Abstract
Background
Alopecia affects millions of individuals globally, with hair loss becoming more common among young people. Various traditional Chinese medicines (TCM) have been used clinically for treating alopecia, however, the effective compounds and underlying mechanism are less known. We sought to investigate the effect of Alpinetin (AP), a compound extracted from Fabaceae and Zingiberaceae herbs, in hair regeneration.
Methods
Animal model for hair regeneration was mimicked by depilation in C57BL/6J mice. The mice were then topically treated with 3 mg/ml AP, minoxidil as positive control (PC), or solvent ethanol as vehicle control (VC) on the dorsal skin. Skin color changes which reflected the hair growth stages were monitored and pictured, along with H&E staining and hair shaft length measurement. RNA-seq analysis combined with immunofluorescence staining and qPCR analysis were used for mechanism study. Meanwhile, Gli1CreERT2; R26RtdTomato and Lgr5EGFP−CreERT2; R26RtdTomato transgenic mice were used to monitor the activation and proliferation of Gli1+ and Lgr5+ HFSCs after treatment. Furthermore, the toxicity of AP was tested in keratinocytes and fibroblasts from both human and mouse skin to assess the safety.
Results
When compared to minoxidil-treated and vehicle-treated control mice, topical application of AP promoted anagen initiation and delayed catagen entry, resulting in a longer anagen phase and hair shaft length. Mechanistically, RNA-seq analysis combined with immunofluorescence staining of Lef1 suggested that Lgr5+ HFSCs in lower bulge were activated by AP via Wnt signaling. Other HFSCs, including K15+, Lef1+, and Gli1+ cells, were also promoted into proliferating upon AP treatment. In addition, AP inhibited cleaved caspase 3-dependent apoptosis at the late anagen stage to postpone regression of hair follicles. More importantly, AP showed no cytotoxicity in keratinocytes and fibroblasts from both human and mouse skin.
Conclusion
This study clarified the effect of AP in promoting hair regeneration by activating HFSCs via Wnt signaling. Our findings may contribute to the development of a new generation of pilatory that is more efficient and less cytotoxic for treating alopecia.
Similar content being viewed by others
Introduction
Alopecia is one of the most common complaints in dermatology clinics, 85% males and 40% of female worldwide are suffering from hair loss [1]. Although it is not a life-threatening disease, alopecia has significant impact on patient’s self-esteem and overall life quality. Notably, alopecia has become more prevalent and affects people at a younger age. While minoxidil and finasteride, two extensively used clinical interventions, could help prevent hair loss by suppressing male hormones [2], their usage is restricted due to a multitude of adverse side effects. Minoxidil has poor efficiency, thus a long-term use is required to warrant desired therapeutic benefits. It also causes mild postural dizziness and peripheral edema in a small number of patients [3]. Finasteride could trigger adverse effects including a decrease in libido, erectile dysfunction, and ejaculatory dysfunction [4]. Therefore, effective treatments that can promote hair growth with minimal side effects are yet to be identified.
Hair follicles grow in repeated cycles with three phases: the growth phase (anagen), the regression phase (catagen), and the rest phase (telogen). The shape of hair follicles and dermal papilla varies greatly in different hair follicle cycle stages. The dermal papilla of the telogen follicle sitting at the bottom of the hair follicle is the first part to respond to growth signal. When the hair follicle enters the anagen phase, the dermal papilla ascends and is surrounded by proliferating hair matrix cells. In catagen follicle, the dermal papilla becomes smaller again and forms an epithelial chain connected with the bulge [5]. These phases are tightly regulated and closely associated with the activation or quiescence of various hair follicle stem cell (HFSC) populations, such as the Gli1+ cells in the upper bulge and hair germ, the Lgr5+ cells in the lower bulge, the Lef1+ cells in the hair germ (telogen) and dermal papilla (anagen), and the K15+ cells in the outer root sheath (ORS) [6,7,8,9]. The activation of HFSCs requires the precise regulation of a variety of signaling pathways. BMP signaling keeps HFSCs in the resting phase during telogen [10]. Several signaling pathways, including the sonic hedgehog (Shh), Wnt/β-catenin, and Notch pathways, are involved in anagen entry, among which the Wnt/β-catenin pathway plays a crucial role not only during the telogen-anagen transition in adults [11], but also in maintaining HFSC stemness [12]. Previous studies have demonstrated that various Wnt ligands (WNT1A, WNT3A, WNT4, WNT7B and WNT10B) could stabilize β-catenin and induce anagen-phase specific gene expression, and therefore promote hair follicle growth and hair shaft elongation [11]. Thus, compounds that can target the Wnt/β-catenin pathway to stimulate HFSCs would be candidates for hair regeneration in treating alopecia.
Clinical studies have shown that prescriptions containing herbs from the Fabaceae (such as Astragalus membranaceus Bunge., Psoralea corylifolia Linn., and Glycyrrhiza uralensis Fisch.) and the Zingiberaceae (such as Davallia mariesii Moore ex Bak. and Salvia miltiorrhiza Bunge.) have significant therapeutic effects on alopecia [13]. Nevertheless, the pharmacodynamic ingredients in these prescriptions are complex, making it difficult to determine the underlying mechanisms and consequently limit their clinical use. Alpinetin (AP) is the main component found in Fabaceae and Zingiberaceae herbs. Studies have shown that AP could possibly target Wnt signaling pathway [14, 15]. However, the effect of AP on hair regeneration hasn’t been checked. In the present study, we sought to investigate the effects of AP in hair regeneration and its underlying mechanism.
Methods
General design of experiments
In animal studies, mice were randomly assigned to control or experimental groups whenever possible. When specific strains of mice were used, the mice with indicated genotypes in the same litter were compared. Five mice in each group (n = 5) were used in most of the experiments, otherwise stated. For immunostaining quantification analysis, RNA-seq library preparation, and sequencing, experimenters were blinded to experimental conditions according to experimental designs [16].
Animals
C57BL/6J mice were purchased from Charles River Laboratories (Jiaxing, China) and Shanghai SLAC Laboratory Animal Co.Ltd (Shanghai, China). Gli1CreERT2, Lgr5EGFP−CreERT2, R26RtdTomato mice were purchased from the Jackson Laboratory. Gli1CreERT2; R26RtdTomato and Lgr5EGFP−CreERT2; R26RtdTomato mice were used to monitor the activation and proliferation of Gli1+ and Lgr5+ HFSCs after treatment. Four or six days after depilation, mice (6–7 weeks old) received three i.p. injections of 200 mg/kg Tamoxifen (MACKLIN, Shanghai, China) dissolved in corn oil (MACKLIN) at 1-day intervals. Mice were housed with a 12 h/12 h light/dark cycle at 22 °C, with free access to food and water. Only male mice were used for the experiments. The experimental study was conducted according to the institutional guidelines with approval from the Review Committee of Zhejiang Chinese Medical University.
Hair cycle synchronization
For hair cycle synchronization, hair on the dorsal skin was manually depilated in 6–7 weeks old mice, when the vast majority of dorsal skin hair follicles were in the telogen phase. Two areas (1.5 cm by 1.5 cm for each, 1 cm apart from each other) on the left and right back skin near the forelimbs of the mice were depilated for each mouse, and the position of depilation areas were the same in all mice. Mice were shaved with a razor, then the remaining hair shafts were plucked twice with beeswax paper.
Topical drug treatment and tissue sampling
Mice were divided into three groups (n = 5 mice per group) as vehicle control (VC, treated with ethanol), positive control (PC, treated with Minoxidil), and Alpinetin group (AP, treated with Alpinetin). 20 µL 65% ethanol (MACKLIN), 5% Minoxidil (Wansheng, H20010714, Linan China) or 3 mg/mL AP (Standard Technology, Shanghai, China) dissolved in 65% ethanol, respectively, were applied topically at 24 h after depilation, mice were treated once a day for each skin areas until sample harvesting. Skin samples were collected at Day 4 (D4), D5, D7, D18, D19, and D22 after depilation from C57BL/6J mice; D4 and D6 from Gli1CreERT2; R26RtdTomato mice; and D4 from Lgr5EGFP−CreERT2; R26RtdTomato mice. Samples were fixed in 4% paraformaldehyde overnight followed by tissue dehydration and then embedded in OCT (SAKURA, Tokyo, Japan). The embedded skin tissues were frozen and stored at − 80 °C until cryostat sectioning. In C57BL/6J mice, hair shafts were collected from all treated areas at D13 and D17 after depilation to measure the length.
EdU administration
Single dose of EdU (Beyotime, Nantong, China) that was dissolved in PBS (GENOM, Jiaxing, China) was administered by intraperitoneal injection (50 mg/kg EdU) 4 h before dorsal skin harvesting at D4 and D19 after depilation [17].
Histology, immunofluorescence, and image analysis
For histology analysis, Hematoxylin and Eosin (H&E) staining were performed according to standard protocols with minor modification: sections were incubated for 5 min in hematoxylin and 30 s in eosin solutions. Image acquisition was performed on a Leica DM4000 microscope. Only follicles with a clear and complete structure were chosen for length analysis. The length of hair follicles was measured by ImageJ.
For immunostaining, the frozen sections were blocked in PBS with 5% Donkey serum or 1% BSA and 0.25% Triton for 1–4 h at room temperature, then incubated with primary antibody at 4 °C overnight and were subsequently incubated with secondary antibodies conjugated with Alexa Fluor 488, 594 or 647 (1:1000, Invitrogen, California, USA). Nuclei were stained with DAPI (Solarbio, Beijing, China). The following primary antibodies were used: P-cadherin antibody (1:1000, AF761, R&D), K15 (1:100, ab52816, Abcam), and Lef1 (1:200, 2230, CST). Image acquisition was performed on a Zeiss microscope. For quantification, 5 sections from each sample were processed and 5 randomly selected areas of each section were quantified in Image J.
RNA sequencing and analysis
Skin samples at D4 after depilation were collected for total RNA extraction. Dermis tissues with hair follicle were used after removing interfollicular epidermal keratinocytes. Briefly, mRNA was enriched with Oligo-dT magnetic beads followed by reverse transcription, fragmentation and library construction. Sequencing was conducted on NovaSeq 6000 at Novogene (Beijing, China). RNA-seq data were deposited in NCBI’s Gene Expression Omnibus (GEO) with GSE193763.
Raw sequencing reads were cleaned and then aligned to the mouse genome (mm10) by using STAR [18] and raw count matrixes were obtained by feature counts [19]. Then, differential gene expression (DGE) analysis was done by R package DESeq2 [20, 21]. The genes with minimum counts of 3, p-value < 0.01 and fold change (FC) > 1.5 were considered as differentially expressed genes. Pathway and process enrichment analysis was carried out with Gene Ontology (GO) Biological Processes, and PaGenBase by using Metascape (http://metascape.org/) [22]. GSEA was performed with gene sets obtained from MSigDB (https://www.gsea-msigdb.org/gsea/msigdb). Chord plots of enriched GO terms were generated in R (v 4.1.2) using GOplot (v 1.0.2).
Cell culture and treatments
Human keratinocytes (hKC) and fibroblasts (hFB) were isolated from foreskin following circumcision (age between 20 and 30 years) with ethical approval (No. 20191104-13) according to standard procedures [23, 24]. Mouse keratinocytes (mKC) and fibroblasts (mFB) were isolated from neonatal dorsal skin as previously described [25, 26]. hKC were cultivated in EpLife (Gibco, NY, USA) supplemented with EDGS as provided by the manufacturer. mKC were cultivated in Defined Keratinocyte-SFM medium (Gibco). Fibroblasts were cultivated in DMEM (GENOM) supplemented with 10% fetal bovine serum (ExCell bio, Shanghai, China). All cells were cultivated in an incubator with 5% CO2 at 37 °C.
Cell viability assay
Cell viability was measured following the manufactory instruction of TransDetect cell counting kit (TransGen Biotech, Beijing, China). Keratinocytes (KC) and fibroblasts (FB) of both human and mouse were seeded into 96-well at a density of 1 × 105 cells/well and 2 × 104 cells/well, respectively, for 24 h before AP exposure. KCs and FBs were cultured with AP (dissolved in DMSO) for 48 h and 72 h, respectively. Then, the cells were washed, 100 µL of fresh medium containing 10 µL CCK8 reagent was added, and cells were incubated until checked. The absorbance was measured at 450 nm by a MD M5 full-wavelength microplate reader (Molecular Devices, California, USA).
Statistical analysis
Statistical analysis was performed using Graph Prism 9 (GraphPad Software, San Diego, California, USA). Data were expressed as mean ± SEM. One-way ANOVA followed by Bonferroni multiple-test and non-parametric test were used to assess the statistical significance of differences between the groups; p-value < 0.05 was considered to be significant [27]. The sample size was calculated by power analysis with p-value 0.05, power 0.8 and effect size estimated by pilot data [28].
Results
Topical application of AP stimulates hair growth
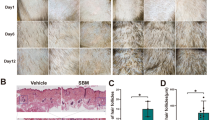
To check the effect of Alpinetin (AP) in promoting hair growth, we closely examined relevant phenotypes after topically applying vehicle control (VC, 65% ethanol treated), positive control (PC, minoxidil treated) or AP on depilated dorsal skin once a day according to experiment design (Fig. 1a). For the skin color of C57BL/6J (C57) mice can indicate the hair follicle cycling stage. As shown in Fig. 1b, skin color change was considerably faster in mice with topical application of AP (D4) compared to the PC- or VC-treated mice (D6). At D8, hair shaft was already observable in AP-treated mice, but not in VC- or PC-treated mice. The skin color of VC- or PC-mice turned white at D18, indicating a transition from anagen to catagen, while that of AP-treated mice did not turn white color until 1 day later (D19).
AP promotes hair follicle and hair shaft growth. a Experimental scheme of topical drug treatment and sampling. b Skin color of depilated skin areas at day 1 to 19 (D1, D4, D6, D8, D18 and D19) after depilation. c Hematoxylin and eosin (H&E) staining of VC-, PC- or AP-treated skin at D7 after depilation. Scale bar, 100 μm. Quantitation of hair follicle length. The average hair follicle length of 3 individual animals (n = 3) in each group was presented. The average length was calculated from 4 to 6 hairs in each animal. d Hair shafts pulled at D13 (upper panel) and D17 (lower panel) after depilation. Quantitation of hair shaft length (n = 50 hair shafts per group from 5 mice). Scale bar, 1 mm. Data are presented as mean ± SEM. *p < 0.05; **p < 0.01
These data suggested that AP could promote anagen entry and prolong the anagen phase. We next examined whether AP promotes the elongation of hair follicle and shafts. Histological analyses showed AP-treatment significantly increased hair follicle length at D7 (Fig. 1c). The hair shafts at both D13 and D17 in AP-treated mice were significantly longer than those in VC- and PC-treated mice (Fig. 1d). These results indicated that AP could stimulate hair growth in mice.
AP promotes anagen entry of hair cycle
Compared with hair follicles in the telogen phase, hair follicles in anagen phase grow longer and dermal papilla is surrounded by hair matrix cells (Fig. 2a). Therefore, we categorized the hair follicles based on the morphological features in different hair follicle cycles. Given that AP treatment accelerated skin color change and promoted hair shaft elongation, we further examined the skin biopsy histologically in telogen–anagen transition at D4 after depilation (Fig. 2b). Histological analysis showed that while only 17% and 26% of the hair follicles entered the anagen stage in the VC- and PC-treated mice, respectively, all the hair follicles (100%) in the AP group were in the anagen stage (Fig. 2c, d, Additional files 1, 2: Fig. S1a). Notably, there seemed to be two types of hair follicles in the AP group: one type was straight and longer (Fig. 2d, AP#1); the other type was shorter and had a smaller hair follicle in their proximity, which called club hair. (Fig. 2d, AP#2), indicating AP might activate de novo hair follicle growth. P-cadherin protein is a marker indicating hair follicles entering the anagen phase [29]. Consistently, P-cadherin was barely detected in the hair germ of hair follicles in VC-treated mice and only expressed in ~ 50% of the hair follicles in PC-treated mice, while most cells in the hair germ and the bulge area expressed P-cadherin at D4 after AP application (Fig. 2e, Additional files 1, 2: Fig. S1b). Next, we examined cell proliferation in hair follicles using the EdU-incorporation assay. As expected, EdU+ cells were mainly detected in dermal papilla and hair matrix in all three groups (Fig. 2f). We observed additional EdU+ cells located in the bulge area of hair follicles in AP-treated mice, but not in the club hair of AP-treated skin. Remarkably, the EdU+ cells in AP-treated group were much more than those in the other groups, suggesting that AP could facilitate hair cycle entry by promoting proliferation of hair follicle epithelium cells.
AP drives anagen entry. a Hair follicle cycle diagram. b Experimental scheme of topical drug treatment and sampling. c Quantification for proportion of hair follicles at anagen stage (n = 228 hair follicles from 4 mice per group). Data are presented as mean ± SEM. **p < 0.01. c H&E staining of VC-, PC- and AP-treated skin at D4 after depilation (n = 5 mice). Scale bar, 100 μm. Immunofluorescence images for P-cadherin (e) and EdU (f) staining in hair follicles of VC-, PC-, and AP-treated mice at D4 after depilation (n = 50 hair follicles per group from 5 mice). Scale bars, 50 μm
AP delays catagen entry by inhibiting cell apoptosis
We further determined whether AP affects anagen–catagen transition (Fig. 3a). As shown in Fig. 2a, compared with the anagen phase, the hair follicle in catagen not only becomes shorter in length, but the dermal papilla also begins to shrink down to the bottom of the hair follicle, while forming an epithelial chain with the bulge, indicating the transition into catagen phase. Histological analyses showed that dermal papilla size reduced in VC- or PC-treated groups at D18 after depilation, indicating hair follicles entering the catagen stage (Fig. 3b, f). On the contrary, all hair follicles following AP treatment remained in the anagen stage at this point. Significantly, most hair follicles in AP-treated mice were still in the late anagen stage at D19. At D22, hair follicles in all the three groups have entered the telogen stage. These results suggested that AP delayed catagen entry as well as shortened catagen duration.
AP delays anagen-catagen transition. a Experimental scheme of topical drug treatment and sampling, n = 5 mice per group. b H&E staining of VC-, PC- and AP-treated skin at D18, D19, and D22 after depilation. n = 5 mice per group. Scale bars, 100 μm for D18, D19, 50 μm for D22. c Immunofluorescence images and quantification of EdU staining within hair follicles of VC-, PC-, and AP-treated mice at D19 after depilation. n = 50 hair follicles per group from 5 mice. Scale bars, 50 μm. d Immunofluorescence images and quantification of capspase3 staining within hair follicles of VC-, PC-, and AP-treated mice at D19 after depilation. n = 50 hair follicles per group from 5 mice. Scale bars, 50 μm. e Immunofluorescence images of K15 staining in hair follicles of VC-, PC-, and AP-treated mice at D19 after depilation. n = 5 mice per group. Scale bars, 100 μm. f Quantification of DP size of VC-, PC-, and AP-treated mice at D19 after depilation. g Comparison of the duration of catagen, anagen and telogen phase in VC-, PC- and AP-treated skin, n = 5 mice per group
Since the AP group was in the anagen stage at D19, we wanted to know whether cells in hair follicles were proliferative. Surprisingly, cells in the dermal papilla in the AP group, but not those in the VC or PC-treated groups were incorporating EdU at D19 (Fig. 3c), suggesting AP treatment could sustain cell proliferation. On the contrary, cleaved caspase 3, a marker for apoptosis, was expressed in the bulge in the VC or PC-treated groups but was not detected in the AP group at D19 (Fig. 3d), indicating AP hadn’t get into catagen entry. Most hair follicles have entered catagen in VC or PC-treated groups, as the hair follicle retracts, the dermal papilla was pulled upward towards the permanent portion of the hair follicle where stem cells reside. K15+ HFSCs in both hair germ and bulge appear quiescent, no EdU staining was observed in VC group, few staining was observed in PC group, while all hair bulb appeared EdU+ in AP group (Fig. 3e). Taken together, these data showed that AP stimulated hair follicle entering anagen earlier and delayed catagen entry (Fig. 3g).
AP activates hair follicle stem cells
We have shown that AP could extend the anagen stage as well as promote hair follicle cell proliferation and wondered if AP could affect hair follicle stem cells (HFSCs). At D4 after treatment, there were more Lef1+ stem cells in the dermal papilla and K15+ stem cells in the ORS in the AP-treated group compared with the VC- or PC-treated groups, as well as for EdU+ proliferating cells (Fig. 4a). Lgr5+ HFSCs are the pioneer stem cell population that are triggered at the onset of anagen [30]. By using Lgr5EGFP−CreERT2; R26RtdTomato transgenic mice, Lgr5+ HFSCs were labeled 8 days before depilation. Lgr5-EGFP+tdtomato+ cells are HFSCs or progenies that express Lgr5, while Lgr5-EGFP−tdtomato+ cells are hair follicle cells that derived from Lgr5-EGFP+tdtomato+ stem cells, however, not expressing Lgr5 anymore as they become transitional amplification cells, participating in the rapid proliferation of hair follicle in the anagen phase [31]. At D4 after depilation, Lgr5+ (EGFP+; Tomato+) cells were detected in the lower bulge and hair germ of telogen hair follicle in the VC-treated group. In the AP treatment group, Lgr5 + (EGFP + ; Tomato +) cells were present in the expanding bulge, and a small number of transitional amplification cells (EGFP−; Tomato+) derived from Lgr5+ cells were observed in ORS (Fig. 4b). These results indicate that AP promotes the proliferation of Lgr5+ HFSCs (EGFP+; Tomato+) and progenies. Similarly, Gli1creERT2; R26RtdTomato transgenic mice is used to label Gli1+ HFSCs, another marker of HFSCs identified previously [9]. Gli1+ stem cells or progenies were rarely detected in follicles following VC treatment, whereas significant amount of Gli1+ stem cells/progenies (tomato+) were detected in the inner root sheath (IRS) from the upper bulge to the matrix after 6-day AP treatment (Additional files 1, 3: Fig. S2). Fewer hair follicles containing Tomato+ cells were detected in VC group either at D4 or D6 post depilation (Additional file 3: Fig. S2). These results indicated that AP could efficiently stimulate different stem cell populations in hair follicles.
AP stimulates Lgr5+ HFSC at the onset of anagen. a Upper panel: experimental scheme of topical drug treatment and sampling. Lower panel: immunofluorescence images for dual-staining of EdU and Lef1, dual-staining of EdU and K15 in hair follicles of VC-, PC-, and AP-treated mice at D4 after depilation. Quantification of K15 staining in hair follicles (n = 50 hair follicles per group from 5 mice). b Upper panel: experimental scheme of topical drug treatment and sampling. Lower panel: lineage tracing of Lgr5+ HFSCs by using Lgr5EGFP−CreERT2; R26RtdTomato mice at D4 after depilation, dual-staining of Tomato and GFP, n = 3 mice per group. Scale bars, 50 μm
AP functions through Wnt signaling
To explore the underlying molecular mechanism, by using RNA-seq we investigated the transcriptional changes in skin induced by AP treatment. Compared to the VC group, there were 1017 up-regulated and 550 down-regulated genes identified in the AP group (Fig. 5a). Enrichment analysis showed that the significantly up-regulated genes in the AP group were enriched for Gene Ontology (GO) terms including tissue morphogenesis, positive regulation of cell migration, skin development and hair cycle, as well as the Wnt signaling pathway (Fig. 5b). In-depth GO analysis revealed potential key regulators which significantly contributed to the enhanced Wnt signaling pathway and hair regeneration. For instance, Egr1, Wnt11 and Hmga2 are important genes involved in Wnt signaling pathway, whose expression are both upregulated over twofold. Meanwhile, the AP-induced upregulation of Fgf20, Itga6 and Csf1 may be the significant regulators promoting hair regeneration and cycle (Fig. 5c). Tissue-specific marker gene enrichment analysis also showed that a list of epidermis-specific genes are upregulated after AP treatment, in line with the morphological findings that AP treatment facilitates hair follicle proliferation (Fig. 5d).
RNAseq analysis reveals the molecular mechanism of AP-treated skin. a Volcano plot of the differentially expressed genes between AP and VC-treated groups (FC > 1, p < 0.01). b Highly enriched Gene ontology (GO) analysis of up-regulated genes in AP group. c GO Chord plot showing the expression spectrum of significantly upregulated gene in the Go terms. Genes are selected according to the log2FC, and they are linked to their assigned term via colored ribbons. d Summary of histospecific gene enrichment analysis in PaGenBase. e Gene set enrichment analysis (GSEA) between AP and VC-treated groups. f Heat map of genes that are significantly up-regulated in epidermis development and Wnt signaling pathways, n = 4 mice per group
GSEA analysis revealed that the gene sets of skin development and epidermis development were clearly enriched in the AP-treated group, which was consistent with GO analysis (Fig. 5e). In addition, the set of genes associated with Wnt signaling pathway were also enriched in AP-treated group. Specifically, genes related to epidermal development (Krt10, Krt80, Atp7a, Krt16, Krt77, Itga6) and Wnt signaling pathway (Fzd1, Apcdd1, Fzd6, Sfrp1, Fzd4, Ror1, Lrp6, Lrp5, Reck, Fzd10, Fzd5, Fzd9) were up-regulated in the AP group (Fig. 5f). The expression of Fzd1 and Lef1, the principle receptor and the core transcription factor involved in canonical Wnt pathway, which are important regulators functioned in the induction of primary hair/follicle, were also induced by AP application among the top list and validated by qPCR (Additional files 1, 4: Fig. S3), though not showed in Fig. 5e. These results suggested that AP regulates hair follicle development through the Wnt-Lgr5-Lef1 axis.
AP exhibits no cytotoxicity in keratinocytes and fibroblasts
While AP showed a promising effect to stimulate hair growth, it is critical to test the toxicity of a potential druggable compound. We did the in vitro pioneer cytotoxicity study for AP in primary cultured keratinocytes and fibroblasts, the two main cell types in skin from both human and mice. To examine the cytotoxicity of AP, we treated primary fibroblasts and keratinocytes obtained from human (Fig. 6a) and mice (Fig. 6b) at a wide range of concentration (0.01 μg/ml, 1 μg/ml, 100 μg/ml). Interestingly, AP increased proliferation of human fibroblasts at the high dose (100 μg/ml) and slightly increased proliferation of mouse fibroblasts. However, AP did not affect cell viability in human keratinocytes at all concentrations and slightly increased mouse keratinocytes proliferation at the high dose (100 μg/ml). These findings suggested that AP exposure had no cytotoxicity in the two main types of skin cells.
No cytotoxicity of AP is detected in keratinocytes and fibroblasts from human and mice. Keratinocytes and fibroblasts obtained from human foreskin or dorsal skin of neonatal mice were treated with different doses of AP (0.01 μg/ml, 1 μg/ml, 100 μg/ml) for 48 h. a Cell viability of human fibroblasts (hFB, left panel) and keratinocytes (hKC, right panel) that treated with different concentrations of AP. b Cell viability of mouse fibroblasts (mFB, left panel) and keratinocytes (mKC, right panel) that treated with different concentrations of AP. Data are presented as mean ± SEM. *p < 0.05, **p < 0.01, compared to the control group
Discussion
AP is mainly found in Zingiberaceae plants, and it is abundant in cardamom and turmeric that are commonly used in clinical treatment for the multi-effects and low toxicity, which is demonstrated in our toxicological studies as well. Alpinia katsumadai Hayata, which is rich in AP ingredients, is a condiment used in daily life, and is classified as medicinal and edible, suggesting its safety as well. The pharmacological effects of AP have so far been mostly anti-inflammatory, including significantly reducing TNF-α, IL-6 and IL-1β expression levels [32]. Other reports have also demonstrated that AP possesses antibacterial, antioxidant, anti-cancer, antithrombotic, antiemetic and analgesic properties, as well as maintaining blood pressure, blood lipid and blood glucose levels [33,34,35]. Additionality, AP was less polar property compound and belongs to fat soluble ingredients, making it can be easily absorbed by the skin. It is therefore feasible to turn it into shampoo and other forms of commodity for daily use, making it marketable. However, 65% ethanol was used as a solvent to dissolve AP in the present study, which is likely to be irritation to the skin. In the future, a more adaptable solvent will be required for therapeutic treatment or daily use product.
To date, medication and hair transplantation are the two most common treatments for hair loss. Two commonly used medicines include minoxidil and finasteride, both of which yet have drawbacks. As for hair transplantation, hair follicles from other cites of the body are needed, skin lumps and scarring may occur, and only around half of the transplanted hair follicles survive [36]. Moreover, hair transplantation is expensive due to its costly operation procedures. Alopecia treatments with efficiency, safe and lower cost are of the urgent need for clinic. Activation of HFSCs is a challenge yet promising therapeutic strategy for curing hair loss. The most common types of hair loss include androgenetic alopecia (AGA) and alopecia areata (AA), which are non-cicatricial alopecia. They are characterized by damaged hair follicle progenitor cells but a relatively intact pool of HFSCs, which makes hair loss treatable [37]. HFSCs, when activated, can promote hair regeneration. Therefore, finding new compounds like AP to directly activate HFSCs will pave the way for hair loss treatment.
Lgr5 is identified to be a Wnt target gene in the HFSCs, and Lgr5+ HFSCs are the first HFSC population to respond to hair growth signals [38]. In this study, we confirmed that Lgr5+ HFSCs were activated and proliferated quickly upon AP application. Canonically, Wnt proteins stabilize β-catenin and activate its downstream genes via Frizzled receptors and low-density lipoprotein-related protein (LRP) co-receptors [39]. According to our RNA-seq data, up-regulated genes were enriched in the pathway of Wnt activation in the AP-treated group with high Frizzleds and LRPs expression. There are other pieces of evidence supporting our finding that AP affects HFSC via Wnt signaling. AP is found to be the agonist of peroxisome proliferator-activated receptor G (PPARG), which functions together with β-catenin [40, 41]. Therefore, AP is likely to stimulate Wnt/β-catenin pathway via upregulating its receptors and co-receptors in telogen–anagen transition, thus other compounds targeting these receptors might also promote hair regeneration.
In the current study, we used a depilation model to mimic the process of hair regeneration, plucking was used to induce synchronous cycle of the hair follicles, thus we could clearly monitor the effect of AP in stimulating hair follicle into growth. We did observe that AP had a strong effect in promoting hair regeneration. However, in the future we sought to check for its effect in other animal models that could mimic alopecia in a pathological condition, including dermal injection of a mixture of cells isolated from AA-affected skin and pre-treatment of IFN-γ in C3H/HeJ mice for AA [42], or subcutaneous injection of dihydrotestosterone in C57BL/6J mice for AGA [43]. Because there are inflammation and oxidative stress in both AGA and AA [43,44,45,46], as AP can function as an anti-inflammatory agent as well as an antioxidant [47], and evidenced as a HFSCs stimulator in our study, we speculate that it might work efficiently in AGA and AA animal models, though need further experiment to address.
Conclusion
For the first time we demonstrated that AP, a primary active component in Alpinia katsumadai Hayata or Alpinia japonica (Thunb.) Miq. plants can promote hair regeneration by stimulating the activation and proliferation of HFSCs via Wnt signaling. Our findings may contribute to the development of a new generation of pilatory that is more efficient and less cytotoxic for treating alopecia.
Availability of data and materials
Datasets related to this article can be found at the National Center for Biotechnology Information Gene Expression Omnibus through https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi. The datasets used in the current study are available upon request for research.
Abbreviations
- AP:
-
Alpinetin
- VC:
-
Vehicle control
- PC:
-
Positive control
- HFSC:
-
Hair follicle stem cell
- ORS:
-
Outer root sheath
- IRS:
-
Inner root sheath
- Shh:
-
Sonic hedgehog
- H&E:
-
Hematoxylin and Eosin
- hKC:
-
Human keratinocytes
- hFB:
-
Human fibroblast
- mKC:
-
Mouse keratinocytes
- mFB:
-
Mouse fibroblast
- Lgr5:
-
Leucine-rich repeat-containing G-protein coupled receptor 5
- Gli1:
-
Glioma-associated oncogene homolog 1
- Fzd1:
-
Frizzled 1
- K15:
-
Cytokeratin 15
- Lef1:
-
Lymphoid enhancer-binding factor 1
- i.p:
-
Intra-peritoneally
- PPARG:
-
Peroxisome proliferator-activated receptor gamma
References
Zito PM, Raggio BS. Hair transplantation. In: StatPearls. Treasure Island (FL); 2022.
Shen YL, Li XQ, Pan RR, Yue W, Zhang LJ, et al. Medicinal plants for the treatment of hair loss and the suggested mechanisms. Curr Pharm Des. 2018;24:3090–100.
Sinclair R, Trindade de Carvalho L, Ferial Ismail F, Meah N. Treatment of male and female pattern hair loss with sublingual minoxidil: a retrospective case-series of 64 patients. J Eur Acad Dermatol Venereol. 2020;34:e795–6.
Rousso DE, Kim SW. A review of medical and surgical treatment options for androgenetic alopecia. JAMA Facial Plast Surg. 2014;16:444–50.
Morgan BA. The dermal papilla: an instructive niche for epithelial stem and progenitor cells in development and regeneration of the hair follicle. Cold Spring Harb Perspect Med. 2014;4: a015180.
Troy TC, Arabzadeh A, Turksen K. Re-assessing K15 as an epidermal stem cell marker. Stem Cell Rev Rep. 2011;7:927–34.
Phan QM, Fine GM, Salz L, Herrera GG, Wildman B, et al. Lef1 expression in fibroblasts maintains developmental potential in adult skin to regenerate wounds. Elife. 2020;9: e60066.
Hoeck JD, Biehs B, Kurtova AV, Kljavin NM, de Sousa EMF, et al. Stem cell plasticity enables hair regeneration following Lgr5(+) cell loss. Nat Cell Biol. 2017;19:666–76.
Brownell I, Guevara E, Bai CB, Loomis CA, Joyner AL. Nerve-derived sonic hedgehog defines a niche for hair follicle stem cells capable of becoming epidermal stem cells. Cell Stem Cell. 2011;8:552–65.
Botchkarev VA, Botchkareva NV, Nakamura M, Huber O, Funa K, et al. Noggin is required for induction of the hair follicle growth phase in postnatal skin. FASEB J. 2001;15:2205–14.
Zhang B, Ma S, Rachmin I, He M, Baral P, et al. Hyperactivation of sympathetic nerves drives depletion of melanocyte stem cells. Nature. 2020;577:676–81.
Lim X, Tan SH, Yu KL, Lim SB, Nusse R. Axin2 marks quiescent hair follicle bulge stem cells that are maintained by autocrine Wnt/beta-catenin signaling. Proc Natl Acad Sci USA. 2016;113:E1498–505.
Wei W. Summary of traditional chinese medicine in diagnosis and treatment of seborrheic alopecia. Occup Health. 2011;27:3.
Kay SK, Harrington HA, Shepherd S, Brennan K, Dale T, et al. The role of the Hes1 crosstalk hub in Notch-Wnt interactions of the intestinal crypt. PLoS Comput Biol. 2017;13: e1005400.
Wang J, Yan Z, Liu X, Che S, Wang C, et al. Alpinetin targets glioma stem cells by suppressing Notch pathway. Tumour Biol. 2016;37:9243–8.
Choi S, Zhang B, Ma S, Gonzalez-Celeiro M, Stein D, et al. Corticosterone inhibits GAS6 to govern hair follicle stem-cell quiescence. Nature. 2021;592:428–32.
Zeng C, Pan F, Jones LA, Lim MM, Griffin EA, et al. Evaluation of 5-ethynyl-2′-deoxyuridine staining as a sensitive and reliable method for studying cell proliferation in the adult nervous system. Brain Res. 2010;1319:21–32.
Dobin A, Gingeras TR. Mapping RNA-seq reads with STAR. Curr Protoc Bioinform. 2015;51:11–4.
Liao Y, Smyth GK, Shi W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics. 2014;30:923–30.
Zytnicki M. mmquant: how to count multi-mapping reads? BMC Bioinform. 2017;18:411.
Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550.
Zhou Y, Zhou B, Pache L, Chang M, Khodabakhshi AH, et al. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat Commun. 2019;10:1523.
Li F, Adase CA, Zhang LJ. Isolation and culture of primary mouse keratinocytes from neonatal and adult mouse skin. J Vis Exp. 2017;14(125): e56027.
Kovacs D, Maresca V, Flori E, Mastrofrancesco A, Picardo M, et al. Bovine colostrum induces the differentiation of human primary keratinocytes. FASEB J. 2020;34:6302–21.
Sumbal J, Koledova Z. FGF signaling in mammary gland fibroblasts regulates multiple fibroblast functions and mammary epithelial morphogenesis. Development. 2019. https://doi.org/10.1242/dev.185306.
Rosenblum MD, Olasz EB, Yancey KB, Woodliff JE, Lazarova Z, et al. Expression of CD200 on epithelial cells of the murine hair follicle: a role in tissue-specific immune tolerance? J Invest Dermatol. 2004;123:880–7.
Kang S, Cox CL, Gulley JM. High frequency stimulation-induced plasticity in the prelimbic cortex of rats emerges during adolescent development and is associated with an increase in dopamine receptor function. Neuropharmacology. 2018;141:158–66.
Murayama K, Usami S, Sakaki M. Summary-statistics-based power analysis: a new and practical method to determine sample size for mixed-effects modeling. Psychol Methods. 2022. https://doi.org/10.1037/met0000330.
Avigad Laron E, Aamar E, Enshell-Seijffers D. The mesenchymal niche of the hair follicle induces regeneration by releasing primed progenitors from inhibitory effects of quiescent stem cells. Cell Rep. 2018;24:909-921.e903.
Wang X, Chen H, Tian R, Zhang Y, Drutskaya MS, et al. Macrophages induce AKT/beta-catenin-dependent Lgr5(+) stem cell activation and hair follicle regeneration through TNF. Nat Commun. 2017;8:14091.
Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–7.
Qiao C, Xu L, Wang Z, Yang Z. Progress in studies of alpinetin and cardamonin. China Wild Plant Resour. 2001;20:11–13+15.
Wu D, Li S, Liu X, Xu J, Jiang A, et al. Alpinetin prevents inflammatory responses in OVA-induced allergic asthma through modulating PI3K/AKT/NF-kappaB and HO-1 signaling pathways in mice. Int Immunopharmacol. 2020;89: 107073.
Tan Y, Zheng C. Effects of alpinetin on intestinal barrier function, inflammation and oxidative stress in dextran sulfate sodium-induced ulcerative colitis mice. Am J Med Sci. 2018;355:377–86.
Liang X, Zhang B, Chen Q, Zhang J, Lei B, et al. The mechanism underlying alpinetin-mediated alleviation of pancreatitis-associated lung injury through upregulating aquaporin-1. Drug Des Dev Ther. 2016;10:841–50.
Kwack MH, Kim MK, You SH, Kim N, Park JH. Comparative graft survival study of follicular unit excision grafts with or without minor injury. Dermatol Surg. 2021;47:e191–4.
Egger A, Tomic-Canic M, Tosti A. Advances in stem cell-based therapy for hair loss. CellR4 Repair Replace Regen Reprogram. 2020;8: e2894.
Jaks V, Barker N, Kasper M, van Es JH, Snippert HJ, et al. Lgr5 marks cycling, yet long-lived, hair follicle stem cells. Nat Genet. 2008;40:1291–9.
Hu XM, Li ZX, Zhang DY, Yang YC, Fu SA, et al. A systematic summary of survival and death signalling during the life of hair follicle stem cells. Stem Cell Res Ther. 2021;12:453.
Jansson EA, Are A, Greicius G, Kuo IC, Kelly D, et al. The Wnt/beta-catenin signaling pathway targets PPARgamma activity in colon cancer cells. Proc Natl Acad Sci USA. 2005;102:1460–5.
Stambolic V, Ruel L, Woodgett JR. Lithium inhibits glycogen synthase kinase-3 activity and mimics wingless signalling in intact cells. Curr Biol. 1996;6:1664–8.
Li Y, Kilani RT, Leung G, Ghahary A. Myeloid adherent cells are involved in hair loss in the alopecia areata mouse model. J Investig Dermatol Symp Proc. 2020;20:S16–21.
Deng W, Zhang Y, Wang W, Song A, Mukama O, et al. Hair follicle-derived mesenchymal stem cells decrease alopecia areata mouse hair loss and reduce inflammation around the hair follicle. Stem Cell Res Ther. 2021;12:548.
Prie BE, Iosif L, Tivig I, Stoian I, Giurcaneanu C. Oxidative stress in androgenetic alopecia. J Med Life. 2016;9:79–83.
Prie BE, Voiculescu VM, Ionescu-Bozdog OB, Petrutescu B, Iosif L, et al. Oxidative stress and alopecia areata. J Med Life. 2015;8(Spec Issue):43–6.
Simakou T, Butcher JP, Reid S, Henriquez FL. Alopecia areata: a multifactorial autoimmune condition. J Autoimmun. 2019;98:74–85.
Zhu Z, Hu R, Li J, Xing X, Chen J, et al. Alpinetin exerts anti-inflammatory, anti-oxidative and anti-angiogenic effects through activating the Nrf2 pathway and inhibiting NLRP3 pathway in carbon tetrachloride-induced liver fibrosis. Int Immunopharmacol. 2021;96: 107660.
Acknowledgements
We thank members of the Laboratory Animal Research Center in Zhejiang University and Zhejiang Chinese Medical University for mice rearing. We are grateful to Ms. Chao Bi and Ms. Xiaoli Hong at the Core Facilities of Institute of Translational Medicine, Zhejiang University School of Medicine for technical support in microscopy and microplate reader.
Funding
This study is supported by the National Nature Science Foundation of China (31900620).
Author information
Authors and Affiliations
Contributions
YX and XJF designed the study; XJF, JC, YJZ, SYW, WQZ, YXZ, XW and HPY performed the experiments and collected and analyzed the data; YX, YW, XJF, JC, YJZ, SYW wrote and revised the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
All steps were implemented in accordance with animal welfare procedures and with permission from the Zhejiang Chinese Medical University Laboratory Animal Research Center (#20211025-10). This study was approved by Medical Ethics Committee, Sir Run Run Shaw Hospital, Zhejiang University (#20191104-13).
Consent for publication
Not applicable.
Competing interests
The authors state that they have no conflict of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Additional methods.
Additional file 2: Figure S1.
AP promotes hair follicle growth.
Additional file 3: Figure S2.
AP affects Gli1+ HFSC at the onset of anagen.
Additional file 4: Figure S3.
AP upregulates the expression of Fzd1 and Lef1 mRNA.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Fan, X., Chen, J., Zhang, Y. et al. Alpinetin promotes hair regeneration via activating hair follicle stem cells. Chin Med 17, 63 (2022). https://doi.org/10.1186/s13020-022-00619-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13020-022-00619-2