290108025.
Farrar MA, Vucic S, Johnston HM, Du Sart D, Kiernan MC. Pathophysiological insights derived by natural history and motor function of spinal muscular atrophy. J Pediatr. 2013;162:155–9. https://doi.org/10.1016/j.jpeds.2012.05.067.
Messina S, Sframeli M, Maggi L, D’Amico A, Bruno C, Comi G, Mercuri E. Spinal muscular atrophy: state of the art and new therapeutic strategies. Neurol Sci. 2021. https://doi.org/10.1007/s10072-021-05258-3.
Nicolau S, Waldrop MA, Connolly AM, Mendell JR. Spinal muscular atrophy. Semin Pediatr Neurol. 2021;37:100878. https://doi.org/10.1016/j.spen.2021.100878.
Singh NK, Singh NN, Androphy EJ, Singh RN. Splicing of a critical exon of human survival motor neuron is regulated by a unique silencer element located in the last intron. Mol Cell Biol. 2006;26:1333–46. https://doi.org/10.1128/MCB.26.4.1333-1346.2006.
Mercuri E, Darras BT, Chiriboga CA, Day JW, Campbell C, Connolly AM, et al. Nusinersen versus sham control in later-onset spinal muscular atrophy. N Engl J Med. 2018;378:625–35. https://doi.org/10.1056/NEJMoa1710504.
Veerapandiyan A, Eichinger K, Guntrum D, Kwon J, Baker L, Collins E, Ciafaloni E. Nusinersen for older patients with spinal muscular atrophy: a real-world clinical setting experience. Muscle Nerve. 2020;61:222–6. https://doi.org/10.1002/mus.26769.
Audic F, de La Banda MGG, Bernoux D, Ramirez-Garcia P, Durigneux J, Barnerias C, et al. Effects of nusinersen after one year of treatment in 123 children with SMA type 1 or 2: a French real-life observational study. Orphanet J Rare Dis. 2020;15:148. https://doi.org/10.1186/s13023-020-01414-8.
Coratti G, Pane M, Lucibello S, Pera MC, Pasternak A, Montes J, et al. Age related treatment effect in type II Spinal Muscular Atrophy pediatric patients treated with nusinersen. Neuromuscul Disord. 2021;31:596–602. https://doi.org/10.1016/j.nmd.2021.03.012.
Mendonça RH, Polido GJ, Matsui C, Silva AMS, Solla DJF, Reed UC, Zanoteli E. Real-world data from nusinersen treatment for patients with later-onset spinal muscular atrophy: a single center experience. JND. 2021;8:101–8. https://doi.org/10.3233/JND-200551.
Szabó L, Gergely A, Jakus R, Fogarasi A, Grosz Z, Molnár MJ, et al. Efficacy of nusinersen in type 1, 2 and 3 spinal muscular atrophy: real world data from Hungarian patients. Eur J Paediatr Neurol. 2020;27:37–42. https://doi.org/10.1016/j.ejpn.2020.05.002.
Darras BT, Chiriboga CA, Iannaccone ST, Swoboda KJ, Montes J, Mignon L, et al. Nusinersen in later-onset spinal muscular atrophy: long-term results from the phase 1/2 studies. Neurology. 2019;92:e2492–506. https://doi.org/10.1212/WNL.0000000000007527.
Pechmann A, König K, Bernert G, Schachtrup K, Schara U, Schorling D, et al. SMArtCARE: a platform to collect real-life outcome data of patients with spinal muscular atrophy. Orphanet J Rare Dis. 2019;14:18. https://doi.org/10.1186/s13023-019-0998-4.
Mercuri E, Finkel R, Scoto M, Hall S, Eaton S, Rashid A, et al. Development of an academic disease registry for spinal muscular atrophy. Neuromuscul Disord. 2019;29:794–9. https://doi.org/10.1016/j.nmd.2019.08.014.
Wijnhoven TM, de Onis M, Onyango AW, Wang T, Bjoerneboe G-EA, Bhandari N, et al. Assessment of gross motor development in the WHO Multicentre growth reference study. Food Nutr Bull. 2004;25:S37-45. https://doi.org/10.1177/15648265040251S105.
Glanzman AM, O’Hagen JM, McDermott MP, Martens WB, Flickinger J, Riley S, et al. Validation of the Expanded Hammersmith Functional Motor Scale in spinal muscular atrophy type II and III. J Child Neurol. 2011;26:1499–507. https://doi.org/10.1177/0883073811420294.
Mazzone ES, Mayhew A, Montes J, Ramsey D, Fanelli L, Young SD, et al. Revised upper limb module for spinal muscular atrophy: Development of a new module. Muscle Nerve. 2017;55:869–74. https://doi.org/10.1002/mus.25430.
Brown EG, Wood L, Wood S. The medical dictionary for regulatory activities (MedDRA). Drug Saf. 1999;20:109–17. https://doi.org/10.2165/00002018-199920020-00002.
Pera MC, Coratti G, Forcina N, Mazzone ES, Scoto M, Montes J, et al. Content validity and clinical meaningfulness of the HFMSE in spinal muscular atrophy. BMC Neurol. 2017;17:39. https://doi.org/10.1186/s12883-017-0790-9.
Pane M, Coratti G, Pera MC, Sansone VA, Messina S, D’Amico A, et al. Nusinersen efficacy data for 24-month in type 2 and 3 spinal muscular atrophy. Ann Clin Transl Neurol. 2022. https://doi.org/10.1002/acn3.51514.
Coratti G, Lucibello S, Pera MC, Duong T, Muni Lofra R, Civitello M, et al. Gain and loss of abilities in type II SMA: A 12-month natural history study. Neuromuscul Disord. 2020;30:765–71. https://doi.org/10.1016/j.nmd.2020.07.004.
Coratti G, Carmela Pera M, Montes J, Scoto M, Pasternak A, Bovis F, et al. Revised upper limb module in type II and III spinal muscular atrophy: 24-month changes. Neuromuscul Disord. 2022;32(1):36–42. https://doi.org/10.1016/j.nmd.2021.10.009.
Pane M, Coratti G, Sansone VA, Messina S, Catteruccia M, Bruno C, et al. Type I SMA “new natural history”: long-term data in nusinersen-treated patients. Ann Clin Transl Neurol. 2021;8:548–57. https://doi.org/10.1002/acn3.51276.
Trucco F, Ridout D, Scoto M, Coratti G, Main ML, Muni Lofra R, et al. Respiratory Trajectories in type 2 and 3 spinal muscular atrophy in the iSMAC cohort study. Neurology. 2021;96:e587–99. https://doi.org/10.1212/WNL.0000000000011051.
Acknowledgements
We would like to thank all patients and families that agreed to share their data within the SMArtCARE registry. AP was supported by the Berta Ottenstein clinician scientist program of the University of Freiburg.
SMArtCARE study group: Lisa Ameshofer, Barbara Andres, Daniela Angelova-Toshkina, Daniela Banholzer, Christina Bant, Petra Baum, SandraBaumann, Ute Baur, Benedikt Becker, Bettina Behring, Julia Bellut, Andrea Bevot, Jasmin Bischofberger, Lisa Bitzan, Bogdan Bjelica, Markus Blankenburg, Sandra Böger, Friederike Bonetti, Anke Bongartz, Svenja Brakemeier, Lisa Bratka, Nathalie Braun, Sarah Braun, Brigitte Brauner, Christa Bretschneider, Nadine Burgenmeister, Bea Burke, Sebahattin Cirak, Andrea Dall, Heike de Vries, Adela Della Marina, Jonas Denecke, Marcus Deschauer, Zylfie Dibrani, Uta Diebold, Lutz Dondit, Jessica Drebes, Joenna Driemeyer, Vladimir Dukic, Matthias Eckenweiler, Mirjam Eminger, Michal Fischer, Cornelia Fischer, Maren Freigang, Philippa Gaiser, Andrea Gangfuß, Stephanie Geitmann, Annette George, Magdalena Gosk-Tomek, Susanne Grinzinger, Kristina Gröning, Martin Groß, Anne-Katrin Güttsches, Anna Hagenmeyer, Hans Hartmann, Julia Haverkamp, Miriam Hiebeler, Annegret Hoevel, Georg Friedrich Hoffmann, Britta Holtkamp, Dorothea Holzwarth, Annette Homma, Viola Horneff, Carolin Hörnig, Anna Hotter, Andrea Hubert, Peter Huppke, Eva Jansen, Lisa Jung, Nadja Kaiser, Stefan Kappel, Bolte Katharina, Johannes Koch, Stefan Kölke, Brigitte Korschinsky, Franziska Kostede, Karsten Krause, Hanna Küpper, Annina Lang, Irene Lange, Thorsten Langer, Yvonne Lechner, Helmar Lehmann, Christine Leypold, Paul Lingor, Jaqueline Lipka, Wolfgang Löscher, Antje Luiking, Gerrit Machetanz, Eva Malm, Kyriakos Martakis, Bettina Menzen, Moritz Metelmann, Gerd Meyer zu Hörste, Federica Montagnese, Kathrin Mörtlbauer, Petra Müller, Anne Müller, Anja Müller, Lars Müschen, Christoph Neuwirth, Moritz Niesert, Josefine Pauschek, Elke Pernegger, Susanne Petri, Veronika Pilshofer, Barbara Plecko, Jürgen Pollok, Martin Preisel, Manuel Pühringer, Anna Lisa Quinten, Sabine Raffler, Barbara Ramadan, Mika Rappold, Christian Rauscher, Kerstin Reckmann, Tabea Reinhardt, Melanie Röder, Doris Roland-Schäfer, Erdmute Roth, Lena Ruß, Afshin Saffari, Mareike Schimmel, Melina Schlag, Beate Schlotter-Weigel, Joanna Schneider, Jan-Christoph Schöne-Bake, David Schorling, Isabella Schreiner, Stephanie Schüssler, Michaela Schwarzbach, Michaela Schwippert, Luisa Semmler, Karin Smuda, Alina Sprenger-Svacina, Theresa Stadler, Paula Steffens, Daniela Steuernagel, Benjamin Stolte, Corinna Stoltenburg, Gehrke Tasch, Andreas Thimm, Elke Tiefenthaler, Raffi Topakian, Matthias Türk, Lieske van der Stam, Katia Vettori, Peter Vollmann, Matthias Vorgerd, Deike Weiss, Stephan Wenninger, Svea Werring, Maria Wessel, Ute Weyen, Sabine Wider, Nils Ole Wiebe, Anna Wiesenhofer, Sarah Wiethoff, Corinna Wirner, Camilla Wohnrade, Gilbert Wunderlich, Daniel Zeller, Michael Zemlin, Joachim Zobel.
Funding
Open Access funding enabled and organized by Projekt DEAL. Biogen and Novartis Gene Therapies provide financial support for the registry but had no influence on data analysis and interpretation.
Author information
Authors and Affiliations
Consortia
Contributions
All authors have made substantial contributions to conception and design of the study; AP wrote the first draft of the manuscript. All authors have been involved in revising the manuscript critically for important intellectual content and they have given final approval of the version to be published. All authors have participated sufficiently in the work to take public responsibility for appropriate portions of the content and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethics approval of the central ethics committee has been obtained [Ethics Committee of the University of Freiburg, Germany (EK 56/18)], and local ethics approvals were obtained from all participating centers. All patients or caregivers signed an informed consent form prior to being enrolled in the registry.
Consent for publication
Not applicable.
Competing interests
A. Pechmann received compensation for advisory boards, training activities and research grants from Novartis and Biogen. C. Weiß received compensation for advisory boards, publications and/or presentations from Novartis, Biogen, and Roche. M. Baumann received compensation for advisory boards and speakers honoraria from Novartis, Biogen and Roche. M. Flotats-Bastardas received compensation for advisory boards from Biogen, Novartis and Roche. R. Trollmann reports personal fees from Desitin Pharma, Novartis, PTC Therapeutics. M. Weiler received compensation for advisory boards, consultant and speaker honoraria and/or financial support for conference attendances from Akcea Therapeutics, Alnylam Pharmaceuticals, Biogen, Pfizer, Roche, and Sobi. S. Illsinger received compensation for advisory board/consultant work from Novartis. A. Bertsche reports grants from UCB Pharma GmbH and honoraria for speaking engagements and advisory boards from Biogen GmbH, Desitin Arzneimittel GmbH, Eisai GmbH, GW Pharma GmbH, Neuraxpharm GmbH, Shire/Takeda GmbH, UCB Pharma GmbH, and ViroPharma GmbH. A. Eisenkölbl received honoraria for advisory boards, interviews and speaking engegements from Biogen, Novartis and Roche. K. Vill received speaker honoraria and travel expenses from Novartis, Biogen and Santhera. M.C. Walter received compensation for advisory boards, funding for travel or speaker honoraria, and research support from Biogen, Novartis, and Roche. J. Johannsen received honoraria for advisory board participation and/or lectures and/or manuscript writing from Avexis/Novartis, Biogen, Roche, PTC, Pfizer and Sarepta Th., respectively. C. Reihle reports travel expenses from Biogen. R. Günther received compensation for advisory boards and research support from Biogen and compensation for advisory boards from Hofmann La-Roche. T. Hagenacker received compensation for advisory boards, lectures and research grants from Novartis, Biogen and Roche. V. Horber participated in workshops and received compensation for advisory boards from Novartis and Biogen. C. Köhler received honoraria from Roche, PTC, Biogen and Genzyme. M. Müller received compensation for expert meetings and training vom Norvartis, Biogen and Roche. O. Schwarz received compensation for advisory boards and training activities and research grants from Novartis and Biogen. I. Cordts received personal fees from Biogen (speaker’s honoraria, participation in advisory boards) outside the submitted work. M. Smitka received funding for educational activities and participation in advisory boards concerning SMA from Novartis Gene Therapies, Biogen and Roche. A. Schwerin-Nagel received compensation for an advisory board from Novartis Gene Therapies. H. Kölbel received compensation for presentations and consultancy from Avexis, Biogen, Sanofi, Pfizer, Novartis, and Roche. K. Schlachter received compensation for advisory boards from Novartis, Biogen and Roche. J. Friese received compensation for an advisory board from Novartis Gene Therapies. A. Ziegler receives honoraria for advisory boards and speaker honoraria from Novartis Gene Therapies, Roche Pharma and Biogen. His institution receives research grants from Biogen. G. Bernert receives research suppert from PTC and honoraria from Novartis Gene Therapies, PTC, Biogen, Roche, Pfizer, Santhera. E. Wilichowski received compensation for advisory boards from Biogen, Novartis and Roche. H. Lochmüller received consultancy and financial support for research projects and clinical trials from Amplo Biotechnology, AMO Pharma, argenx, Biogen, Desitin, Fulcrum Therapeutics, Harmony Biosciences, KYE Pharmaceuticals, Milo Biotechnology, Novartis, Pfizer, PTC Therapeutics, Hoffman-La Roche Limited, Sanofi-Genzyme, Santhera, Sarepta, Satellos, Spark Therapeutics and Ultragenyx. HL is the Editor-in-chief for the Journal of Neuromuscular Diseases (IOS Press). J. Kirschner received support from Biogen, Novartis, Roche and ScholarRock for consultancy, educational activities and/or clinical research.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
One of the authors first and last names’ tagging (Gerd Meyer zu Hörste, the SMArtCARE study group) has been corrected.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Pechmann, A., Behrens, M., Dörnbrack, K. et al. Improved upper limb function in non-ambulant children with SMA type 2 and 3 during nusinersen treatment: a prospective 3-years SMArtCARE registry study. Orphanet J Rare Dis 17, 384 (2022). https://doi.org/10.1186/s13023-022-02547-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13023-022-02547-8