Abstract
Background
Thinning practices are useful measures in forest management and play an essential role in maintaining ecological stability. However, the effects of thinning on the soil properties and microbial community in large Chinese fir timber plantations remain unknown. The purpose of this study was to investigate the changes in soil physicochemical properties and microbial community composition in topsoil (0–20 cm) under six different intensities (i.e., 300 (R300), 450 (R450), 600 (R600), 750 (R750) and 900 (R900) trees per hectare and 1650 (R1650) as a control) in a large Chinese fir timber plantation.
Results
Compared with the CK treatment, thinning significantly altered the contents of soil organic carbon (SOC) and its fractions but not in a linear fashion; these indicators were highest in R900. In addition, thinning did not significantly affect the soil microbial community diversity indices but significantly affected the relative abundance of the core microbial community. Proteobacteria, Acidobacteria, and Actinobacteria were the dominant bacterial phyla; the relative abundances of Proteobacteria and Acidobacteria were highest in R900, and that of Actinobacteria was lowest in R900. The dominant fungal phyla were Ascomycota, Basidiomycota and Mucoromycota; the relative abundance of Ascomycota was lowest in R900, and that of Mucoromycota was highest in R900. The fungal microbial community composition was more sensitive than the bacterial community composition. The activity of the carbon-cycling genes was not linearly correlated with thinning, and the abundance of C-cycle genes was highest in R900.
Conclusions
These findings are important because they show that SOC and its fractions and the abundance of the soil microorganism community in large Chinese fir timber plantations can be significantly altered by thinning, thus affecting the capacity for carbon storage. These results may advance our understanding of how the density of large timber plantations could be modified to promote soil carbon storage.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Background
Soil carbon (C) storage is considered to be the greatest form of carbon storage in terrestrial ecosystems and has received much interest in recent years because of its potential for carbon storage and its important role in the global carbon cycle [1]. Soil organic carbon (SOC) is an important component of carbon storage. Cotrufo and Lavallee [2] reported that the C pool contained fast-cycling SOC and slow-cycling SOC; fast-cycling SOC mainly comprises plant derivatives, and slow-cycling SOC primarily comprises microbial necromass. Nevertheless, SOC is very sensitive to environmental variations, such as management measures and climate variability [1, 3]. Thinning is a primary measure of forest management that changes stand density, soil properties and understory vegetation, which ultimately affect soil nutrients and microbial activity and thus affect carbon storage in forest ecosystems [1, 4, 5]. Thinning can significantly affect plant communities and then influence litter, roots and plant exudates, which directly affect SOC and its fractions, thus affecting the structure of the soil microbial community in forest ecosystems [6]. In contrast, soil microorganisms release mineral elements near the rhizosphere to affect the growth of plants [7]. Owing to the complex interactions among thinning, soil physiochemical and microbial communities, the relationships among them are still largely unknown; thus, determining the effects of thinning practices on the soil carbon storage capacity is necessary.
Soil microorganisms are involved in most soil ecological processes, such as atmospheric CO2 fixation, energy flow, soil fertility and carbon cycling [8,9,10]. Studies have reported that the effects of thinning on microbial diversity are based on different forest types, thinning times, and thinning intensities [11, 12]. Chinese fir (Cunninghamia lanceolata (Lamb.) Hook) is an excellent fast-growing species that is widely used in southern China as a commercial wood source, with the largest planting area among artificial forests in China [13]. Owing to its special quality, it is considered an essential factor in the timber supply for human society, environmental protection, and maintaining stability in the global ecosystem [14]. During the past few decades, many Chinse fir trees have been planted, and some plantations, including large timber tree plantations, have been preserved. However, after decades of growth, high stand density, a low-diversity plant community and poor regeneration capacity are the main problems for maintaining forest ecosystems. Therefore, proper management measures should be taken to achieve sustainable development of these ecosystems. Previous studies have reported that forest thinning changes microbial activity, fine root production, and biomass, resulting in effects on the carbon inputs and outputs of Chinese fir [15, 16]. On the basis of these findings, we hypothesized that (1) thinning may influence the soil properties and microbial community of large Chinese fir timber plantations and that (2) the function of carbon cycling in Chinese fir ecosystems can be affected by thinning. However, the influence of forest thinning on soil microbes in large Chinese fir timber plantations has not yet been investigated. Furthermore, how soil characteristics vary with stand density and the main drivers of carbon storage remain unknown.
In this study, eighteen experimental plots were subjected to six intensities of thinning in a 28-year-old large Chinese fir timber plantation, that is, 300 (R300), 450 (R450), 600 (R600), 750 (R750), 900 (R900) and 1650 (R1650, control treatment) trees per hectare. Five years after thinning, the soils under the different thinning intensities were studied to investigate (1) the variations in SOC and its fractions, the microbial community structure, and soil C storage under different the intensities; (2) the association between soil carbon storage and microorganism communities; and (3) the functional genes involved in C-cycling under the different intensities.
Materials and methods
Experimental site and thinning treatments
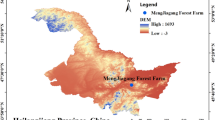
The study was performed in a 28-year-old Chinese fir plantation at Shijingshan State-owned Forest Farm (with 2797.17 hm2 of pure Chinese fir forest) (109° 7′ 14″ E, 26° 11′ 17″ N) in Liping County, Southeast Guizhou Province, China (Fig. 1). The plantation has rich biodiversity, including Taxus chinensis, Eurya nitida Korthals, Smilax china L., Michelia figo, Acer oblongum, Kalopanax septemlobus and several other plant species with crucial scientific value and ecological status in China. The soil type is characterized by yellow or red‒yellow soil. The area has a central subtropical monsoon humid climate with a mean temperature of 15.6 ℃ and annual average precipitation of 1325.9 mm, and the elevation is between 650 and 750 m.
Eighteen experimental plots (20 m × 30 m) were subjected to six thinning treatments in a completely randomized design with three replicates of each treatment. Each plot has a 15-m-wide perimeter buffer zone in the middle. The eighteen experimental plots had the same initial tree number before thinning. The thinning treatments were conducted in October 2017 after all small trees (with a breast diameter < 15 cm) and trees in close proximity (distance < 50 cm) were removed, with the exception of those showing strong growth. The residual stand densities after thinning were 300 (R300), 450 (R450), 600 (R600), 750 (R750), and 900 (R900) Chinese fir trees per hectare. The remaining three plots without thinning were used as controls (1650 trees per hectare).
Soil sample collection
Forest soil sampling was conducted in September 2022. Ten sampling points were randomly chosen in each treatment. The topsoil (0–20 cm soil depth) was collected with a cutting ring (5 cm in diameter, 10 cm deep) to obtain soil samples. The 10 soil samples from each plot were thoroughly mixed to obtain one composite soil sample for each stand via a soil auger, resulting in 18 soil samples from the six different thinning densities. Roots were carefully removed from the soil samples, insects and gravels were removed, and the samples were then sieved through a 2 mm mesh. Each soil sample was mixed and divided into three portions: one portion was air-dried at room temperature and stored for soil physiochemical detection, one portion was stored at 4 ℃ for the analysis of microbial carbon (MBC) and organic carbon contents, and the third portion was stored at − 80 ℃ for microbial DNA extraction and high-throughput sequencing [17,18,19].
Determination of soil physicochemical factors
SOC was detected via potassium dichromate oxidation with external heating. The concentration of easily oxidizable carbon (EOC) was determined in accordance with the process of Carter [20]. In brief, dissolved organic carbon (DOC) was determined from fresh soil with deionized water at a 1:5 ratio (soil:water) and detected with a multi-N/C 3100 TOC analyzer [21]. The concentration of particulate organic carbon (POC) was analyzed according to the methods of Elliott and Cabbardella [22]. Heavy fraction organic carbon (HFOC) and light fraction organic carbon (LFOC) analyses were performed according to the methods of Wu et al. [23]. In addition, soil microbial biomass carbon (MBC) was extracted from fresh soil samples via chloroform fumigation—extraction and measured via a total organic carbon (TOC) analyzer [24, 25].
Microbial necromass C
Microbial necromass C is a component of SOC, and its content (fungal and bacterial necromass C) was measured via the method of Zhang and Amelung [26]. Amino sugar compounds were detected via a chromatographic column (250 mm* 4.6 mm, 5 μm) on a gas chromatograph. We tested glucosamine (GlcN) and muramic acid (MurA) in the microbial cell wall, and the concentrations of microbial necromass carbon were calculated via the following equations:
where 45 is the conversion factor from MurN to the bacterial necromass C, and 251.23 and 179.17 are the molecular weights of MurA and GlcN, respectively. In addition, 9 represents the conversion value of fungal GlcN to fungal necromass. The microbial necromass C was calculated as the sum of the bacterial necromass C and fungal necromass C. The GlcN-to-MurA ratio represents the proportion of fungal and bacterial residue contributions to the SOC content.
DNA extraction and sequencing
Total DNA from each soil sample was extracted via a FastDNA Spin Kit according to the manufacturer’s instructions. The quality and quantity were analyzed via 1% agarose gel electrophoresis and TBS-380, respectively, and the DNA purity was tested via a NanoDrop 2000 (Nanodrop Technologies Inc., CA, USA). And the qualitied samples were sent to Shanghai Majorbio Biopharm Technology Co., Ltd. (Shanghai, China) to obtain metagenomic sequence data via Illumina HiSeq 4000 instruments.
De novo metagenomic assembly and functional annotations
The adaptor sequences and poor-quality regions of the raw data were removed via Trimmomatic software (version 0.35). A total of 18 separate assemblies were obtained via de novo assembly. Contigs and scaffold sequences were constructed via De Bruijn graph, and a comprehensive summary of assembly statistics was generated for the different metagenomes. CD-HIT-EST software was used to remove the duplicated and suboptimal contigs, resulting in the final assembly of each soil sample. Furthermore, the complete metagenomic sequences (length ≥ 300 bp) were submitted to the National Center for Biotechnology Information (NCBI) database (No. PRJNA994818).
The BLAST analyses of the unigenes were performed via the nonredundant protein sequence database (NR) and the Kyoto Encyclopedia of Genes and Genomes (KEGG) repositories via Diamond software [27]. The annotated genes were subsequently assigned to different functional hierarchies via the KEGG Orthology (KO) database. Furthermore, the results of BLAST analyses of the unigenes were analyzed via the carbohydrate-active enzymes database (CAZy) by Hmmscan (http://hmmer.janelia.org/search/hmmscan).
Statistical analyses
The differences in the physiochemical properties of the soil and the relative abundances of functional genes were analyzed via one-way ANOVA and the Kruskal‒Wallis test. The differences among the different thinning intensities were compared via Tukey’s test. P < 0.05 was considered significant. Constrained redundancy analysis (RDA) software was used to analyze the correlations between environmental changes and microbial communities. Nonmetric multidimensional scaling (NMDS) software was used to analyze the soil microbial communities based on Bray‒Curtis distance metrics. A heatmap was constructed with TBtools.
Results
Soil physiochemical analyses under the different thinning densities
The soil characteristics significantly differed under the different thinning intensities (Table 1), and they did not display a linear relationship. Interestingly, all the tested soil physiochemical indicators were highest in R900, i.e., 35.71 g kg−1 (SOC), 13.61 g kg−1 (POC), 2.62% (EOC), 66.17 mg kg−1 (DOC), 25.87 g kg−1 (HFOC), 9.69 g kg−1 (LFOC) and 6508.9 mg kg−1 (MBC). Compared with those in R1650, the SOC content in R900 was 85.89% higher, the POC content was 86.69% higher, the DOC content was 36.63% higher, the LFOC content was 26% higher, the HFOC content was 134.54% higher, and the MBC content was 11.71% higher. These results may indicate that R900 is more beneficial for SOC fractions than the other intensities are.
Contents of microbial necromass C under the different thinning intensities
The microbial necromass C varied significantly under the six different thinning intensities (P < 0.05, Fig. 2b) and was highest in R900, which contributed to an essential portion of the SOC. The content of the microbial necromass C was lowest in R1650, which may indicate that high-density trees were not beneficial for microorganism processes. However, the change trends of fungal necromass C were similar to those of microbial necromass C (Fig. 2a), whereas the change trends of bacterial necromass C differed. In addition, there was no significant difference in the bacterial necromass C among R750, R900 and R1650 (P > 0.05, Fig. 2a).
Genomic sequencing results
A total of 820.4 million clean reads were obtained for the eighteen soil samples, with an average of 45.6 million reads per sample obtained through metagenomic sequencing after the low-quality data were filtered out. The clean data were assembled via MEGAHIT. An average of 280,956, 271,815, 240,138, 301,979, 258,264 and 297,749 contigs were generated in the R300, R450, R600, R750, R900, and R1650 metagenomes, respectively. A total of 5,511,966 nonredundant open reading frames (ORFs) were obtained. Taxonomy-based annotations were collected for microbial community analysis from all unigenes.
Soil microbial community characteristics under the different thinning densities
A total of 223 phyla, 416 classes and 4440 genera were identified via whole-metagenome sequencing from all the soil samples. The results indicated that the microbial communities were not linearly correlated but rather sharply differed under the different thinning density levels. At the phylum level, the bacterial communities with a relative abundance > 1% were Proteobacteria (40.71% to 46.28%), Acidobacteria (19.30% to 27.67%), Actinobacteria (20.03% to 27.35%), Verrucomicrobia (2.48% to 3.99%) and Chloroflexi (1.14% to 1.53%), which are the core phyla accounting for more than 80.0% of the total bacterial abundance (Fig. 3a). However, the relative abundance of Acidobacteria was highest in R900, and that of Actinobacteria was lowest in R900. Planctomycetes, unclassified bacteria, Gemmatimonadetes, Candidatus Rokubacteria and Candidatus Binatota had relative abundances of < 1%. In addition, Acidobacteria, Alphaproteobacteria, Actinomycetia and Hyphomicrobiales were the four most abundant genera in all the samples (Fig. 4a).
Within the fungal community, Ascomycota, Basidiomycota and Mucoromycota were the dominant phyla, with relative abundances ranging from 45.63% to 62.16%, 10.75% to 33.03% and 1.835% to 3.81%, respectively (Fig. 3b). The relative abundance of Ascomycota was lowest in R900, and that of Mucoromycota was highest in R900. Moreover, Rhizopus arrhizus, Rhizophagus irregularis, and Lipomyces starkeyi were the most abundant species in all the samples (Fig. 4b).
NMDS analysis indicated that the phylogenetic distances of the soil microorganisms varied among the different thinning densities. The microbes associated with the different Chinese fir densities formed separate aggregations that segregated across the first and second coordinate axes (Fig. 5). RDA reflected the impacts of thinning and soil physicochemical properties on the soil microbial phyla and species community structure under the six different thinning densities (Fig. 6). The first two axes explained 74.78% of the microbial phyla composition (Fig. 6a). Pearson correlation analysis revealed that among the tested environmental factors, DOC, MBC, LFOC, and EOC were positively correlated with Proteobacteria and Candidatus Binatota (P < 0.05) (Fig. 6c). Actinobacteria was negatively correlated with all the tested environmental factors. At the species level, the first two axes explained 63.69% of the microbial species composition (Fig. 6b). Pearson correlation analysis revealed that SOC and EOC were positively correlated with Hyphomicrobiales and Gammaproteobacteria (P < 0.05). Additionally, pH was negatively correlated with Verrucomicrobia and Deltaproteobacteria (Fig. 6d).
Carbon cycling functional characteristics of the soil microbial communities
In this study, we analyzed the relative abundances of C-cycling functional genes under the different Chinese fir densities using the KEGG database. A total of 7203 KO genes were obtained that were distributed into the different KEGG level-two pathways, including carbohydrate metabolism, energy metabolism, cellular community-prokaryotes, signal transduction, xenobiotic biodegradation, and metabolism (Fig. 7a).
PCoA results indicated that the level 2 KEGG pathways formed distinct clusters under the different thinning densities, indicating that the thinning of Chinese fir trees resulted in different functional traits under the different thinning densities (Fig. 7b). Thinning and soil microorganisms play pivotal roles in tree growth and ecosystem balance. In addition, the practice of forest thinning may considerably affect the composition of the microbial community.
The results of the analysis of soil characteristics revealed that the carbon content in the soil varied widely. We focused mostly on the genes associated with carbon fixation and carbon metabolism with the purpose of studying potential microbial variations in soil C cycling. In particular, the number of KO genes associated with carbon metabolism related to the pathways of glycolysis/gluconeogenesis, the citrate cycle, the pentose phosphate pathway, pyruvate metabolism and glyoxylate and dicarboxylate metabolism significantly differed among the different thinning densities (Fig. 7c), along with genes associated with the Calvin cycle (PRK, gapA, rbcS, rbcL, and rpiA), the reductive TCA cycle (porB, porD, porG, and korD), the 3-hydroxypropionic acid cycle (accA), and reducing acetyl-CoA pathway circulation (fdhB) (Fig. 7d). The heatmap results revealed that the abundance of most of the genes was markedly higher in R900, which may indicate that pathways related to C-cycling were active in R900.
With the aim of identifying the effects of soil microorganisms on organic matter in the environment, the distribution of soil microbial carbohydrate-active enzymes was analyzed at different thinning densities via the CAZy database. The results revealed that the distribution of carbohydrate-active enzyme-encoding genes varied among the different thinning densities and represented 294 CAZy enzyme-encoding gene families. These families are distributed in major protein functional modules, including glycoside hydrolases (GHs), glycosyl transferases (GTs), polysaccharide lyases (PLs), carbohydrate-binding modules (CBMs), carbohydrate esterases (CEs) and auxiliary activities (AAs). Furthermore, there were 165 enzyme-encoding genes belonged to GHs, 107 enzyme-encoding genes belong to GTs, 49 enzyme-encoding genes belong to CBMs, and 36 genes belong to PLs. We analyzed the relative abundances of the 50 highest-ranking CAZy gene families under the different Chinese fir densities. The abundance of genes in each family noticeably differed under the different thinning densities. The number of enzyme-encoding genes under R900 was significantly greater than that under the other densities, indicating that the microorganisms in this region participated in carbon cycle activities more actively (Fig. 8). This finding is similar to the results of the KEGG database analysis.
Association analysis between functional genes and soil properties
The correlations between the carbon-cycling functional genes and soil characteristics were analyzed via RDA (Fig. 9). RDA1 and RDA2 constituted 26.50% and 11.39% of the carbon metabolism pathways, respectively. Among the soil environmental factors, TC and HFOC significantly influenced carbon metabolism (Fig. 9a). In terms of carbon fixation, RDA1 and RDA2 constituted 35.32% and 8.34%, respectively. pH, TC, HFOC, and POC significantly influenced carbon fixation (Fig. 9b).
Discussion
Response of soil characteristics to the different thinning densities
Appropriate thinning operations can maintain the structural and functional features of plantation ecosystems [28]. Compared with those in the CK treatment, the SOC content and its fractions did not display a linear relationship under the different thinning densities in the large Chinese fir plantation, the SOC content in the R900 treatment was sharply (85.89%) higher, and the other fractions were differently elevated with thinning (Table 1). These results indicated that proper thinning had a positive effect on soil physiochemical properties in the large Chinese fir timber plantation, supporting our first hypothesis. The variation in SOC was related mainly to the change in litter carbon input caused by thinning [29]. In this study, the SOC concentration was highest in R900; however, the litterfall was not highest in R900 (Fig. S1). On the basis of these findings, we suggest that the shrubs and herbs had higher decomposition rates than did the other treatments in this study. Forest age can be an important factor influencing SOC. Zhou et al. [30] reported that SOC increased from the young to the overmature stage in Chinese fir plantations; however, Song et al. [31] reported that there was no significant correlation between SOC and stand age in Chinese fir. Therefore, measuring SOC in Chinese fir forests of different ages is important for obtaining more information about its ecosystem value. POC is a portion of the SOC fraction and decomposes relatively easily during soil C storage. In this study, the POC contents significantly differed under the six different thinning intensities and were highest in R900 (Table 1).
MBC is an important fraction of SOC that is sensitive to short-term changes in soil ecosystems, and increasing MBC is beneficial to the stability of SOC [1]. In this study, the results revealed that under the different thinning intensities, the MBC content and ratio to SOC obviously changed in large Chinese fir timber plantation, which may reveal that proper thinning practices are helpful for the carbon input and fixation of microbial necromass C. The proportion of MBC in this study was greater than that reported by Zhang et al. [1], indicating that the ratio of MBC to SOC is related to forest type. MBC mainly consists of microbial necromass C, and cell wall compounds contribute to at least 50% of SOC sequestration during microbial death in soil [1, 32]. The results of this study revealed that both fungal and bacterial necromass C were highest in R900, which contributed greatly to the SOC. Guizhou is a special place with karst landforms, most mountains are steep cliffs, and proper management may significantly affect the microclimate of the forest. In this study, the results revealed that thinning intensity strongly affected the SOC content and its fractions (Table S1). This finding indicates that thinning is an effective measure for managing large Chinese fir timber plantations.
Taxonomic changes in the soil-based microbiome
A previous study suggested that the structure of the microbial community is highly sensitive to environmental changes, including the practice of thinning [33]. In this study, clear patterns emerged in the soil microbial community structure, confirming that the diversity and richness did not differ significantly among the treatments, whereas the relative abundance of the soil microbial community of large Chinese fir timber plantations can be altered by thinning (Figs. 3, 4), supporting our first hypothesis. The results revealed that five bacterial phyla (Proteobacteria, Acidobacteria, Actinobacteria, Verrucomicrobia and Chloroflexi) contributed more than 80.0% of the total bacterial abundance (Fig. 3a). The dominant phyla in the fungal community were Ascomycota, Basidiomycota and Mucoromycota, and their relative abundances varied significantly across the different thinning intensities. These results suggested that thinning affected the relative abundance of key phyla, particularly in the fungal community. These findings may indicate that the fungal community structure in large Chinese fir timber plantations is more sensitive to thinning than the bacterial community structure is; this result is similar to that reported for mature Chinese pine plantations [11].
Soil microbes are essential for nutrient element cycling, organic matter degradation, mineral dissolution and other biogeochemical processes [34, 35]. Thinning altered the microbial community structure by changing soil nutrients and understory vegetation in mature Chinese pine plantations [11]. Zhang et al. [7] revealed that the net rate of carbon mineralization in the soil can be predicted by changes in the abundances of Proteobacteria and Acidobacteria. In our study, the relative abundance of Acidobacteria was greater in R900 than in the other treatments, and Acidobacteria are commonly considered oligotrophic bacteria [11]. This result indicated that Acidobacteria had opposing effects in the large Chinese fir timber plantation.
Fungi survive on carbon from the surrounding plants and soil, and tree density and understory plants may affect the dominant fungal composition. In our study, the abundance of Ascomycota in R900 was lower than that in the other treatments in the large Chinese fir timber plantation, and that of Mucoromycota was highest in R900. These findings indicated that thinning decreased the amount of litter and Chinese fir root and increased those of other understory plants, which may slightly increase the abundance of Ascomycotain low densities. This result was similar to that reported for mature Chinese pine plantations [11].
Additionally, microbial necromass C has a positive effect on soil C pool stability [1, 36]. Our results are similar to previously reported findings; the practice of thinning continually increased the microbial necromass C among total SOC. We suggest that microorganisms are essential drivers of SOC among large Chinese fir timber plantations under different thinning densities. However, the mechanism by which bacterial and fungal communities mediate SOC fractions under thinning is still unknownnand needs further research.
Responses of soil microbial functional genes involved in carbon cycling under the different thinning densities
Microbial biomass plays an essential role in carbon degradation, and the decomposition process cannot be predicted based on microbial biomass alone [37, 38]. Our study revealed that the microbial community and gene abundance related to carbon cycling clearly differed under the different thinning densities (Figs. 7 and 8), supporting our second hypothesis. Additionally, KO genes related to carbon metabolism (ilvB, ACAT, fdoG, echA, ACO, and sdhA) and carbon fixation (e.g., ppc, PRK, gapA, porB, and porD) were enriched under the different intensities, and most of the genes presented high C cycling activity in R900, indicating that R900 was a suitable intensity for carbon cycling in the large Chinese fir timber plantation. This finding may suggest that microorganisms were more active at this density, which promoted the degradation of litterfall. This result was consistent with the soil physiochemical properties. In contrast, the functional genes were significantly related to the soil properties, such as total C, DOC, and HFOC contents, which was similar to the findings of other studies [39]. This correlation may be because microorganisms degrade litterfall and plant communities, and the soil provides substrate for the growth of microorganisms and plants [40].
Conclusions
Chinese fir is an important tree species in China. It is a fast-growing tree species that produces wood and sequesters carbon, and large timber trees have been preserved. To this end, it is important to understand the effects of thinning on soil properties and microorganism communities in large Chinese fir timber plantations. The results obtained from this research revealed that (1) thinning has a significant effect on the soil C balance of large Chinese fir timber plantations and may alter the SOC content and its fractions; furthermore, the R900 thinning intensity can be considered for carbon storage. (2) The abundances of the dominant microorganisms significantly differed under thinning; however, the fungal community was more sensitive than the bacterial community. The SOC, POC and HFOC contents were positively correlated with Acidobacteria. (3) Additionally, the relative abundance of C-cycling genes corresponds to different soil characteristics under the different thinning densities, and most of the genes in R900 were significantly more abundant than those in the other densities. Our findings contribute to a better understanding of the effects of thinning on large Chinese fir timber plantations, which are a resource for carbon storage in the environment.
Availability of data and materials
The metagenomic assembly data has been deposited in the Sequence Read Archive (SRA) at the National Center for Biotechnology Information (NCBI). The accession number is PRJNA994818.
Abbreviations
- R300, R450, R600, R750, R900 and R1650:
-
Indicates trees of Chinese fir are 300, 450, 600, 750, 900 and 1650 per hectare, respectively
- C:
-
Carbon
- SOC:
-
Soil organic carbon
- POC:
-
Particulate organic carbon
- EOC:
-
Easily oxidizable carbon
- DOC:
-
Dissolved organic carbon
- HFOC:
-
Heavy fraction organic carbon
- LFOC:
-
Light fraction organic carbon
- MBC:
-
Microbial biomass carbon
- NCBI:
-
National center for biotechnology information
- NR:
-
Non-redundant protein sequence database
- KEGG:
-
Kyoto encyclopedia of genes and genomes
- KO:
-
KEGG Orthology
- CAZy:
-
Carbohydrate-active enzymes database
- RDA:
-
Redundancy analysis
- NMDS:
-
Nonmetric multidimensional scaling
References
Zhang ZX, Hao M, Yu QH, Dun XJ, Xu JW, Gao P. The effect of thinning intensity on the soil carbon pool medicated by soil microbial communities and necromass carbon in coastal zone protected forests. Sci Total Environ. 2023;881: 163492.
Cotrufo MF, Lavallee JM. Soil organic matter formation, persistence, and functioning: a synthesis of current understanding to inform its conservation and regeneration. Cambridge: Academic Press; 2022.
Gong C, Tan Q, Liu G, Xu M. Forest thinning increased soil carbon stocks in China. Forest Ecol Manag. 2021;482:118812–23.
Wang D, Chen XL, Chen HYH, Olatunji OA, Guan QW. Contrasting effects of thinning on soil CO2 emission and above-and belowground carbon regime under a subtropical Chinese fir plantation. Sci Total Environ. 2019;690:361–9.
Zhou T, Wang C, Zhou Z. Impacts of forests thinning on soil microbial community structure and extracellular enzyme activities: a global meta-analysis. Soil Biol Biochem. 2020;149: 107915.
Urbanová M, Šnajdr J, Baldrian P. Composition of fungal and bacterial communities in forest litter and soil is largely determined by dominant trees. Soil Biol Biochem. 2015;84:53–64.
Zhang C, Liu G, Xue S, Wang G. Soil bacterial community dynamics reflect changes in plant community and soil properties during the secondary succession of abandoned farmland in the loess plateau. Soil Biol Biochem. 2016;97:40–9.
Liu B, Arlotti D, Huyghebaert B, Tebbe CC. Disentangling the impact of contrasting agricultural management practices on soil microbial communities importance of rare bacterial community members. Soil Biol Biochem. 2022;166:108573–84.
Soong JL, Fuchslueger L, Marañon-Jimenez S, Torn MS, Janssens IA, Penuelas J, Richter A. Microbial carbon limitation: the need for integrating microorganisms into our understanding of ecosystem carbon cycling. Glob Chang Biol. 2016;26(4):1953–61.
Condron L, Stark C, O’callaghan M, Clinton P, Huang Z. Soil microbiology and sustainable crop production. Berlin: Springer Press; 2010.
Dang P, Gao Y, Liu J, Yu S, Zhao Z. Effects of thinning intensity on understory vegetation and soil microbial communities of a mature Chinese pine plantation in the Loess Plateau. Sci Total Environ. 2018;630:171–80.
Ye Y, Sun X, Zhao J, Chen X, Wang M, Li J. Thinning alters the network patterns and keystone taxa of rhizosphere soil microbial communities in Chinese fir plantation. Appl Soil Ecol. 2023;189:104956–66.
Yuan Y, Li J, Yao L. Soil microbial community and physicochemical properties together drive soil organic carbon in Cunninghamia lanceolata plantations of different stand ages. PeerJ. 2022;10:e13873–87.
Huang YQ, Deng XW, Zhao ZH, Xiang WH, Yan WD, Ouyang S, Lei PF. Monthly radial growth model of Chinese Fir (Cunninghamia lanceolata (Lamb.) Hook.), and the relationships between radial increment and climate factors. Forests. 2019;10:757–71.
Wang D, Olatunji OA, Xiao J. Thinning increased fine root production, biomass, turnover rate and understory vegetation yield in a Chinese fir plantation. Forest Ecol Manag. 2019;440:92–100.
Lei L, Xiao W, Zeng L, Frey B, Huang Z, Zhu J, Cheng R, Li MH. Effects of thinning intensity and understory removal on soil microbial community in Pinus massoniana plantations of subtropical China. Appl Soil Ecol. 2021;167:104055–64.
Li YF, Deng XW, Zhang YF, Huang YQ, Wang CY, Xiang WH, Xiao FM, Wei XC. Chemical characteristics of heartwood and sapwood of red heart Chinese Fir (Cunninghamia lanceolata). For Prod J. 2019;69:103–9.
Lu Y, Coops NC, Wang T, Wang G. A process-based approach to estimate Chinese fir (Cunninghamia lanceolata) distribution and productivity in southern China under climate change. Forests. 2015;6:360–79.
Zhang YF, Deng XW, Huang YQ, Li YF, Xiang WH, Yan WD. Quantification of individual tree competition index taking Chinese-fir plantations in subtropical low hilly area as an example. Pol J Ecol. 2019;67:1–16.
Carter MR, Gregorich E. Soil sampling and methods of analysis. Boca Raton: CRC Press; 1993.
Gong W, Yan XY, Wang JY, Hu TX, Gong YB. Long-term manuring and fertilization effects on soil organic carbon pools under a wheat-maize cropping system in North China Plain. Plant Soil. 2009;314:67–76.
Elliott ET, Cambardella CA. Physical separation of soil organic atter. Argic Ecosyst Environ. 1991;34(1–4):407–19.
Wu T, Schoenau JJ, Li F, Qian P, Malhi SS, Shi Y, Xu F. Influence of cultivation and fertilization on total organic carbon and carbon fractions in soils from the Loess Plateau of China. Soil Till Res. 2004;77(1):59–68.
Brookes PC, Kragt JF, Powlson DS, Jenkinson DS. Chloroform fumigation and the release of soil nitrogen: the effects of fumigation time and temperature. Soil Biol Biochem. 1985;17(6):831–5.
Vance ED, Brookes PC, Jenkinson DS. An extraction method for measuring soil microbial biomass C. Soil Biol Biochem. 1987;19(6):703–7.
Zhang X, Amelung W. Gas chromatographic determination of muramic acid, glucosamine, mannosamine, and galactosamine in soils. Soil Biol Biochem. 1996;28:1201–6.
Buchfink B, Xie C, Huson DH. Fast and sensitive protein alignment using DIAMOND. Nat Methods. 2015;12(1):59–60.
Trentini CP, Campanell PI, Villagra M, Ritter L, Ares A, Goldstein G. Thinning of loblolly pine plantations in subtropical Argentina: impact on microclimate and understory vegetation. Forest Ecol Manag. 2017;384:236–47.
Nath PC, Sileshi GW, Ray P, Das AK, Nath AJ. Variations in soil properties and stoichiometric ratios with stand age under agarwood monoculture and polyculture on smallholder farms. CATENA. 2022;213:106174–85.
Zhou L, Li S, Liu B, Wu P, Heal KV, Ma X. Tissue-specific carbon concentration, carbon stock, and distribution in Cunninghamia lanceolata (Lamb.) Hook plantations at various developmental stages in sybtropical China. Ann For Sci. 2019;76:70–86.
Song X, Kinberley MO, Zhou G, Wang H. Soil carbon dynamics in successional and plantation forests in subtropical China. J Soil Sediment. 2017;17(9):2250–6.
Liang C, Amelung W, Lehmann J, Kästner M. Quantitative assessment of microbial necromass contribution to soil organic matter. Glob Chang Biol. 2019;25:3578–90.
Coban O, De Deyn GB, Van der Ploeg M. Soil microbiota as game-changers in restoration of degraded lands. Science. 2022;375:eabe0725-eabe735.
Formina M, Skorochod I. Microbial interaction with clay minerals and its environmental and biotechnological implications. Minerals. 2020;10(10):861–914.
Xiong X, Xing Y, He J, Wang L, Shen Z, Chen W, Huang Q. Keystone species determine the “selection mechanism” of multispecies biofilms for bacteria from soil aggregates. Sci Total Environ. 2021;773:145069–76.
Yu W, Huang W, Weintraub-Leff SR, Hall SJ. Where and why do particulate organic matter (POM) and mineral-associated organic matter (MAOM) differ among diverse soils? Soil Biol Biochem. 2022;172:108756–68.
Deng JJ, Zhu WX, Zhou YB, Yin Y. Soil organic carbon chemical functional groups under different revegetation types are coupled with changes in the microbial community composition and the functional genes. Forests. 2019;10(3):240–65.
Manzoni S, Porporato A. Soil carbon and nitrogen mineralization: theory and models across scales. Soil Biol Biochem. 2009;41:1355–79.
Zhong Y, Yan W, Wang R, Shangguan ZP. Decreased occurrence of carbon cycle functions in microbial communities along with long-term secondary succession. Soil Biol Biochem. 2018;123:207–17.
Cleveland CC, Reed SC, Keller AB, Nemergut DR, O’Neill SP, Ostertag R, Vitousek PM. Litter quality versus soil microbial community controls over decomposition: a quantitative analysis. Oecologia. 2014;171(1):283–94.
Acknowledgements
The authors would like to thank Yanyan Gao for assistance with samples collection.
Funding
This work was supported by the “13th Five-year Plan” for National Key Research and Development project “Research on Efficient Cultivation Technology of Cunninghamia lanceolata L.” (2016YFD0600301).
Author information
Authors and Affiliations
Contributions
Lei Huang methodology, designed, analyzed and wrote the manuscript. Yunchao Zhou designed the research work and provided critical suggestions for revising the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Huang, L., Zhou, Y. Influence of thinning on carbon storage mediated by soil physicochemical properties and microbial community composition in large Chinese fir timber plantation. Carbon Balance Manage 19, 29 (2024). https://doi.org/10.1186/s13021-024-00269-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13021-024-00269-x