Abstract
Background
Treacher Collins syndrome (TCS, OMIM 154500) is an autosomal disorder of craniofacial development with an incidence rate of 1/50,000 live births. Although TCOF1, POLR1D, and POLR1C, have been identified as the pathogenic genes for about 90% TCS patients, the pathogenic variants of about 8–11% cases remain unknown. The object of this study is to describe the molecular basis of 14 clinically diagnosed TCS patients from four families using Whole-exome sequencing (WES) followed by Sanger sequencing confirmation, and to analyze the effect of bone conduction hearing rehabilitation in TCS patients with bilateral conductive hearing loss.
Results
Four previously unreported heterozygous pathogenic variants (c.3047-2A > G, c.2478 + 5G > A, c.489delC, c.648delC) were identified in the TCOF1 gene, one in each of the four families. Sanger sequencing in family members confirmed co-segregation of the identified TCOF1 variants with the phenotype. The mean pure-tone threshold improvements measured 3 months after hearing intervention were 28.8 dB for soft-band BAHA, 36.6 ± 2.0 dB for Ponto implantation, and 27.5 dB SPL for Bonebridge implantation. The mean speech discrimination improvements measured 3 months after hearing intervention in a sound field with a presentation level of 65 dB SPL were 44%, 51.25 ± 5.06, and 58%, respectively. All six patients undergoing hearing rehabilitation in this study got a satisfied hearing improvement.
Conclusions
WES combined with Sanger sequencing enables the molecular diagnosis of TCS and may detect other unknown causative genes. Bone conduction hearing rehabilitation may be an optimal option for TCS patients with bilateral conductive hearing loss.
Similar content being viewed by others
Background
Treacher Collins syndrome (TCS, OMIM 154500) is an autosomal disorder of craniofacial development that has an incidence rate of 1/50,000 live births [1, 2]. TCS is characterized by typical bilateral craniofacial malformations, such as hypoplasia of the mandible and zygomatic complex, downward-slanting palpebral fissures, coloboma of the lower eyelids, antimongoloid slant of the eyes, micrognathia, cleft palate and microtia, and most cases are associated with conductive hearing loss [3], which affect patients both in cosmetic and functional aspects. Diagnosis and subsequent genetic counseling may be very difficult because some individuals are only mildly affected, and there are clinical overlaps between TCS, Goldenhar syndrome, Miller syndrome and Nagar syndrome, all of which are thought to be caused by impaired development of the first and second branchial arches between the 5th and 8th weeks of embryonic development.
Historically, a diagnosis of TCS has been based on the clinical identification of a minimal clinical phenotype: downward slanting palpebral fissures and hypoplasia of the zygomatic arch. However, this can overlook some mildly affected patients. The use of molecular diagnosis could enable the range of TCS phenotypes to be determined with less bias [4]. TCS is genetically heterogeneous, having been associated with pathogenic changes in three causative genes: TCOF1 (OMIM 606847), POLR1D (OMIM 613715), and POLR1C (OMIM 610060). More than 200 distinct mutations have been reported in TCOF1, accounting for about 70–93% of TCS individuals, which are inherited via an autosomal dominant pattern, while POLR1D and POLR1C mutations have been found in about 11–23% of the remaining patients, which are inherited via autosomal dominant and autosomal recessive patterns, respectively [3, 5,6,7].
To date, molecular diagnosis of TCS has focused on Sanger sequencing of these three known pathogenic genes, a method that is now the recommended first-tier test for TCS. The causative pathogenic variants of ~ 8–11% of TCS cases remain unknown, suggesting that there may be other TCS-related genes [8]. No phenotype-genotype correlation has been found in TCS patients [3]. Although nonpenetrance is rare, there is high inter-and intra-familial phenotypic variation, ranging from mildly affected cases to perinatal death due to severe craniofacial malformations that cause airway obstruction [3, 9]. With the development of next generation sequencing (NGS) technology, the cost of Whole-exome sequencing (WES) has become progressively lower in the past few years. WES could help to screen new causative genes compared to Sanger sequencing of TCOF1, POLR1D, and POLR1C. In this study, we used WES combined with Sanger confirmation to screen for causative genes in TCS families in China.
The occurrence rate of TCS in China is low, which has hindered genetic counseling for Chinese TCS patients. Although there have been several genetic studies of TCS in Chinses populations [8, 10, 11], most of the enrolled TCS cases are sporadic. Here, we describe four Chinese families containing 14 TCS patients. We performed WES in the four probands of these unrelated families and identified one previously undescribed pathogenic TCOF1 variant in each family, followed by Sanger sequencing to perform familiar segregation analysis. Our findings provide relevant information to diagnose TCS patients and counsel their families.
TCS is not a progressive disease. The primary concern in a newborn TCS patient is respiratory failure arising from craniofacial malformation-associated airway narrowing. Early interventions may be required to clear and maintain the airway, enable feeding, protect the eyes, improve the auditory ability, and allow for the development of speech. Later operations may include aesthetic and functional reconstructions of the mouth, face, and external ear [12]. With respect to the ear, 50% of TCS patients suffer from anomalies of the middle ear ossicular chain and a size reduction of the middle ear cavity, which can lead to bilateral conductive hearing loss. Bone conduction hearing aids or middle ear surgery are usually used to improve hearing in these patients [13, 14]. In the families studied herein, various hearing interventions were conducted for six TCS patients suffering from bilateral conductive hearing loss. We evaluated and compared their effects.
Results
Patients
The present study included nine female and four male patients from four families, each of which included at least two patients and were of Han nationality. The major clinical features of all patients were evaluated (Table 1). Downward slanting palpebral fissures and mandibular hypoplasia were observed in all patients. All patients had conductive hearing loss of different degrees. For the six patients that underwent hearing intervention during the study period, the average air-conducted hearing thresholds ranged from 56.25 dB HL to 60 dB HL and the bone-conducted hearing thresholds were ≥ 30 dB HL at frequencies of 0.5–4 kHz. HRCT scans demonstrated hypoplasia of the facial bones in all patients, including the zygomatic arch, mandible, and external ear canals. Temporal bone CT revealed malformation of the ossicles with fusion between rudiments of the malleus and incus.
Pathogenic variants
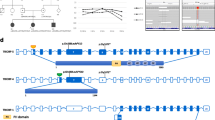
Four different and previously undescribed pathogenic variants of TCOF1 were identified in the four families (Fig. 1): c.3047-2A > G, c.2478 + 5G > A, c.489delC, and c.648delC, corresponding to two deletion mutations and two splicing mutations. The mutational spectrum of the four families are shown in Fig. 2. Sanger sequencing confirmed that all affected family members carried the relevant pathogenic mutation, whereas their unaffected relatives did not. The pathogenic variants found in this study are presented in Table 2.
(F1) Sequence of the patients of Family 1 showed a heterozygous mutation c.3047-2A > G. (F2) Sequence of the patient of Family 2 showed a heterozygous mutation c.2478 + 5G > A. (F3) Sequence of the patient of Family 3 showed a reported mutation c.489delC. (F4) Sequence of the patients of Family 4 showed a heterozygous mutation c.648delC
Hearing improvement
The mean pure-tone threshold improvements measured 3 months after hearing intervention were 28.8 dB for soft-band BAHA, 36.6 ± 2.0 dB for Ponto implantation, and 27.5 dB for Bonebridge implantation. The frequency specialized hearing thresholds unaided and with bone conduction hearing aid of the six patients were shown in Fig. 3. The mean speech discrimination improvements measured 3 months after hearing intervention in a sound field with a presentation level of 65 dB SPL were 44%, 51.25 ± 5.06, and 58%, respectively. The speech discrimination scores of each patient unaided and with bone conduction hearing aid were shown in Fig. 4. All six patients undergoing hearing rehabilitation in this study got a satisfied hearing improvement.
Discussion
TCS is caused by abnormal formation of the first and second branchial arches during the 5th to 8th weeks of fetal development, which leads to profound facial dysmorphism. Phenotypic expressivity varies between families and within families, and the disease is known to be both genetically and phenotypically heterogeneous. No phenotype-genotype correlation has been established to date. About 60% of patients arises as the result of de novo mutations without family history of this disease. Although there have been several genetic studies of TCS in Chinese populations [2, 8, 10], most of the enrolled TCS cases are sporadic. Family studies allow segregation analysis to support the identified genetic variants as pathogenic. We report the molecular characterization and bone conduction hearing rehabilitation effect on four Chinese TCS families, including a four- generation family with ten affected members.
Molecular diagnosis is of great significance for TCS families which can provide information for their genetic counseling. Han et al. (2018) reported the clinical findings and molecular diagnostics of a Chinese family with TCS. They concluded that the progeny of the proband and her mother have a 50% risk of suffering from TCS and therefore genetic counseling is necessary [15]. However, their study enrolled only one TCS family focused on molecular diagnosis using Sanger sequencing of TCOF1 and did not give any intervention to the patients. TCOF1, POLR1C, and POLR1D have been identified as causative genes for TCS, but mutations in these genes have not been found in ~ 8–11% of TCS cases. This, along with the time-consuming nature of Sanger sequencing, has limited the direct analysis of these genes for the systematic molecular diagnosis of TCS [4, 16]. Considering that the cost of WES has become progressively lower in the past few years, we used WES to detect the new causative gene in four TCS families. Although the identified mutations were in the known TCOF1 gene, it’s still of significance for the molecular diagnosis of TCS families.
Over 50% of TCS patients have bilateral conductive hearing loss due to abnormal development of the external/middle ear [12, 17]. These patients often require a multidisciplinary treatment approach that includes hearing intervention. In this study, hearing rehabilitations were performed for six patients with bone conductive hearing loss. We evaluated and compared the outcomes. This work, which describes clinical and molecular aspects of 14 Chinese TCS patients, and evaluates the effect of bone conduction hearing aids in TCS patients, could help facilitate the molecular diagnosis and treatment of TCS.
Mutations detected in this study
Most cases of TCS represent an autosomal dominant disorder of craniofacial development. Positional cloning enabled researchers to identify TCOF1 as the major causative gene, and a series of mutations within its coding sequence have been identified [18]. The most common causative mutations in TCS are small deletions (60%) and duplications (25%), all of which lead to frameshift variations [19]. Splicing, missense, and nonsense mutations are also seen, the vast majority of which are predicted to introduce a termination codon into the mRNA [17, 18, 20, 21]. The mutations associated with TCS are consistently predicted to shorten treacle, which is the gene product of TCOF1, and thus might cause dominant-negative effects. Alternatively, it has been suggested that mutation of one TCOF1 allele may cause TCS by haploinsufficiency [22, 23]. In addition to these mutations in TCOF1, mutations in genes encoding two subunits of POLR1C and POLR1D have also been associated with TCS [6].
The 14 patients studied herein were all found to have pathogenic variants in TCOF1. The high detection rate of pathogenic variants achieved in this study suggests that WES combined with Sanger sequencing may be a useful method for detecting pathogenic variants in TCS families. Four different pathogenic TCOF1 variants were identified and all haven’t been previously reported, which broadens the mutational spectrum in the Asian population. Among the studied patients, 71.4% (10/14) had one nucleotide deletion that were predicted to yield haploinsufficiency of the treacle protein. This finding is consistent with most of the previous studies. In the present work, each family harbored a different mutation. The most frequent mutation was a deletion (C. 648delC) in exon 6A, which was found in eight patients of the same family. Although the literature suggests that exons 10, 15, 16, 23, and 24 are mutational hotspots in TCOF1, the mutations identified in the present work were located in exon 6A, exon 5, intron 14, and intron 17 [7, 24, 25].
The proband of one family (F4) was initially suspected to have autosomal recessive (AR) inheritance at the time of clinical diagnosis, as both of his parents showed normal physical features. Our work ruled out this potential AR inheritance, however, as we found that the proband’s seemingly normal mother carried a 1-bp deletion C in exon 6A of TCOF1, as did her affected mother and other affected individuals of this family. The clinical diagnosis was missed in the proband’s mother because she showed mild downslanting palpebral fissures that were rendered nearly inconspicuous by the droop of her aging eyelids. This serves to emphasize that the severity of the TCS phenotype is highly variable, and some subjects are so mildly affected that it is almost impossible to make a clinical diagnosis without molecular analysis.
TCOF1 mutation spectrum in TCS patients
The majority of TCS patients are heterozygous for mutations in TCOF1, which is located at 5q32–q33.1.11–18 and has an open reading frame that encodes 4465 base pairs and 28 exons. The gene product, treacle, contains at least 1411 amino acids and acts as a nucleolar phosphoprotein that travels between the nucleolus and the cytoplasm. Treacle is a low-complexity protein with a 14-residue N-terminus followed by 11 repeated units with potential phosphorylation sites and a C-terminus with multiple putative nuclear and nucleolar localization signals. It has been suggested that correct expression of treacle is essential for the survival of cephalic neural crest cells. The pathogenic TCOF1 mutations can reduce the number of neural crest cells (NCCs), which are needed for craniofacial embryological development, by impacting the involvement of treacle in ribosomal DNA gene transcription. Nonsense mutations of TCOF1 can lead to an immediate termination of translation, producing a shortened protein. The location of the mutation affects the length of the produced protein, and all of the shortened proteins are likely to be degraded via nonsense-mediated decay. In addition, the C-terminus of treacle contains multiple putative nuclear localization signals, which can be disrupted by two constructs that split the C-terminal region [20, 26, 27]. In the present study, we identified four mutations, including two deletions and two splicing mutations, all of which could lead to the production of a shortened treacle protein.
No clear phenotype-genotype correlation is seen in TCS, but the severity is related to the type of mutation. Although penetrance is high, there is intra- and interfamilial variation. 11–23% of patients have mutations in POLR1C or POLR1D, which encode proteins that are important in the ribosomal transcription of RNA and affect ribosomal biogenesis [6]. However, in this study we did not identify any mutation in POLR1C or POLR1D.
Advantages of WES in identifying pathogenic variants
Most suspected cases of TCS can be molecularly confirmed by Sanger sequencing of three causative genes: TCOF1, POLR1D, and POLR1C. The major causative gene, TCOF1, has a total of 27 coding exons and adjacent splice junctions, rendering such analysis time-consuming and costly. We thus set out to use WES to quickly screen the causative exons, followed by Sanger sequencing of specific exons that appeared to confirm mutations.
The pathogenic variants in about 8–11% of TCS cases remain undetected. There are four main possible explanations for this. First, some of these cases may have been clinically misdiagnosed. However, we note that in most such cases, CT scans and clinical analysis have been used to confirm the diagnosis. Second, the causative mutations might be located in untranslated (and thus unexamined) regions of the three known TCS genes. Although such pathogenic variants are rare in the literature, these regions should be checked in future studies. Third, the causative mutations may be large deletions or insertions within the known TCS genes, which may not be detected by Sanger sequencing. This is especially true in dominant diseases, in which patients also possess the normal allele. Finally, there may be additional, yet-undiscovered genes responsible for TCS. These could potentially be identified by WES.
Molecular diagnosis of syndromes with overlapping phenotypes
TCS, Goldenhar syndrome, Miller syndrome, and Nagar syndrome exhibit overlaps in their variable phenotypic expression, asymmetric involvement of facial structures, and familial occurrence of microtia or related anomalies (e.g., preauricular tags and pits). This complicates the diagnosis of such diseases according to the patient’s clinical manifestation. WES could help overcome this limitation. The common phenotypes and causative genes of these syndromes are shown in Table 3. The WES allowed us to exclude the involvement of these non-TCS causative genes and confirmed the molecular diagnosis of TCS in the 14 enrolled patients. It is helpful to conduct WES for clinical tentative TCS patients to identify the pathogenic variants and to differentiate from other syndromes sharing common clinical features.
Intervention for TCS patients
TCS is characterized by a complex presentation of mandibulofacial dysplasia that requires a multidisciplinary intervention from birth to adulthood. Although the presenting features are predictable, there is considerable individual variability, and the functional, aesthetic, and psychosocial needs of each patient will differ. Patients can be given a general scheme of operations and a broad description of the potential help that is available, but a more individually tailored approach is needed [12,13,14, 28]. Bilateral conductive hearing loss is seen in 50% of TCS patients, arising from a wide range of anomalies of the middle ear ossicular chain and a reduction in the size of the middle ear cavity. Deformities in the ossicular chain can be corrected surgically if the external meatus is patent; otherwise, bone conduction hearing aids are usually used [12]. Bone conduction hearing device implantation surgery requires that the cranial bone have a thickness of at least 4 mm, which is usually achieved at 6 years of age. Prior to this, patients are given banded bone conduction hearing aids. This is ideally initiated before 12 months of age, to enable proper central auditory neurological development. As CT may be indicated to assess the status of the middle ear and external meatus, the patient’s craniofacial and auditory ear, nose, and throat (ENT) teams should discuss their scanning protocols and intentions to ensure minimal exposure to radiation over time. The present study included six patients with bilateral conductive hearing loss who were given hearing interventions during the study period. All obtained optimal results.
It has been suggested that genetic or pharmacological blockade of the p53 gene could reduce neuroepithelial apoptosis during embryogenesis and restore the migrating population of NCCs, potentially preventing the TCS phenotype. However, this would also block the ability of p53 to act as a tumor suppressor, so it would be necessary to interrogate its downstream targets to find a safe point for intervention. This would have to occur in the first trimester, which would make it challenging to detect the need for and properly time the treatment [12, 29].
Conclusions
We herein show that mutational analysis based on WES was useful for the definitive diagnosis of TCS Chinese families and may also provide more information for molecular diagnosis. We also report that bone conduction hearing rehabilitation was consistently helpful for TCS patients with bilateral conductive hearing loss.
Methods
Patients and families
This single-center prospective study, which involved four Chinese families with 14 clinically diagnosed TCS patients, was performed at Peking Union Medical College Hospital (PUMCH) in Beijing, China. Approval was obtained from the Institutional Review board of PUMCH and written informed consent was obtained from each studied family member. A comprehensive clinical history was taken and a complete physical examination was performed on all subjects to exclude Goldenhar, Nager, and Miller syndromes. The scoring system developed by Ozge Altug and Teber was used to clarify the phenotypic expression of TCS in these patients [4, 9]. All patients were identified as severely or mildly affected. Of the 14 patients, six underwent hearing rehabilitation between January 2017 and January 2018. All six patients received hearing measurements consisting of pure tone auditory (PTA) testing at 0.5, 1, 2, 4 kHz before and after hearing intervention. Clinical data, photographs of the patients, and temporal bone high-resolution computed tomography (HRCT) data were collected.
WES and mutational analysis
Genomic DNA was extracted from peripheral blood samples using a TIANamp Blood DNA Kit (Tiangen, Beijing, China) according to the manufacturer’s protocol. WES was performed on the four TCS probands at Beijing Allwegene (Beijing, China). Exome enrichment was performed using a Sure Select Human All Exon v6 kit (65 Mb) (Agilent, Santa Clara, CA, USA), which yielded an average sequencing depth of 100-fold and a coverage of 97.7%. Enriched shotgun libraries were sequenced on a HiseqX platform (Illumina, San Diego, CA, USA).
Sequenced reads were collected, filtered for quality, and aligned to the human reference sequence (UCSC Genome Browser hg19, http://genome.ucsc.edu/) using the Burrows-Wheeler Aligner. Genotypes were called using SAMtools, Picard, and GATK. Sequence variants, including single-nucleotide variants (SNVs) and small insertions or deletions (InDels), were annotated using the ANNOVAR software (http://annovar.openbioinformatics.org) (TCOF1 reference: NM_001135243). For coding or splice-site mutations, the conservation at the variant site and the predicted effect on protein function were evaluated using the in silico tools, SIFT (http://sift.jcvi.org/), PolyPhen-2 (http://genetics.bwh.harvard.edu/pph2/), MutationTaster (http://www.mutationtaster.org/), and CADD (http://cadd.gs.washington.edu/).
A list of qualifying genotypes was generated using the following criteria: First, only protein-altering variants, such as missense variants, frameshift, InDels, and intron-exon boundary variants were included. Second, mutations were excluded as common variants if they were present at a frequency of 10% or more in at least one of the following databases: dbSNP (v.144); the 1000 Genomes Project; the HapMap CHB (Han Chinese in Beijing, China) population; the National Heart, Lung, and Blood Institute Exome Sequencing Project (ESP); and the Exome Aggregation Consortium (ExAC) Browser. Finally, missense variants were excluded if they were not predicted to be deleterious by the SIFT, PolyPhen-2, MutationTaster, or CADD analyses.
The mutations identified in the four families were prioritized for Sanger confirmation. The relevant sequences were PCR amplified from the probands and their family members, and the amplified fragments were purified using an Agencourt AMPure XP kit (Beckman Coulter, USA). Sanger sequencing was performed with an ABI3730xl DNA Sequencer (Applied Biosystems | Thermo Fisher Scientific, USA) and the results were analyzed using the Sequencing Analysis 5.2 software (Applied Biosystems | Thermo Fisher Scientific, USA). We referred to the HGVS nomenclature guidelines (http://www.hgvs.org/mutnomen) when naming the identified variants.
Hearing interventions and audiometric data
Of the 14 TCS patients, six were given hearing interventions: one received a soft-band bone anchored hearing aid (BAHA), four received Ponto implantation, and one received bonebridge implantation. Pure-tone audiograms and speech discrimination tests were performed before and after the hearing interventions. Loudspeakers were placed 1 m in front of each subject and free sound field hearing thresholds were evaluated at 0.5, 1, 2 and 4 kHz frequencies. Speech discrimination scores (in quiet) were measured using the Mandarin Speech Test Materials (MSTM) [30], which comprised 10 lists of 50 Chinese characters or spondaic words. Speech stimuli were presented at 65 dB SPL. All test materials were presented without repetition. The average hearing gains at 0.5, 1, 2, and 4 kHz were calculated.
Availability of data and materials
All data generated during this study are included in this published article [and its supplementary information files.
Abbreviations
- AR:
-
Autosomal recessive
- TCS:
-
Treacher Collins syndrome
- WES:
-
Whole-exome sequencing
References
Trainor PA, Andrews BT. Facial dysostoses: etiology, pathogenesis and management. Am J Med Genet C Semin Med Genet. 2013;163c(4):283–94.
Zhang X, Fan Y, Zhang Y, Xue H, Chen X. A novel mutation in the TCOF1 gene found in two Chinese cases of Treacher Collins syndrome. Int J Pediatr Otorhinolaryngol. 2013;77(9):1410–5.
Vincent M, Genevieve D. Treacher Collins syndrome: a clinical and molecular study based on a large series of patients. Genet Med. 2016;18(1):49–56.
Teber OA, Gillessen-Kaesbach G, Fischer S, Bohringer S, Albrecht B, Albert A, Arslan-Kirchner M, Haan E, Hagedorn-Greiwe M, Hammans C, et al. Genotyping in 46 patients with tentative diagnosis of Treacher Collins syndrome revealed unexpected phenotypic variation. Eur J Hum Genet. 2004;12(11):879–90.
Splendore A, Silva EO, Alonso LG, Richieri-Costa A, Alonso N, Rosa A, Carakushanky G, Cavalcanti DP, Brunoni D, Passos-Bueno MR. High mutation detection rate in TCOF1 among Treacher Collins syndrome patients reveals clustering of mutations and 16 novel pathogenic changes. Hum Mutat. 2000;16(4):315–22.
Dauwerse JG, Dixon J, Seland S, Ruivenkamp CA, van Haeringen A, Hoefsloot LH, Peters DJ, Boers AC, Daumer-Haas C, Maiwald R, et al. Mutations in genes encoding subunits of RNA polymerases I and III cause Treacher Collins syndrome. Nat Genet. 2011;43(1):20–2.
Bowman M, Oldridge M, Archer C, O'Rourke A, McParland J, Brekelmans R, Seller A, Lester T. Gross deletions in TCOF1 are a cause of Treacher-Collins-Franceschetti syndrome. Eur J Hum Genet. 2012;20(7):769–77.
Chen Y, Guo L, Li CL, Shan J, Xu HS, Li JY, Sun S, Hao SJ, Jin L, Chai G, et al. Mutation screening of Chinese Treacher Collins syndrome patients identified novel TCOF1 mutations. Mol Genet Genomics. 2018;293(2):569–77.
Dixon MJ, Marres HA, Edwards SJ, Dixon J, Cremers CW. Treacher Collins syndrome: correlation between clinical and genetic linkage studies. Clin Dysmorphol. 1994;3(2):96–103.
Li H, Zhang X, Li Z, Chen J, Lu Y, Jia J, Yuan H, Han D. Clinical and genetic analysis of a patient with Treacher Collins syndrome in TCOF1 gene. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2012;26(10):459–62.
Wang Y, Yin XJ, Han T, Peng W, Wu HL, Liu X, Feng ZC. A novel silent deletion, an insertion mutation and a nonsense mutation in the TCOF1 gene found in two Chinese cases of Treacher Collins syndrome. Mol Gen Genomics. 2014;289(6):1237–40.
Cobb AR, Green B, Gill D, Ayliffe P, Lloyd TW, Bulstrode N, Dunaway DJ. The surgical management of Treacher Collins syndrome. Br J Oral Maxillofac Surg. 2014;52(7):581–9.
Posnick JC, Tiwana PS, Costello BJ. Treacher Collins syndrome: comprehensive evaluation and treatment. Oral Maxillofac Surg Clin North Am. 2004;16(4):503–23.
Plomp RG, van Lieshout MJ, Joosten KF, Wolvius EB, van der Schroeff MP, Versnel SL, Poublon RM, Mathijssen IM. Treacher Collins syndrome: a systematic review of evidence-based treatment and recommendations. Plast Reconstr Surg. 2016;137(1):191–204.
Yan Z, Lu Y, Wang Y, Zhang X, Duan H, Cheng J, Yuan H, Han D. Identification of a novel TCOF1 mutation in a Chinese family with Treacher Collins syndrome. Exp Ther Med. 2018;16(3):2645–50.
Lim EC, Brett M, Lai AH, Lee SP, Tan ES, Jamuar SS, Ng IS, Tan EC. Next-generation sequencing using a pre-designed gene panel for the molecular diagnosis of congenital disorders in pediatric patients. Hum Genomics. 2015;9:33.
Marszalek B, Wojcicki P, Kobus K, Trzeciak WH. Clinical features, treatment and genetic background of Treacher Collins syndrome. J Appl Genet. 2002;43(2):223–33.
Gladwin AJ, Dixon J, Loftus SK, Edwards S, Wasmuth JJ, Hennekam RC, Dixon MJ. Treacher Collins syndrome may result from insertions, deletions or splicing mutations, which introduce a termination codon into the gene. Hum Mol Genet. 1996;5(10):1533–8.
So RB, Gonzales B, Henning D, Dixon J, Dixon MJ, Valdez BC. Another face of the Treacher Collins syndrome (TCOF1) gene: identification of additional exons. Gene. 2004;328:49–57.
Edwards SJ, Gladwin AJ, Dixon MJ. The mutational spectrum in Treacher Collins syndrome reveals a predominance of mutations that create a premature-termination codon. Am J Hum Genet. 1997;60(3):515–24.
Wise CA, Chiang LC, Paznekas WA, Sharma M, Musy MM, Ashley JA, Lovett M, Jabs EW. TCOF1 gene encodes a putative nucleolar phosphoprotein that exhibits mutations in Treacher Collins syndrome throughout its coding region. Proc Natl Acad Sci U S A. 1997;94(7):3110–5.
Marsh KL, Dixon J, Dixon MJ. Mutations in the Treacher Collins syndrome gene lead to mislocalization of the nucleolar protein treacle. Hum Mol Genet. 1998;7(11):1795–800.
Isaac C, Marsh KL, Paznekas WA, Dixon J, Dixon MJ, Jabs EW, Meier UT. Characterization of the nucleolar gene product, treacle, in Treacher Collins syndrome. Mol Biol Cell. 2000;11(9):3061–71.
Splendore A, Fanganiello RD, Masotti C, Morganti LS, Passos-Bueno MR. TCOF1 mutation database: novel mutation in the alternatively spliced exon 6A and update in mutation nomenclature. Hum Mutat. 2005;25(5):429–34.
Conte C, D'Apice MR, Rinaldi F, Gambardella S, Sangiuolo F, Novelli G. Novel mutations of TCOF1 gene in European patients with Treacher Collins syndrome. BMC Med Genet. 2011;12:125.
Robbins J, Dilworth SM, Laskey RA, Dingwall C. Two interdependent basic domains in nucleoplasmin nuclear targeting sequence: identification of a class of bipartite nuclear targeting sequence. Cell. 1991;64(3):615–23.
Woodgett JR, Gould KL, Hunter T. Substrate specificity of protein kinase C. use of synthetic peptides corresponding to physiological sites as probes for substrate recognition requirements. Eur J Biochem. 1986;161(1):177–84.
Aljerian A, Gilardino MS. Treacher Collins syndrome. Clin Plast Surg. 2019;46(2):197–205.
Jones NC, Lynn ML, Gaudenz K, Sakai D, Aoto K, Rey JP, Glynn EF, Ellington L, Du C, Dixon J, et al. Prevention of the neurocristopathy Treacher Collins syndrome through inhibition of p53 function. Nat Med. 2008;14(2):125–33.
Badran K, Bunstone D, Arya AK, Suryanarayanan R, Mackinnon N. Patient satisfaction with the bone-anchored hearing aid: a 14-year experience. Otol Neurotol. 2006;27(5):659–66.
Acknowledgements
Not applicable.
Funding
This work was supported by grant to Xiaowei Chen from the General Programs of National Natural Science Foundation of China (No. 81271053) and National Natural Emergency management project (No. 81450026).
Author information
Authors and Affiliations
Contributions
XWC conceived and designed the experiments. XMF and YBW ascertained the probands and collected the DNA samples. XWC acted as head surgeon for the bone conduction hearing devices implantations performed in this study. XMF, HQD and NNL analyzed the data and wrote the paper. XWC, YF and SYZ revised it critically for important intellectual content. All authors read and approved the final manuscript and collected data.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Approval was obtained from the Institutional Review board of PUMCH and written informed consent was obtained from each studied family member.
Consent for publication
Consent for publication of individuals’ details was obtained from each subjects or their parents.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Fan, X., Wang, Y., Fan, Y. et al. TCOF1 pathogenic variants identified by Whole-exome sequencing in Chinese Treacher Collins syndrome families and hearing rehabilitation effect. Orphanet J Rare Dis 14, 178 (2019). https://doi.org/10.1186/s13023-019-1136-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13023-019-1136-z