Abstract
Background
Ginsenosides are pharmacologically active compounds that are often extracted from the Panax plant for their medicinal properties. Ginsenosides have multiple effects, including antitumor effects which have been widely studied. In recent years, studies have found that ginsenosides promote proliferation and osteogenesis of osteoblast-related cells, as well as inhibit the activity of osteoclasts.
Main body
We briefly introduces the molecules and BMP, WNT, and RANKL signalling pathways involved in bone formation and bone resorption. Next, recent studies on the mechanism of action of ginsenosides in bone remodelling are reviewed from three perspectives: the effects on proliferation of osteoblast-related cells, effects on osteogenesis and effects on osteoclasts. To expedite the development of drugs containing ginsenosides, we summarize the multiple beneficial roles of various types of ginsenosides in bone remodelling; including the promotion of bone formation, inhibition of bone resorption, and anti-inflammatory and antioxidant effects.
Conclusion
Many ginsenosides can promote bone formation and inhibit bone resorption, such as Rb1, Rb2 and Re. Ginsenosides have the potential to be new drugs for the treatment of osteoporosis, promote fracture healing and are strong candidates for cytokines in the tissue-engineered bone. This review provides a theoretical basis for clinical drug applications and proposes several future directions for exploring the beneficial role of ginseng compounds in bone remodelling.
Similar content being viewed by others
Background
Ginsenosides are the main pharmacologically active compounds present in plants of the genus Panax (ginseng), which belongs to the Araliaceae family. The main medicinal plants of Panax include P. ginseng C. A. Mey, P. quinquefolius L, P. notoginseng (Burk.) F. H Chen, P. japonicus C.A. Mey, P. japonicus C.A. Mey. var. major (Burk) C. Y. Wu et KM. Feng and P. japonicus C. A. Mey. var. bipinnatifidus (Seem.) C. Y. Wu et KM. Feng; among these, P. ginseng C. A. Mey., P. quinquefolium L., and P. notoginseng (Burk.) F. H. Chen. are most widely used. Ginsenosides are mostly concentrated in the roots, leaves, and flower buds of ginsengs. Petkov [1] first reported the pharmacological properties of P. ginseng extracts in the 1950s and since then, over 6000 articles have been published on the traditional uses, chemical constituents, and biological and pharmacological effects of ginseng. Ginsenosides are almost non-toxic to normal human cells, while their natural properties (Fig. 1) include resistance to tumours [2], inhibition of neurodegeneration in patients with Alzheimer’s disease [3], promotion of brain development and memory enhancement [4], exhibition of anti-inflammatory [5] and antioxidant effects [6], prevention of diabetes [7], resistance to fatigue [8], and protection of the heart [9], etc. In recent years, studies have found that ginsenosides also promote cell proliferation and osteogenesis, as well as inhibit osteoclasts.
The review explores the molecular mechanisms of ginsenosides that affect bone remodelling, and provide a theoretical basis for novel applications of ginsenosides as drugs. Based on the type and molecular structure of ginsenosides, this review discusses the effects and mechanisms of ginsenosides while considering their ability to promote cell proliferation and osteogenesis, as well as inhibit osteoclast formation.
Main types of ginsenosides that affect bone remodelling
Ginsenosides are mainly extracted from P. ginseng C. A. Mey, P. quinquefolium L, and P. notoginseng (Burk.) F. H Chen. Based on their diverse structural characteristics, ginsenosides can be divided into the following three types: protopanaxadiol (PPD), protopanaxatriol (PPT), and oleanane. Typical PPD, also called 3β,12β,20-trihydroxydammar-24-ene type saponins, includes the ginsenosides Rb1, Rb2, Rc, and Rd. As shown in Fig. 2, PPD-type ginsenosides involve the attachment of the saccharide(s) C-3 and/or C-20. Rh2 and Rg3 are also PPD-type ginsenosides. In P. ginseng, the two most abundant PPT, also called 3β,6α,12β,20-tetrahydroxydammar-24-ene type saponins, are Re and Rg1. A variety of saponins can be biosynthesized via the O-glycosylation of PPT, which involves the attachment of saccharide(s) to C-6 and/or C-20. Typically, the hydroxyl group at C-3 remains free in PPT-type ginsenosides. Rf and Rh1 also belong to PPT-type ginsenosides.
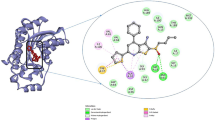
The chemical name of ginsenoside compound K (C-K, Compound 1) is 20-O-β- D-glucopyranosyl-20(S)-protopanaxadiol; it is a non-natural protopanaxadiol ginsenoside. P. ginseng C. A. Mey, P. quinquefolium L, and P. notoginseng (Burk.) F. H. Chen, which contain the natural ginsenosides Rb1, Rb2, Rc, and Rd, are the main sources of C-K. C-K can only be produced through biotransformation; microbial fermentation and enzymatic methods are mainly used, with the latter being the preferred method. C-K is one of the major metabolites detected in blood after the oral administration of the ginsenosides Rb1, Rb2, or Rc; it is also speculated to be the major form of protopanaxadiol saponins absorbed through the intestine [10, 11]. Figure 2 shows the main components and structural formulae of ginsenosides that can affect bone remodelling [12,13,14,15,16,17,18,19,20,19,21,].
Bone remodelling
The bone is one of the most important tissues in the human body. Bone remodelling plays a critical role in maintaining the skeletal system and involves the processes of bone formation and bone absorption [22, 23].
There are two types of bone development. The first is intra-membrane osteogenesis, which includes the proliferation and differentiation of mesenchymal stem cells into pre-osteoblasts that differentiate into osteoblasts and secrete extracellular matrix. The cells are embedded into the calcification matrix turn into osteocytes, become ossification centers, and form bone trabeculae. The other type of bone development is intrachondral osteogenesis, which involves the proliferation and differentiation of mesenchymal stem cells into chondrocytes. The chondrocytes produce a cartilage matrix, which forms the cartilage and is gradually replaced with bone tissue. Osteoclasts are derived from hematopoietic stem cells and can perform bone resorption. Osteoblasts and osteoclasts complement each other and participate in bone development and remodelling. The entire process requires many intracellular signals as well as interactive molecules and signalling pathways to promote proliferation and differentiation.
Osteogenesis and related molecules
The two most active pathways that regulate osteogenesis involve bone morphogenetic protein (BMP) and wingless/int-1 (WNT), as shown in Fig. 3.
The role of the Bone morphogenetic proteins (BMP) family, including BMP-2, BMP-4, and BMP-7, have been extensively studied in osteogenesis. There are two types of BMP receptors: BMPRI and BMPRII. Type I is a high-affinity receptor, while type II receptors bind specialized BMP ligands, including the three aforementioned BMPs [24]; both types are expressed on cell membranes. When BMP-2/4/7 binds to type II receptors, they activate type I receptors that in turn phosphorylate Smad1, 5, and 8 [25, 26], followed by the binding of the type I receptors to Smad4, the entry in the nucleus and recruitment of different transcription factors to regulate transcription. The Smad cascade can activate core-binding factor a1 (Cbfal), also known as Runt-related gene 2 (Runx2), which binds to the osteoblast-specific cis-acting element-2 (OSE2) of the promoter region of bone-specific genes and induces the expression of many bone marker genes [27], including alkaline phosphatase (ALP), osteocalcin (OCN), osteopontin (OPN), bone sialoprotein and bone-specific collagens. The expression of Runx2 marks the beginning of osteogenic differentiation; it is the first and most specific marker of bone formation [28]. ALP is an early phenotypic marker and an essential enzyme in osteoblast differentiation [29]. OCN is an important non-collagen component secreted by osteoblasts into the bone matrix during the mineral formation stage [30]; it is closely related to hydroxyapatite in bone tissue and participates in the regulation of bone calcium deposition. Col-1, one of the main components of the extracellular matrix secreted by osteoblasts, provides a structural framework for the maturation of the extracellular matrix and formation of calcified nodules [31,32,33]. In addition, BMP-2 can also induce ALP without Smad proteins by various pathways, such as the p38-mitogen-activated protein kinase (MAPK) [34]. Noggin (Nog) prevents BMP from binding to BMPR, thereby inhibiting the rate of bone formation.
WNT signalling consists of two major pathways: a canonical signalling pathway and a non-canonical signalling pathway. In canonical signalling, proteins such as Wnt3A, Wnt8 and Wnt10b bind to frizzled (Fz) receptors to generate dishevelled (DSH) and then transfer the signal to glycogen synthase kinase 3 (GSK-3), adenomatous polyposis coli (APC), axin and β-catenin. β-catenin enters the nucleus and binds to the lymphatic enhancer factor/T cell-specific transcription factor (TCF/LEF), so that the target gene is expressed [35, 36]. On the other hand, the non-canonical pathway involves intracellular secondary messengers: one to activate specific transcription factors through Ca2+ [37] and the other to control planar cell polarity (PCP) signalling. After binding to the Fz receptor, proteins activate Rho to regulate the cytoskeleton [38]. The canonical WNT pathway plays an important role in bone development. Wnt proteins are produced by osteoblasts and Wnt genes are upregulated during the osteogenic differentiation of BMSCs [39, 40]. Wnt10b, Wnt1, Wnt2 and Wnt3a regulate bone marker genes such as Runx2, ALP, Osx and OCN through canonical pathways [41]. Upregulation of Wnt signalling in osteoblasts can promote osteoblast proliferation and osteogenic activity [42]. The lack of Wnt10b or Wnt5a in mice leads to insufficient bone mass and abnormal bone tissue structure [43, 44]. In addition, non-canonical Wnt signalling also participates in osteogenesis and can interact with other signalling pathways [45]. For example, Wnt5a can promote osteogenic differentiation by promoting the Ror2-JNK and PLC-PKC-CaMKII pathways, while the former can stimulate RANKL in osteoclasts to regulate osteoclasis. This is of great significance for maintaining bone homeostasis [46,47,48]. The Wnt signalling pathway plays an important role in promoting the proliferation and differentiation of osteoblasts and stem cells. It can also promote bone formation by regulating the FGF and BMP pathways [49].
The BMP and WNT signalling pathways described above are active and play important roles in regulating osteogenesis.
Osteoclast and related molecules
Osteoclasts are required for bone remodelling. RANK is a homotrimeric receptor that interacts with its ligand RANKL to induce downstream signal transduction, such as the activation of nuclear factor-kappa B (NF-κB), which can enter the nucleus to induce proliferation, differentiation and activation of osteoclasts [50]. The canonical Wnt pathway can produce OPG and inhibits the activity of osteoclasts by increasing the ratio of OPG to RANK [51]. Tartrate-resistant acid phosphatase (TRAP), mainly derived from osteoclasts, has a protective effect against tartaric acid. During the process of bone resorption, TRAP and other isoenzymes degrade the solid calcium phosphate mineralized substrate in the bone matrix. Moreover, TRAP marks the activity of osteoclasts and plays a role in osteoclastic bone resorption. Among the bone resorption indicators, TRAP has the highest specificity and is not easily affected by diurnal changes and liver or kidney diseases [52].
Therefore, changes in the content of the above molecules can objectively reflect the activity of osteoclasts and degree of activity of bone resorption.
Relationship between bone development, fracture healing and osteoporosis
Bone development involves continuous bone remodelling which includes bone regeneration, enhancement, absorption and atrophy, to facilitate bone tissue growth and changes in its anatomical structure. The healing of fractures can be divided into three stages: acute inflammation, repair and remodelling. Inflammatory reactions include vasodilation, increased permeability, exudation of plasma proteins and inflammatory cell infiltration in the tissue of the fractured area during the acute inflammation phase. During repair, osteoblasts in the fractured area secrete collagen, synthesize the matrix and deposit calcium salts. In the bone remodelling phase, osteoblasts and osteoclasts work together to rebuild the bone trabecula of spongy bone along the direction of stress lines, strengthen the cortical bone, and connect the bone marrow cavity to restore the normal structure of the bone. The similarity between fracture healing and bone development is that they both undergo bone remodelling. The difference is that the former is a physiological process and the latter is a pathological process after trauma.
The balance between osteoclasts and osteoblasts results in bone homeostasis. However, in case of abnormal metabolism, bone metabolic diseases such as osteoporosis tend to occur. During osteoporosis, bone mass and bone density are reduced, and the microstructure of bone tissue is damaged, which results in increased bone fragility and risk of fracture. The treatment of osteoporosis also requires bone remodelling to promote bone formation and inhibit bone resorption.
Effect of ginsenosides on bone remodelling
Role and mechanism of ginsenosides in promoting osteoblast-related cell proliferation
In general, the ginsenosides Rb1, Rb2, Rg1, Rh1, Rg5, and Rk1 promote the proliferation of osteoblasts and stem cells [53,54,55,56,57,58,59,60]. Mesenchymal stem cells are multipotent cells capable of differentiating into osteoblasts and play an important role in the growth, development, and reconstruction of bone tissue. The research methods in this part mainly include MTT assay, CCK8, and [3H]-thymidine incorporation assay. Table 1 shows the role and mechanisms of ginsenosides in promoting osteoblast-related cell proliferation. The mixture of protopanaxadiol ginsenoside Rg5:Rk1 was obtained by Siddiqi et al. [60] by repeatedly cooking and drying fresh ginseng root. At present, there are few studies on the mechanism of ginsenosides promoting osteoblasts or MSCs. Ginsenoside Rg1 significantly increased the proportion of hDPCs and hDPSCs in the proliferative phase (S phase) and decreased the cells in the resting phase (G0/G1 phase) [57, 58]. Moreover, a gene expression profile microarray analysis was performed; results showed that compared to the control, ginsenoside Rg1 had more than 2000 gene expression differences. Through analysis, it was revealed that these genes are mainly related to the following functions: cell cycle, cell proliferation, growth factor and receptor activity, cellular metabolism, biosynthetic process, signal transduction, and apoptosis. The molecular mechanism of ginsenosides in promoting cell proliferation needs further study.
Effect and mechanism of ginsenoside on bone formation
Today, people are becoming increasingly interested in how drugs promote osteogenesis. The conventional in vitro research methods include ALP activity determination, calcium nodule staining, PCR and western blot, to detect the expression of osteogenesis-related factors. There are further studies on research methods. In vivo research methods include the establishment of animal models, imaging detection of bone reconstruction indicators, and the histological observation of bone structure. Many in vivo and in vitro experiments have shown that ginsenosides can up-regulate the expression of intracellular osteogenic transcription factors and osteogenic related gene products, induce osteogenic differentiation of pre-osteoblasts, stimulate osteoblast proliferation and promote bone nodule formation and matrix mineralization. They modulate various intracellular signalling pathways to exert these effects. Table 2 will introduce the roles and mechanisms of various major types of ginsenosides in bone remodelling.
Ginsenoside mixture
At present, there are only a few studies on the osteogenesis of ginsenoside mixtures. Siddiqi et al. obtained ginsenoside mixture Rg5:Rk1 after repeatedly cooking and drying fresh ginseng root [60]. Ginsenosides Rg5:Rk1 can promote osteogenic differentiation of MC3T3-E1 cells by increasing the activity of ALP, the content of Col-1 and the intracellular calcium deposition. Siddiqi et al. speculated that this osteogenesis-promoting effect is achieved through the BMP-2/Runx2 pathway [60]. Lee et al. [61] used chromatography to verify that the main part of ginseng water extract included 1.19% Rb1, 0.12% Rb2, 0.57% Rg1, 0.07% Rc, 0.64% Re and 0.04% Rf. It was found that in ovariectomized (OVX) rats treated with ginseng water extract, the sharp decrease in bone mineral density (BMD) and the deterioration of trabecular bone structures can be significantly reduced. In the presence of ginseng water extract, other bone remodelling markers such as Tb.N, Tb.Th and Tb.Sp were also significantly restored. Hence, ginseng can prevent bone loss and trabecular microstructure deterioration caused by OVX and is a potential drug for the prevention and treatment of postmenopausal osteoporosis. Lee and Siddiqi have proved the role of ginseng mixture in promoting bone tissue. However, the question remains whether the individual ingredients exert similar effects.
Ginsenoside Rb1
Ginsenoside Rb1 can promote the osteogenic activity of hADSCs and rMSCs in a dose-dependent manner (0.5–6.0 μmol/L and 0.01–1 μmol/L), and promote ALP activity, mineralization, and expression of osteoblast-related proteins [53, 62]; it however, has no significant effect on bone loss in OVX rats. However, in their experiments, the high-dose Rb1 group was injected with 6 mg/kg/day, and an increase in the dose could be considered in animal experiments in the future.
Ginsenoside Rb2
Ginsenoside Rb2 (0.1–10 μmol/L) can improve the ALP activity; it increases the degree of calcium mineralization and mRNA expression of ALP, Col-1, OCN, and OPN to resist oxidative damage caused by H2O2 in MC3T3-E1 cells [54]. Besides, Rb2 reduced the expression levels of receptor activator of nuclear factor kappa-B ligand (RANKL) and IL-6 and inhibited the H2O2-induced production of ROS. In vivo studies showed that in OVX mice, the continuous administration of Rb2 for 12 weeks partially decreased the malondialdehyde (MDA) activity in the blood and increased the activity of reduced glutathione (GSH). MDA is a parameter for assessing the state of oxidative damage, and GSH is an intracellular oxidant, which can relieve the oxidative stress of cells. In addition, Rb2 improved the microstructure of the trabecular bone and increased BMD of the fourth lumbar spine (L4) and the distal femur.
Ginsenoside Rd
Ginsenoside Rd can improve the ALP activity and mineralization ability of MC3T3-E1 cells. The expression levels of ALP, OCN, Col-1, and BMP-2 can be increased, and the mRNA expression level of BMP-2 can be inhibited by noggin, AMPK inhibitor (Ara-A) or transfection of smad4-targeted siRNA. Therefore, Kim believes that ginsenoside Rd promotes osteogenesis through the AMPK-BMP-2-Smad pathway [63].
Ginsenoside Re
Ginsenoside Re can promote osteoblast differentiation and mineralization of zebrafish scales. Ginsenoside Re was non-cytotoxic to MC3T3-E1 cells and enhanced ALP staining and activity, as well as mRNA levels of osteoblast markers Col-1, Alp and OCN in MC3T3-E1 cells. The calcium concentration of ginsenoside Re-treated zebrafish scales was detected by Alizarin Red S staining [64].
Ginsenoside Rg1
At present, there are more and more in-depth studies on ginsenoside Rg1. It is known to promote the osteogenic differentiation of hPDLSCs [56], hDPCs [57], rBMSCs [55], as well as the ALP activity and the formation of mineralized calcium nodules. It can not only stimulate the secretion of BMP-2 and FGF-2 from hDPSCs [58], but also promote the protein expression of DSPP, ALP, BMP -2, FGF-2, Runx-2, Col-1, OCN and OPN [55,56,57,58]. Wang et al. [58] further compared the representative gene expression profiles of DPSCs using Roche’s full human genome expression profile microarray chip. In the ginsenoside Rg1 (5 μmol/L) group, there were 1498 upregulated genes and 561 downregulated genes. Pathway analysis found seven statistically significant pathways, such as the cell cycle pathway, the MAPK signalling pathway and the TGF-β signalling pathway. Moreover, Gu et al. [55] further explored the mechanisms and found that Rg1 promotes the osteogenesis of rBMSCs through the GR-dependent BMP/Smad-signalling pathway. In addition to the progress of in vitro experiments, in vivo experiments were performed. A rat tibial fracture model was established, and a Micro-CT scanner revealed that Rg1 stimulates fracture healing. Then H&E staining and Safranin O/Light green red staining were used to examine the bone section. It was found that Rg1 promoted the transformation from fibrous to osteogenic callus, increased bone strength and accelerated fracture healing. These results suggest that Rg1 promotes cartilage calcification and osteogenesis at a later stage. Whether or not Rg1 can regulate bone metabolism through other signal pathways that Wang analyzed needs further verification.
Ginsenoside Rh1
Ginsenoside Rh1 can promote osteogenic differentiation and stimulate ALP activity of MC3T3-E1 cells [59], promote mineralization and increase glutathione content. Rh1 was also found to increase BMP-2, Runx2, ALP, Col-1 and OCN expression levels. During mitochondrial electron transport, reactive oxygen species (ROS) keep cells in a state of oxidative stress by producing high levels of oxidants, which destroy proteins, lipids and DNA [65]. Oxidative stress may also destroy osteoblasts and cause osteoporosis [66, 67]. New evidence suggests that ROS increases bone resorption by enhancing osteoclast development and activity [68]. ROS also cause apoptosis and reduce their activity, which leads to osteoblasts apoptosis [69]. Additionally, Ginsenoside Rh1 shows an inhibitory effect on AMA-enhanced ROS production levels in MC3T3-E1 cells. The level of glutathione after Rh1 treatment significantly increases in a concentration-dependent manner, indicating that the increase in the activity of osteoblasts induced by Rh1 was related to the increase in glutathione content. AMA-treated MC3T3-E1 cells significantly increased ROS production, while Rh1 treatment strongly inhibited this effect of AMA.
Ginsenoside Rh2 (S)
Ginsenoside Rh2 (S) stimulated the differentiation and the mineralization of osteoblasts MC3T3-E1, which were expressed by their differentiation markers (ALP, Runx2, OSX, OCN, OPN and Col-1) and upregulation of von Kossa/Alizarin red staining [70, 71]. Ginsenoside Rh2 (S) activated protein kinase D (PKD), p38 mitogen-activated protein kinase (MAPK) and AMP-activated protein kinase (AMPK) in a time- and concentration-dependent manner, which can be inhibited by corresponding inhibitors. Therefore, Rh2 (S) induced differentiation and mineralization of MC3T3-E1 cells by activating the PKD/p38 MAPK signalling pathway [70] and the PKD/AMPK signalling pathway [71].
C-K
C-K inhibited H2O2-induced ROS and NO production in MC3T3-E1 cells in a dose-dependent manner. Cultured H2O2 stimulated MC3T3-E1 cells exposed to C-K showed a sharp increase in the expression of osteoblast differentiation markers, such as ALP activity, Col-1 content and mineralization. In addition, C-K reduced inflammation-related genes including IKK and interleukin 1β (IL-1β) expression [72]. Zhou et al. [73] converted four compounds from ginsenoside Rb1, including C-K, compounds 2, 3, and 4. The compounds significantly increased ALP activity in a dose-dependent manner. The mRNA expression of the osteoblast differentiation markers ALP, Col-1, and Runx2 increased significantly in a dose-dependent manner. C-K and these new derivatives significantly upregulated the mRNA expression of genes of Wnt/β-catenin signalling pathway-related regulators, including Wnt10b, Wnt11, Lrp5 and β-catenin. These minor ginsenosides can induce osteogenic differentiation of MC3T3-E1 cells by partial or independent controlling of the classical Wnt signalling pathway.
The above section introduces the role of various types of ginsenosides in promoting bone formation, such as up-regulating the expression of intracellular osteogenic gene products, inducing osteogenic differentiation, promoting bone nodule formation and matrix mineralization, and regulating various intracellular signal pathways.
Ginsenosides inhibit the mechanism of osteoclastogenesis
Ginseng water extract and 14 kinds of ginsenosides (Rb1, Rb2, Rb3, Rg1, Rg2, Rg3, Rc, Rd, Re, Rf, CK, F11, Rh1, Rh2) can slow bone resorption. During bone resorption, ginsenosides inhibit osteoclast differentiation, TRAP activity and staining, the activation of signalling pathways such as NF-κB induced by RANKL. Among them, Rb1, Rb2 and Re have better effects, and their mechanism of action has been further explored.
Ginsenoside mixture
Ginseng extract effectively inhibits RANKL-induced osteoclast differentiation. Lee et al. [61] exposed RAW 264.7 cells to RANKL and M-CSF receptor activator for 5 days, and each group was added with different concentrations of ginseng water extract. It was found that Rb1, Rg1, Rc, Re, and Rf can inhibit osteoclast differentiation, TRAP activity and staining.
Ginsenoside Rb1
Similar results were found for the independent components of ginsenosides. Ginsenoside Rb1 can inhibit osteoclastogenesis by inhibiting RANKL-induced activation of JNK, p38 MAPKs and the NF-κB pathways, thereby downregulating the gene expression of c-Fos and NFATc1 in osteoclast precursors [74].
Ginsenoside Rb2
The inhibitory effect of Rb2 on osteoclast differentiation may be related to blocking the NF-κB and STAT3 signalling pathways [75]. Rb2 can promote OPN expression and bone resorption. On the other hand, Rb2 inhibits the formation of TRAP-positive multinucleated cells and the expression of TRAP in a dose-dependent manner, and significantly inhibits RANKL-induced NF-κB activation. Furthermore, Rb2 can also significantly inhibit the expression of osteoclast marker genes, including NFATc1, c-Fos, OSCAR and cathepsin K nuclear factors. In addition, knocking down STAT3 can significantly enhance the inhibitory effect of Rb2 on osteoclast differentiation, which indicates that Rb2 also remarkably inhibits the activation of signal transductors and the STAT3 signalling pathway.
Ginsenoside Re
Park et al. [76] treated RANKL-induced bone marrow-derived macrophages (BMMs) with 14 kinds of ginsenosides (including the 14 above) and osteoclast differentiation was evaluated by TRAP activity. To some extent, TRAP activity in all the 14 ginsenoside groups was inhibited. At a concentration of 2.5 μmol/L, ginsenosides Rd, Re, C-K and fraction 11 inhibited osteoclast differentiation by about 60%. All types of ginsenosides except ginsenoside Rd were found to be non-toxic. Among various ginsenosides, ginsenoside Re showed the strongest inhibitory effect on osteoclast differentiation. The mechanism of Re was further explored and it was found that ginsenoside Re affects osteoclast differentiation by inhibiting the RANKL-induced signalling pathways. In particular, ginsenoside Re blocked ERK signalling, a known mechanism of osteoclast differentiation. Re was applied to a zebrafish model to study its effects on osteoclast differentiation in vivo. The results demonstrated that ginsenoside Re inhibited osteoclast differentiation, and osteoclast marker genes were significantly reduced in zebrafish scales. Treatment with Re reduced the mRNA expression levels of TRAP and cathepsin K, although it did not significantly affect the expression of osteoblast marker genes.
In summary, ginsenosides can inhibit osteoclast differentiation and slow bone resorption. The effects of Rb1, Rb2 and Re have been well described; the effect and mechanism of other types of ginsenosides on bone resorption need to be further explored.
Other effects related to bone remodelling
As earlier discussed, there are two ways of bone formation—intra-membrane osteogenesis, and intra-chondral osteogenesis. Having discussed how ginsenosides promote intra-membrane osteogenesis, we turn our attention to the potential effects of ginsenosides on cartilage.
Ginsenosides F4 and Rg3 prevent cartilage destruction in rabbit cartilage tissue culture. Lee et al. [77] studied how 11 kinds of ginsenosides (Rb1, Rb2, Rc, Rd, Re, Rf, Rg1, Rg3, Rg5, Rk1 and F4) affect the induction of MMP-13 in the human chondrocyte cell line SW1353. In osteoarthritis, MMP-13 plays an important role in the degradation of major collagens embedded into the cartilage. Among them, ginsenosides Rc, Rd, Rf, Rg3 and F4, were found to inhibit the expression of MMP-13 in IL-1β-treated SW1353 cells at a non-cytotoxic concentration (1–50 μmol/L). The most prominent inhibitors are ginsenosides F4 and Rg3. Ginsenoside F4 was found to strongly inhibit p38 mitogen-activated protein kinase (p38 MAPK) activation in the signalling pathway. Ginsenosides F4 and Rg3 also reduced the release of glycosaminoglycan in rabbit joint cartilage culture treated with IL-1α. The mechanism of action of ginsenosides on cartilage remains to be fully elucidated.
Dental tissue and bone tissue are homologous in tissue origin (both derived from neural crest). Ginsenoside can promote bone remodelling in bone tissue, and some studies proposed similar effects in tooth tissue.
Ginsenoside Rg1 promotes the differentiation of hDPCs and increases the expression of DSPP and DMP1 genes [57]. DSPP is a type of extracellular matrix protein of dentin. It is considered as an odontoblastic marker, and can trigger the mineralization of dentin. DMP1, a non-collagen matrix protein, is an important component of mineralized tissues and can be expressed in the bone, dentin, and cementum. DMP1 plays an important role in regulating the differentiation of odontoblasts, formation of dentin tubules and mineralization of dentin. These two genes can promote the differentiation of dental pulp cells into odontoblasts and dentin regeneration. Therefore, ginsenoside Rg1 may become a new pulp capping agent, which will be important in dental pulp biotherapy and can provide new strategies for preventing and treating dental caries. Further detailed studies of ginsenoside Rg1 are needed.
Conclusion and outlook
Bone homeostasis is tightly regulated to retain a balance between bone formation and bone resorption. Many anabolic drugs are used as bone-targeting therapeutic agents for the promotion of osteoblast-mediated bone formation or the inhibition of osteoclast-mediated bone resorption. The functions and mechanisms of various types of ginsenosides in bone remodelling are shown in Table 2 and Fig. 3. Many ginsenosides can promote bone formation and inhibit bone resorption, such as Rb1, Rb2 and Re. They play an important role in promoting bone remodelling. These ginsenosides have multiple beneficial roles in bone remodelling. In addition to promoting bone formation and inhibiting bone resorption, they also have anti-inflammatory and antioxidant effects, which can alleviate oxidative stress. These functions are complementary to bone reconstruction.
Many future directions can be taken with this. Firstly, there is little research on the mechanism of ginsenosides on the proliferation of BMSC or osteoblasts, which can be further explored. Secondly, since ginseng from different sources and processing methods have different extracted components, a novel approach for maintaining stability needs to be found. Thirdly, although it is known that Rb1 has the effect of promoting osteogenesis in vitro, whether or not it promotes the formation of bone in animals is still undiscovered. At present, the effect of Rb1 on OVX rats is relatively small, perhaps because the dose is insufficient, and increasing the dose could be a consideration in future research. Fourthly, the role of Rc in bone reconstruction has not been studied in detail and it may be worth further exploration. Many pathways are involved in Wang’s research [58], and further verification is needed. Further, there are many in vitro studies on various types of ginsenosides affecting bone remodelling, but in vivo studies need to be conducted to corroborate the in vitro findings. Lastly, the current research on ginsenosides in the oral cavity has only explored the promoting effect of Rg1 on dentin. In the future, whether other types of ginsenosides have similar effects and more functions is worthy of in-depth study.
With the growing interest in bone tissue engineering, the selection of appropriate cytokines for bone remodelling has become a hotspot of research. In recent years, the effect of ginsenosides on bone remodelling has received increasing attention. Ginsenosides have the potential to be new drugs for the treatment of osteoporosis, promote fracture healing and are strong candidates for cytokines in the tissue-engineered bone.
Availability of data and materials
Not applicable.
Abbreviations
- ALP:
-
Alkaline phosphatase
- Runx2:
-
Runt-related gene 2
- OCN:
-
Osteocalcin
- OPN:
-
Osteopontin
- Col-1:
-
Type I collagen
- TRAP:
-
Tartrate-resistant acid phosphatase
- ROS:
-
Reactive oxygen species
- MSCs:
-
Mesenchymal stem cells
- hADSCs:
-
Human adipose stem cells
- hPDLSCs:
-
Human periodontal ligament stem cells
- hDPCs:
-
Human dental pulp cells
- hDPSCs:
-
Human dental pulp stem cells
- BMD:
-
Bone mineral density
- OVX:
-
Ovariectomized
- MDA:
-
Malondialdehyde
- GSH:
-
Glutathione
References
Petkov W. Pharmacological studies of the drug P. ginseng C.A. Meyer. Arzneim Forsch. 1959;9:305–11.
Chae S, Piao MJ, Kang KA, Zhang R, Kim KC, Youn UJ, Nam KW, Lee JH, Hyun JW. Inhibition of matrix metalloproteinase-1 induced by oxidative stress in human keratinocytes by mangiferin isolated from Anemarrhena asphodeloides. Biosci Biotechnol Biochem. 2011;75(12):2321–5.
Choi RJ, Roy A, Jung HJ, Ali MY, Min BS, Park CH, Yokozawa T, Fan TP, Choi JS, Jung HA. BACE1 molecular docking and anti-Alzheimer’s disease activities of ginsenosides. J Ethnopharmacol. 2016;22(190):219–30.
Kim HS, Hwang SL, Oh S. Ginsenoside Rc and Rg1 differentially modulate NMDA receptor subunit mRNA levels after intracerebroventricular infusion in rats. Neurochem Res. 2000;25(8):1149–54.
Yu T, Yang Y, Kwak YS, Song GG, Kim MY, Rhee MH, Cho JY. Ginsenoside Rc from Panax ginseng exerts anti-inflammatory activity by targeting TANK-binding kinase 1/interferon regulatory factor-3 and p38/ATF-2. J Ginseng Res. 2017;41(2):127–33.
Liu ZQ, Luo XY, Sun YX, Chen YP, Wang ZC. Can ginsenosides protect human erythrocytes against free-radical-induced hemolysis? Biochim Biophys Acta. 2002;1572(1):58–66.
Lee MS, Hwang JT, Kim SH, Yoon S, Kim MS, Yang HJ, Kwon DY. Ginsenoside Rc, an active component of Panax ginseng, stimulates glucose uptake in C2C12 myotubes through an AMPK-dependent mechanism. J Ethnopharmacol. 2010;127(3):771–6.
Yang QY, Lai XD, Ouyang J, Yang JD. Effects of Ginsenoside Rg3 on fatigue resistance and SIRT1 in aged rats. Toxicology. 2018;1(409):144–51.
Yuan SM. Potential cardioprotective effects of Ginseng preparations. Pak J Pharm Sci. 2015;28(3):963–8.
Karikura M, Miyase T, Tanizawa H, Taniyama T, Takino Y. Studies on absorption, distribution, excretion and metabolism of ginseng saponins. VII. Comparison of the decomposition modes of ginsenoside-Rb1 and -Rb2 in the digestive tract of rats. Chem Pharm Bull. 1991;39(9):2357–61.
Bae EA, Choo MK, Park EK, Park SY, Shin HY, Kim DH. Metabolism of ginsenoside Rc by intestinal bacteria and its related antiallergic activity. Biol Pharm Bull. 2002;25(6):743–7.
Sanada S, Kondo N, Shoji J, Tanaka O, Shibata S. Studies on the saponins of ginseng. I. Structures of ginsenoside-Ro, -Rb1, -Rb2, -Rc and -Rd. Chem Pharm Bull. 1974;22:421–8.
Kasai R, Besso H, Tanaka O, Saruwatari Y, Fuwa T. Saponins of red ginseng. Chem Pharm Bull. 1983;31:2120–5.
Yahara S, Kaji K, Tanaka O. Further study on dammarane-type saponins of roots, leaves, flower-buds, and fruits of Panax ginseng C. A. Meyer. Chem Pharm Bull. 1979;27:88–92.
Kondo N, Shoji J, Tanaka O. Studies on the constituents of Himalayan ginseng, Panax pseudoginseng. I. The structures of the saponins. (1). Chem Pharm Bull. 1973;21:2705–11.
Yahara S, Matsuura K, Kasai R, Tanaka O. Saponins of buds and flowers of Panax ginseng C. A Meyer. (1). Isolation of ginsenosides-Rd, -Re, and -Rg1. Chem Pharm Bull. 1976;24:3212–3.
Sanada S, Kondo N, Shoji J, Tanaka O, Shibata S. Studies on the saponins of ginseng. II. Structures of ginsenoside-Re, -Rf and -Rg2. Chem Pharm Bull. 1974;22:2407–12.
Iida Y, Tanaka O, Shibata S. Studies on saponins of ginseng: the structure of ginsenoside-Rg1. Tetrahedron Lett. 1968;9:5449–53.
Kitagawa I, Yoshikawa M, Yoshihara M, Hayashi T, Taniyama T. Chemical studies on crude drug precession. I. On the constituents of Ginseng Radix rubra (1). Yakugaku Zasshi. 1983;103:612–22.
Zhou J, Wu M, Taniyasu S, Besso H, Tanaka O, Saruwatari Y, Fuwa T. Dammarane-saponins of sanchi-ginseng, roots of Panax notoginseng (BURK.) F.H. Chen (Araliaceae): structures of new saponins, notoginsenosides-R1 and -R2, and identification of ginsenosides-Rg2 and -Rh1. Chem Pharm Bull. 1981;29:2844–50.
Hasegawa H. Proof of the mysterious effificacy of ginseng: basic and clinical trials: metabolic activation of ginsenoside: deglycosylation by intestinal bacteria and esterification with fatty acid. J Pharmacol Sci. 2004;95(2):153–7.
Rodan GA, Martin TJ. Therapeutic approaches to bone diseases. Science. 2000;289(5484):1508–14.
Rodan GA. Introduction to bone biology. Bone. 1992;13(Suppl 1):S3–6.
Wan M, Cao X. BMP signalling in skeletal development. Biochem Biophys Res Commun. 2005;328(3):651–7.
Kawabata M, Imamura T, Miyazono K. Signal transduction by bone morphogenetic proteins. Cytokine Growth Factor Rev. 1998;9(1):49–61.
Nohe A, Hassel S, Ehrlich M, Neubauer F, Sebald W, Henis Y, Knaus P. The mode of bone morphogenetic protein (BMP) receptor oligomerization determines different BMP-2 signalling pathways. J Biol Chem. 2002;277(7):5330–8.
Ziros PG, Gil AP, Georgakopoulos T, Habeos I, Kletsas D, Basdra EK, Papavassiliou AG. The bone-specific transcriptional regulator Cbfa1 is a target of mechanical signals in osteoblastic cells. J Biol Chem. 2002;277(26):23934–41.
Zhao Z, Zhao M, Xiao G, Franceschi RT. Gene transfer of the Runx2 transcription factor enhances osteogenic activity of bone marrow stromal cells in vitro and in vivo. Mol Ther. 2005;12(2):247–53.
Serigano K, Sakai D, Hiyama A, Tamura F, Tanaka M, Mochida J. Effect of cell number on mesenchymal stem cell transplantation in a canine disc degeneration model. J Orthop Res. 2010;28(10):1267–75.
Cosman F, Shen V, Morgan D, Gordon S, Parisien M, Nieves J, Lindsay R. Biochemical responses of bone metabolism to 1,25-dihydroxyvitamin D administration in black and white women. Osteoporos Int. 2000;11(3):271–7.
Pochampally RR, Horwitz EM, DiGirolamo CM, Stokes DS, Prockop DJ. Correction of a mineralization defect by overexpression of a wild-type cDNA for COL1A1 in marrow stromal cells (MSCs) from a patient with osteogenesis imperfecta: a strategy for rescuing mutations that produce dominant-negative protein defects. Gene Ther. 2005;12(14):1119–25.
Rauch F, Glorieux FH. Osteogenesis imperfecta. Lancet. 2004;363(9418):1377–85.
Sakkers R, Kok D, Engelbert R, van Dongen A, Jansen M, Pruijs H, Verbout A, Schweitzer D, Uiterwaal C. Skeletal effects and functional outcome with olpadronate in children with osteogenesis imperfecta: a 2-year randomised placebo-controlled study. Lancet. 2004;363(9419):1427–31.
Guicheux J, Lemonnier J, Ghayor C, Suzuki A, Palmer G, Caverzasio J. Activation of p38 mitogen-activated protein kinase and c-Jun-NH2-terminal kinase by BMP-2 and their implication in the stimulation of osteoblastic cell differentiation. J Bone Miner Res. 2003;18(11):2060–8.
Logan CY, Nusse R. The Wnt signalling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810.
Milat F, Ng KW. Is Wnt signalling the final common pathway leading to bone formation? Mol Cell Endocrinol. 2009;310(1–2):52–62.
Chen AE, Ginty DD, Fan CM. Protein kinase A signalling via CREB controls myogenesis induced by Wnt proteins. Nature. 2005;433(7023):317–22.
Piters E, Boudin E, Van Hul W. Wnt signalling: a win for bone. Arch Biochem Biophys. 2008;473(2):112–6.
Kato M, Patel MS, Levasseur R, Lobov I, Chang BH, Glass DA 2nd, Hartmann C, Li L, Hwang TH, Brayton CF, Lang RA, Karsenty G, Chan L. Cbfa1-independent decrease in osteoblast proliferation, osteopenia, and persistent embryonic eye vascularization in mice deficient in Lrp5, a Wnt coreceptor. J Cell Biol. 2002;157(2):303–14.
Gregory CA, Gunn WG, Reyes E, Smolarz AJ, Munoz J, Spees JL, Prockop DJ. How Wnt signalling affects bone repair by mesenchymal stem cells from the bone marrow. Ann N Y Acad Sci. 2005;1049:97–106.
Takada I, Kouzmenko AP, Kato S. Wnt and PPARgamma signalling in osteoblastogenesis and adipogenesis. Nat Rev Rheumatol. 2009;5(8):442–7.
Joeng KS, Lee YC, Lim J, Chen Y, Jiang MM, Munivez E, Ambrose C, Lee BH. Osteocyte-specific WNT1 regulates osteoblast function during bone homeostasis. J Clin Invest. 2017;127(7):2678–88.
Brunet LJ, McMahon JA, McMahon AP, Harland RM. Noggin, cartilage morphogenesis, and joint formation in the mammalian skeleton. Science. 1998;280(5368):1455–7.
Takada I, Mihara M, Suzawa M, Ohtake F, Kobayashi S, Igarashi M, Youn MY, Takeyama K, Nakamura T, Mezaki Y, Takezawa S, Yogiashi Y, Kitagawa H, Yamada G, Takada S, Minami Y, Shibuya H, Matsumoto K, Kato S. A histone lysine methyltransferase activated by non-canonical Wnt signalling suppresses PPAR-gamma transactivation. Nat Cell Biol. 2007;9(11):1273–85.
Tan Z, Ding N, Lu H, Kessler JA, Kan L. Wnt signalling in physiological and pathological bone formation. Histol Histopathol. 2019;34(4):303–12.
Maeda K, Kobayashi Y, Udagawa N, Uehara S, Ishihara A, Mizoguchi T, Kikuchi Y, Takada I, Kato S, Kani S, Nishita M, Marumo K, Martin TJ, Minami Y, Takahashi N. Wnt5a-Ror2 signalling between osteoblast-lineage cells and osteoclast precursors enhances osteoclastogenesis. Nat Med. 2012;18(3):405–12.
Keller KC, Ding H, Tieu R, Sparks NR, Ehnes DD, Zur Nieden NI. Wnt5a supports osteogenic lineage decisions in embryonic stem cells. Stem Cells Dev. 2016;25(13):1020–32.
Martineau X, Abed É, Martel-Pelletier J, Pelletier JP, Lajeunesse D. Alteration of Wnt5a expression and of the non-canonical Wnt/PCP and Wnt/PKC-Ca2 + pathways in human osteoarthritis osteoblasts. PLoS ONE. 2017;12(8):e0180711.
Hill TP, Später D, Taketo MM, Birchmeier W, Hartmann C. Canonical Wnt/beta-catenin signalling prevents osteoblasts from differentiating into chondrocytes. Dev Cell. 2005;8(5):727–38.
Khosla S. Minireview: the OPG/RANKL/RANK system. Endocrinology. 2001;142(12):5050–5.
Suzuki A, Ozono K, Kubota T, Kondou H, Tachikawa K, Michigami T. PTH/cAMP/PKA signalling facilitates canonical Wnt signalling via inactivation of glycogen synthase kinase-3beta in osteoblastic Saos-2 cells. J Cell Biochem. 2008;104(1):304–17.
Shidara K, Inaba M. Bone metabolic marker for osteoporosis. Nihon Rinsho. 2009;67(5):927–31.
Luo ZJ, Li HM, Wang HG, Liu DL, Nan H. Ginsenoside Rb1 affects the proliferation and osteogenic differentiation of human adipose-derived stem cells in vitro. Zhongguo Zuzhi Gongcheng Yanjiu. 2013;17(32):5799–805.
Huang Q, Gao B, Jie Q, Wei BY, Fan J, Zhang HY, Zhang JK, Li XJ, Shi J, Luo ZJ, Yang L, Liu J. Ginsenoside-Rb2 displays anti-osteoporosis effects through reducing oxidative damage and bone-resorbing cytokines during osteogenesis. Bone. 2014;66:306–14.
Gu YQ, Zhou JC, Wang Q, Fan WM, Yin GY. Ginsenoside Rg1 promotes osteogenic differentiation of rBMSCs and healing of rat tibial fractures through regulation of GR dependent BMP-2/SMAD signalling. Sci Rep. 2016;4(6):25282.
Yin LH, Cheng WX, Qin ZS, Sun KM, Zhong M, Wang JK, Gao WY, Yu ZH. Effects of ginsenoside Rg-1 on the proliferation and osteogenic differentiation of human periodontal ligament stem cells. Chin J Integr Med. 2015;21(9):676–81.
Wang P, Wei X, Zhou Y, Wang YP, Yang K, Zhang FJ, Jiang R. Effect of ginsenoside Rg1 on proliferation and differentiation of human dental pulp cells in vitro. Aust Dent J. 2012;57:157–65.
Wang P, Wei X, Zhang FJ, Yang K, Qu C, Luo HQ, He LZ. Ginsenoside Rg1 of Panax ginseng stimulates the proliferation, odontogenic/osteogenic differentiation and gene expression profiles of human dental pulp stem cells. Phytomedicine. 2014;21(2):177–83.
Siddiqi MH, Siddiqi MZ, Ahn S, Kim YJ, Yang DC. Ginsenoside Rh1 induces mouse osteoblast growth and differentiation through the bone morphogenetic protein2/runt-related gene 2 signalling pathway. J Pharm Pharmacol. 2014;66(12):1763–73.
Siddiqi MH, Siddiqi MZ, Ahn S, Kang S, Kim YJ, Veerappan K, Yang DU, Yang DC. Stimulative effect of ginsenosides Rg5:Rk1 on murine osteoblastic MC3T3-E1 cells. Phytother Res. 2014;28(10):1447–55.
Lee HY, Park SH, Chae SW, Soung NK, Oh MJ, Kim JS, Kim YO, Chae HJ. Aqueous ginseng extract has a preventive role in RANKL-induced osteoclast differentiation and estrogen deficiency-induced osteoporosis. J Funct Food. 2015;13:192–203.
Bei JX, Zhang XL, Wu JK, Hu ZQ, Xu BL, Lin SE, Cui L, Wu T, Zou LY. Ginsenoside Rb1 does not halt osteoporotic bone loss in ovariectomized rats. J Ginseng Res. 2017;41(2):127–33.
Kim DY, Park YG, Quan HY, Kim SJ, Jung MS, Chung SH. Ginsenoside Rd stimulates the differentiation and mineralization ofosteoblastic MC3T3-E1 cells by activating AMP-activated protein kinase via the BMP-2 signalling pathway. Fitoterapia. 2012;83(1):215–22.
Kim HM, Kim DH, Han HJ, Park CM, Ganipisetti SR, Valan Arasu M, Kim YO, Park CG, Kim BY, Soung NK. Ginsenoside Re promotes osteoblast differentiation in mouse osteoblast precursor MC3T3-E1 cells and a Zebrafish model. Molecules. 2016;22(1):42.
Finkel T, Holbrook NJ. Oxidants, oxidative stress and biology of ageing. Nature. 2000;408(6809):239–47.
Mody N, Parhami F, Sarafian TA, Demer LL. Oxidative stress modulates osteoblastic differentiation of vascular and bone cells. Free Radic Biol Med. 2001;31(4):509–19.
Bai XC, Lu D, Bai J, Zheng H, Ke ZY, Li XM, Luo SQ. Oxidative stress inhibits osteoblastic differentiation of bone cells by ERK and NF-kappaB. Biochem Biophys Res Commun. 2004;314(1):197–207.
Lee NK, Choi YG, Baik JY, Han SY, Jeong DW, Bae YS, Kim N, Lee SY. A crucial role for reactive oxygen species in RANKL-induced osteoclast differentiation. Blood. 2005;106(3):852–9.
Arai M, Shibata Y, Pugdee K, Abiko Y, Ogata Y. Effects of reactive oxygen species (ROS) on antioxidant system and osteoblastic differentiation in MC3T3-E1 cells. IUBMB Life. 2007;59(1):27–33.
Kim DY, Jung MS, Park YG, Yuan HD, Quan HY, Chung SH. Ginsenoside Rh2(S) induces the differentiation and mineralization of osteoblastic MC3T3-E1 cells through activation of PKD and p38 MAPK pathways. BMB Rep. 2011;44(10):659–64.
Kim DY, Park KH, Jung MS, Huang B, Yuan HD, Quan HY, Chung SH. Ginsenoside Rh2(S) induces differentiation and mineralization of MC3T3-E1 cells through activation of the PKD/AMPK signalling pathways. Int J Mol Med. 2011;28(5):753–9.
Kang S, Siddiqi MH, Yoon SJ, Ahn S, Noh HY, Kumar NS, Kim YJ, Yang DC. Therapeutic potential of compound K as an IKK inhibitor with implications for osteoarthritis prevention: an in silico and in vitro study. Vitro Cell Dev Biol Anim. 2016;52(9):895–905.
Zhou W, Huang H, Zhu H, Zhou P, Shi X. New metabolites from the biotransformation of ginsenoside Rb1 by Paecilomyces bainier sp.229 and activities in inducing osteogenic differentiation by Wnt/β-catenin signalling activation. J Ginseng Res. 2018;42(2):199–207.
Cheng B, Li J, Du J, Lv X, Weng L, Ling C. Ginsenoside Rb1 inhibits osteoclastogenesis by modulating NF-κB and MAPKs pathways. Food Chem Toxicol. 2012;50(5):1610–5.
Cong F, Liu J, Wang C, Yuan Z, Bi L, Liang J, Su K, Qiu Y, Song T, Fan J, Chao G. Ginsenoside Rb2 inhibits osteoclast differentiation through nuclear factor-kappaB and signal transducer and activator of transcription protein 3 signalling pathway. Biomed Pharmacother. 2017;92:927–34.
Park CM, Kim HM, Kim DH, Han HJ, Noh H, Jang JH, Park SH, Chae HJ, Chae SW, Ryu EK, Lee S, Liu K, Liu H, Ahn JS, Kim YO, Kim BY, Soung NK. Ginsenoside re inhibits osteoclast differentiation in mouse bone marrow-derived macrophages and zebrafifish scale model. Mol Cells. 2016;39(12):855–61.
Lee JH, Lim H, Shehzad O, Kim YS, Kim HP. Ginsenosides from Korean red ginseng inhibit matrix metalloproteinase-13 expression in articular chondrocytes and prevent cartilage degradation. Eur J Pharmacol. 2014;5(724):145–51.
Acknowledgements
Not applicable.
Funding
This paper was sponsored by the Special Projects in Cooperation between Changchun City and Local Universities, 17DY024; the Key Scientific and Technological Research and Development Projects in Jilin Province, 20180201056YY; the Science and Technology Project of the 13th Five-Year Plan of Jilin Provincial Department of Education, JJKH20180236KJ.
Author information
Authors and Affiliations
Contributions
NY, DL, XZ, JL and ZL conducted this review. NY, DL, JL, MW, TX and ZL wrote the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
All authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yang, N., Liu, D., Zhang, X. et al. Effects of ginsenosides on bone remodelling for novel drug applications: a review. Chin Med 15, 42 (2020). https://doi.org/10.1186/s13020-020-00323-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13020-020-00323-z