Abstract
Background
Combining pemetrexed with bevacizumab may have some potential in improving the efficacy in patients with non-small-cell lung cancer (NSCLC), and this meta-analysis aims to explore the impact of pemetrexed addition to bevacizumab on treatment efficacy for NSCLC.
Methods
PubMed, EMbase, Web of science, EBSCO, and Cochrane library databases were systematically searched, and we included randomized controlled trials (RCTs) assessing the effect of pemetrexed addition to bevacizumab on treatment efficacy in patients with NSCLC. Overall survival and progression-free survival were included in this meta-analysis.
Results
Four RCTs were finally included in the meta-analysis. Overall, compared with bevacizumab for NSCLC, pemetrexed addition showed significantly improved overall survival (hazard ratio [HR] = 0.87; 95% confidence interval [CI] = 0.76 to 0.99; P = 0.03), survival rate (odd ratio [OR] = 1.41; 95% CI = 1.06 to 1.86; P = 0.02), progression-free survival (HR = 0.63; 95% CI = 0.55 to 0.72; P < 0.00001) and progression-free survival rate (OR = 1.92; 95% CI = 1.38 to 2.67; P < 0.00001), but led to the increase in grade ≥ 3 adverse events (OR = 2.15; 95% CI = 1.62 to 2.84; P < 0.00001).
Conclusions
Pemetrexed addition may be effective to improve treatment efficacy for NSCLC compared to bevacizumab treatment.
Similar content being viewed by others
Introduction
Molecular-targeted anticancer drugs and immune checkpoint inhibitors (ICIs) were commonly used to improve the outcomes of patients with non-small-cell lung cancer (NSCLC) [1,2,3,4,5], while platinum-based chemotherapy was one key therapeutic option for NSCLC without epidermal growth factor receptor (EGFR) mutation [6,7,8,9,10]. Especially, bevacizumab and pemetrexed displayed an important role in treating NSCLC [11,12,13,14].
In the subgroup analysis of one phase III study, cisplatin and pemetrexed resulted in a significant improvement in overall survival (OS) compared to cisplatin and gemcitabine in patients with advanced NSCLC [15]. In the JMEN trial, maintenance therapy with pemetrexed supplementation significantly prolonged OS and progression-free survival (PFS) in patients with NSCLC without disease progression [16]. These suggested that maintenance therapy with pemetrexed may be a promising option for patients with NSCLC.
Several RCTs showed that pemetrexed addition to bevacizumab may have the capability to improve the outcomes for patients with NSCLC, but the results were not well established [17,18,19]. We therefore conducted this meta-analysis of RCTs to evaluate the effectiveness of pemetrexed addition to bevacizumab on treatment efficacy for NSCLC.
Materials and methods
Study selection and data collection
This meta-analysis was conducted by using previously studies, so ethical approval and patient consent were not needed. It was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-analysis statement and Cochrane Handbook for Systematic Reviews of Interventions [20, 21].
We have searched PubMed, EMbase, Web of science, EBSCO and the Cochrane library up to September 2023, by using the search terms “pemetrexed” AND “bevacizumab” AND “lung cancer” OR “NSCLC”. The inclusion criteria were as follows: (1) study design was RCT; (2) patients were diagnosed with NSCLC; (3) intervention treatments were pemetrexed plus bevacizumab versus bevacizumab. Patients with uncontrolled hypertension, major hemoptysis within 4 weeks, recent major surgery within 6 weeks, significant cardiovascular disease, and cavitary lung lesions were excluded.
Assessment for risk of bias
The risk of bias tool was used to assess the quality of individual studies in accordance with the Cochrane Handbook for Systematic Reviews of Interventions [22], and the following sources of bias were considered: selection bias, performance bias, attrition bias, detection bias, reporting bias, and other potential sources of bias. The overall risk of bias for each study was evaluated and rated: low, unclear, and high [23]. Two investigators independently searched articles, extracted data, and assessed the quality of included studies. Any discrepancy was solved by consensus.
Outcome measures
The following information was extracted: first author, publication year, sample size, age, weight, body mass index, adenocarcinoma and methods of two groups. The primary outcomes were overall survival and survival rate. Secondary outcomes included progression-free survival, progression-free survival rate and grade ≥ 3 adverse events.
Statistical analysis
A team consisting of three authors did the statistical analyses. Hazard ratio (HR) with 95% confidence interval [CI] was used to assess continuous outcomes and odd ratio (OR) with 95% CI was used to assess dichotomous outcomes. I2 statistic was used to assess the heterogeneity, and significant heterogeneity was observed when I2 > 50% [24, 25]. The random-effect model was used regardless of the heterogeneity. We conducted the sensitivity analysis through detecting the influence of a single study on the overall estimate via omitting one study in turn or using the subgroup analysis. P < 0.05 indicated statistical significance and Review Manager Version 5.3 was used in all statistical analyses.
Quality of evidence
The quality of evidence for each outcome was evaluated based on the methodological quality and the confidence in the results, and it was assessed by GRADE recommendations as high quality, moderate quality, low quality, or very low quality [26].
Results
Literature search, study characteristics and quality assessment
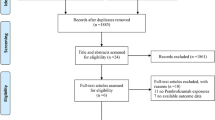
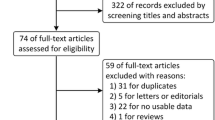
The flow chart for the selection process and detailed identification was presented in Fig. 1. 452 publications were searched after the initial search of databases. 145 duplicates and 301 papers after checking the titles/abstracts were excluded. Three studies were removed because of the study design. Ultimately, four RCTs were included in the meta-analysis [17,18,19, 27].
The baseline characteristics of four eligible RCTs in the meta-analysis were summarized in Table 1. The four studies were published between 2013 and 2020, and total sample size was 1467. There were similar baseline characteristics between pemetrexed group and control group. The treatment duration of pemetrexed addition ranged from 8 to 63 months. The methods of chemotherapies included bevacizumab 7.5 mg/kg or/and pemetrexed 500 mg/m2 once every 3 weeks (Table 2).
Among the four RCTs, four studies reported overall survival [17,18,19, 27], two studies reported survival rate [18, 27], four studies reported progression-free survival [17,18,19, 27], two studies reported progression-free survival rate [17, 18], and three studies reported grade ≥ 3 adverse events [17, 19, 27]. Risk of bias analysis showed that four studies had unclear risk of performance bias and detection bias [17,18,19, 27], while one study showed unclear risk of selection bias (Fig. 2) [27]. However, all four RCTs generally had high quality.
Primary outcomes: overall survival and survival rate
Compared to control group for NSCLC, pemetrexed addition was associated with significantly prolonged overall survival (moderate quality, HR = 0.87; 95% CI = 0.76 to 0.99; P = 0.03) with no heterogeneity among the studies (I2 = 0%, heterogeneity P = 0.93, Fig. 3) and increased survival rate (moderate quality, OR = 1.41; 95% CI = 1.06 to 1.86; P = 0.02) with no heterogeneity among the studies (I2 = 0%, heterogeneity P = 0.91, Fig. 4).
Sensitivity analysis
No heterogeneity was observed for the primary outcomes, and thus we did not perform the sensitivity analysis by omitting one study in turn for the meta-analysis. The funnel plot was relatively symmetrical for overall survival (Fig. 5A) and survival rate (Fig. 5B), and all studies almost fell within the 95% CI axis. There was little evidence of publication bias.
Secondary outcomes
Compared with control group for NSCLC, pemetrexed addition showed substantially improved progression-free survival (moderate quality, HR = 0.63; 95% CI = 0.55 to 0.72; P < 0.00001; Fig. 6) and progression-free survival rate (moderate quality, OR = 1.92; 95% CI = 1.38 to 2.67; P < 0.00001; Fig. 7). With regard to the safety, pemetrexed addition resulted in the increase in grade ≥ 3 adverse events (moderate quality, OR = 2.15; 95% CI = 1.62 to 2.84; P < 0.00001; Fig. 8).
Discussion
In the PARAMOUNT trial, pemetrexed supplementation was able to significantly prolong OS and PFS [28, 29]. Pemetrexed plus bevacizumab was significantly associated with improved PFS versus maintenance therapy with single-agent bevacizumab [27, 30]. In contrast, one recent study reported no increase in OS after the treatment with pemetrexed plus bevacizumab (P = 0.28) in patients with advanced NSCLC [19].
Considering these inconsistent results, our meta-analysis aimed to confirm the efficacy of pemetrexed plus bevacizumab versus bevacizumab for patients with NSCLC. We included four RCTs and 1467 patients. The results suggested that compared to bevacizumab intervention, pemetrexed plus bevacizumab substantially improved overall survival, survival rate, progression-free survival and progression-free survival rate for patients with NSCLC.
In terms of sensitivity analysis, although there was no significant heterogeneity, several factors may produce some bias. Firstly, the stages of NSCLC were different among the included patients, including metastatic and advanced cancers. Secondly, subgroup histologic types of NSCLC included squamous and non-squamous types, which may have different sensitivity to pemetrexed. Thirdly, the treatment duration of pemetrexed addition varied from 8 months to 63 months, which may affect the efficacy assessment of pemetrexed plus bevacizumab.
With regards to the safety, pemetrexed addition was associated with increased incidence of grade ≥ 3 adverse events for NSCLC patients. The most common adverse events mainly included neutropenia, thrombopenia and anemia, leukopenia. They were generally tolerant after corresponding treatments [18]. The prognosis of NSCLC was poor, especially for metastatic NSCLC [31]. Many novel signatures such as lncRNAs and autophagy-related genes may be able to evaluate the prognosis of cancers [32, 33]. For instance, dual homeoboxes A pseudogene 8 (DUXAP8) was closely related to poor overall survival in several cancers, suggesting its ability to serve as a prognostic biomarker and potential therapeutic target for cancers [34]. As the development of immunohistochemical markers in the subclassification of NSCLC, immunotherapy emerged as an increasingly important option [35, 36].
We should also consider several limitations. Firstly, our analysis was based on only four RCTs and more studies with large patient samples should be conducted to confirm our findings. Secondly, the treatment duration of pemetrexed treatment were different in the included studies, and may lead to some heterogeneity. Thirdly, NSCLC patients with different stages and subgroup histologic types may produce some bias.
Conclusion
Pemetrexed addition to bevacizumab may improve the treatment efficacy for NSCLC patients.
Data availability
Not applicable.
References
Nagano T, Tachihara M, Nishimura Y. Molecular mechanisms and targeted therapies including immunotherapy for Non-small Cell Lung Cancer. Curr Cancer Drug Targets. 2019;19(8):595–630.
Hack SP, Zhu AX, Wang Y. Augmenting anticancer immunity through combined targeting of angiogenic and PD-1/PD-L1 pathways: challenges and opportunities. Front Immunol. 2020;11:598877.
Ishihara M, Ochiai R, Haruyama T, Sakamoto T, Tanzawa S, Honda T, Ota S, Ichikawa Y, Ishida T, Watanabe K, Seki N. Pretreatment neutrophil-to-lymphocyte ratio predicts treatment efficacy and prognosis of cytotoxic anticancer drugs, molecular targeted drugs, and immune checkpoint inhibitors in patients with advanced non-small cell lung cancer. Translational lung cancer Res. 2021;10(1):221–32.
Chen H, Feng Y, Zhou Y, Tao Y, Tang L, Shi Y. Brain metastases and immune checkpoint inhibitors in non-small cell lung cancer: a systematic review and meta-analysis. Cancer immunology, immunotherapy: CII; 2022.
Brueckl WM, Ficker JH, Zeitler G. Clinically relevant prognostic and predictive markers for immune-checkpoint-inhibitor (ICI) therapy in non-small cell lung cancer (NSCLC). BMC Cancer. 2020;20(1):1185.
Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, Gemma A, Harada M, Yoshizawa H, Kinoshita I, Fujita Y, Okinaga S, Hirano H, Yoshimori K, Harada T, Ogura T, Ando M, Miyazawa H, Tanaka T, Saijo Y, Hagiwara K, Morita S, Nukiwa T. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362(25):2380–8.
Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe S, O’Brien M, Rao S, Hotta K, Leiby MA, Lubiniecki GM, Shentu Y, Rangwala R, Brahmer JR. Pembrolizumab versus Chemotherapy for PD-L1-Positive non-small-cell Lung Cancer. N Engl J Med. 2016;375(19):1823–33.
Remon J, Steuer CE, Ramalingam SS, Felip E. Osimertinib and other third-generation EGFR TKI in EGFR-mutant NSCLC patients, annals of oncology: official journal of the European Society for Medical Oncology 29(suppl_1) (2018) i20–7.
Leonetti A, Sharma S, Minari R, Perego P, Giovannetti E, Tiseo M. Resistance mechanisms to osimertinib in EGFR-mutated non-small cell lung cancer. Br J Cancer. 2019;121(9):725–37.
Le X, Nilsson M, Goldman J, Reck M, Nakagawa K, Kato T, Ares LP, Frimodt-Moller B, Wolff K, Visseren-Grul C, Heymach JV, Garon EB. Dual EGFR-VEGF pathway inhibition: a promising strategy for patients with EGFR-Mutant NSCLC. J Thorac Oncology: Official Publication Int Association Study Lung Cancer. 2021;16(2):205–15.
Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, Lilenbaum R, Johnson DH. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355(24):2542–50.
Reck M, von Pawel J, Zatloukal P, Ramlau R, Gorbounova V, Hirsh V, Leighl N, Mezger J, Archer V, Moore N, Manegold C. Phase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer: AVAil. J Clin Oncology: Official J Am Soc Clin Oncol. 2009;27(8):1227–34.
Zhou Q, Xu CR, Cheng Y, Liu YP, Chen GY, Cui JW, Yang N, Song Y, Li XL, Lu S, Zhou JY, Ma ZY, Yu SY, Huang C, Shu YQ, Wang Z, Yang JJ, Tu HY, Zhong WZ, Wu YL. Bevacizumab plus Erlotinib in Chinese patients with untreated, EGFR-mutated, advanced NSCLC (ARTEMIS-CTONG1509): a multicenter phase 3 study. Cancer Cell. 2021;39(9):1279–e12913.
Saito H, Fukuhara T, Furuya N, Watanabe K, Sugawara S, Iwasawa S, Tsunezuka Y, Yamaguchi O, Okada M, Yoshimori K, Nakachi I, Gemma A, Azuma K, Kurimoto F, Tsubata Y, Fujita Y, Nagashima H, Asai G, Watanabe S, Miyazaki M, Hagiwara K, Nukiwa T, Morita S, Kobayashi K, Maemondo M. Erlotinib plus Bevacizumab versus erlotinib alone in patients with EGFR-positive advanced non-squamous non-small-cell lung cancer (NEJ026): interim analysis of an open-label, randomised, multicentre, phase 3 trial, the Lancet. Oncology. 2019;20(5):625–35.
Scagliotti GV, Parikh P, von Pawel J, Biesma B, Vansteenkiste J, Manegold C, Serwatowski P, Gatzemeier U, Digumarti R, Zukin M, Lee JS, Mellemgaard A, Park K, Patil S, Rolski J, Goksel T, de Marinis F, Simms L, Sugarman KP, Gandara D. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncology: Official J Am Soc Clin Oncol. 2008;26(21):3543–51.
Ciuleanu T, Brodowicz T, Zielinski C, Kim JH, Krzakowski M, Laack E, Wu YL, Bover I, Begbie S, Tzekova V, Cucevic B, Pereira JR, Yang SH, Madhavan J, Sugarman KP, Peterson P, John WJ, Krejcy K, Belani CP. Maintenance pemetrexed plus best supportive care versus placebo plus best supportive care for non-small-cell lung cancer: a randomised, double-blind, phase 3 study. Lancet (London England). 2009;374(9699):1432–40.
Yoshida H, Kim YH, Sakamori Y, Nagai H, Ozasa H, Kaneda T, Yoshioka H, Nakagawa H, Tomii K, Okada A, Yoshimura K, Hirabayashi M, Hirai T. A randomized phase II study of maintenance Bevacizumab, Pemetrexed or Bevacizumab Plus Pemetrexed for Advanced non-squamous non-small cell Lung Cancer. Anticancer Res. 2020;40(5):2981–7.
Seto T, Azuma K, Yamanaka T, Sugawara S, Yoshioka H, Wakuda K, Atagi S, Iwamoto Y, Hayashi H, Okamoto I, Saka H, Mitsuoka S, Fujimoto D, Nishino K, Horiike A, Daga H, Sone T, Yamamoto N, Nakagawa K, Nakanishi Y. Randomized phase III study of continuation maintenance Bevacizumab with or without Pemetrexed in Advanced Nonsquamous Non-small-cell Lung Cancer: COMPASS (WJOG5610L). J Clin Oncology: Official J Am Soc Clin Oncol. 2020;38(8):793–803.
Ramalingam SS, Dahlberg SE, Belani CP, Saltzman JN, Pennell NA, Nambudiri GS, McCann JC, Winegarden JD, Kassem MA, Mohamed MK, Rothman JM, Lyss AP, Horn L, Stinchcombe TE, Schiller JH. Pemetrexed, Bevacizumab, or the combination as maintenance therapy for Advanced Nonsquamous Non-small-cell Lung Cancer: ECOG-ACRIN 5508. J Clin Oncology: Official J Am Soc Clin Oncol. 2019;37(26):2360–7.
Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535.
HigginsJPT G. Cochrane handbook for systematic reviews of interventions version 5.1. 0 [updated March 2011], The cochrane collaboration (2011).
Higgins GS. JPT, Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011], The Cochrane Collaboration (2011. www.cochrane-handbook.org).
Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA. Cochrane Bias methods, G. Cochrane Statistical methods, the Cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928.
Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–58.
Wang Y, Fu L, Lu T, Zhang G, Zhang J, Zhao Y, Jin H, Yang K, Cai H. Clinicopathological and prognostic significance of long non-coding RNA MIAT in human cancers: a review and Meta-analysis. Front Genet. 2021;12:729768.
Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, Schunemann HJ, Group GW. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–6.
Barlesi F, Scherpereel A, Rittmeyer A, Pazzola A, Ferrer Tur N, Kim JH, Ahn MJ, Aerts JG, Gorbunova V, Vikström A, Wong EK, Perez-Moreno P, Mitchell L, Groen HJ. Randomized phase III trial of maintenance bevacizumab with or without pemetrexed after first-line induction with bevacizumab, cisplatin, and pemetrexed in advanced nonsquamous non-small-cell lung cancer: AVAPERL (MO22089. J Clin Oncology: Official J Am Soc Clin Oncol. 2013;31(24):3004–11.
Paz-Ares L, de Marinis F, Dediu M, Thomas M, Pujol JL, Bidoli P, Molinier O, Sahoo TP, Laack E, Reck M, Corral J, Melemed S, John W, Chouaki N, Zimmermann AH, Visseren-Grul C, Gridelli C. Maintenance therapy with pemetrexed plus best supportive care versus placebo plus best supportive care after induction therapy with pemetrexed plus cisplatin for advanced non-squamous non-small-cell lung cancer (PARAMOUNT): a double-blind, phase 3, randomised controlled trial, the Lancet. Oncology. 2012;13(3):247–55.
Paz-Ares LG, de Marinis F, Dediu M, Thomas M, Pujol JL, Bidoli P, Molinier O, Sahoo TP, Laack E, Reck M, Corral J, Melemed S, John W, Chouaki N, Zimmermann AH, Visseren-Grul C, Gridelli C. PARAMOUNT: final overall survival results of the phase III study of maintenance pemetrexed versus placebo immediately after induction treatment with pemetrexed plus cisplatin for advanced nonsquamous non-small-cell lung cancer. J Clin Oncology: Official J Am Soc Clin Oncol. 2013;31(23):2895–902.
Barlesi F, Scherpereel A, Gorbunova V, Gervais R, Vikström A, Chouaid C, Chella A, Kim JH, Ahn MJ, Reck M, Pazzola A, Kim HT, Aerts JG, Morando C, Loundou A, Groen HJM, Rittmeyer A. Maintenance bevacizumab–pemetrexed after first-line cisplatin–pemetrexed–bevacizumab for advanced nonsquamous nonsmall-cell lung cancer: updated survival analysis of the AVAPERL (MO22089) randomized phase III trial. Ann Oncol. 2014;25(5):1044–52.
Ettinger DS, Wood DE, Aisner DL, Akerley W, Bauman J, Chirieac LR, D’Amico TA, DeCamp MM, Dilling TJ, Dobelbower M, Doebele RC, Govindan R, Gubens MA, Hennon M, Horn L, Komaki R, Lackner RP, Lanuti M, Leal TA, Leisch LJ, Lilenbaum R, Lin J, Loo BW Jr., Martins R, Otterson GA, Reckamp K, Riely GJ, Schild SE, Shapiro TA, Stevenson J, Swanson SJ, Tauer K, Yang SC, Gregory K. Hughes, Non-small Cell Lung Cancer, Version 5.2017, NCCN Clinical Practice guidelines in Oncology. J Natl Compr Cancer Network: JNCCN. 2017;15(4):504–35.
Wang Y, Zhang D, Li Y, Wu Y, Ma H, Jiang X, Fu L, Zhang G, Wang H, Liu X, Cai H. Constructing a novel signature and predicting the immune landscape of colon cancer using N6-methylandenosine-related lncRNAs. Front Genet. 2023;14:906346.
Wang Y, Lin K, Xu T, Wang L, Fu L, Zhang G, Ai J, Jiao Y, Zhu R, Han X, Cai H. Development and validation of prognostic model based on the analysis of autophagy-related genes in colon cancer. Aging. 2021;13(14):19028–47.
Wang Y, Jiang X, Zhang D, Zhao Y, Han X, Zhu L, Ren J, Liu Y, You J, Wang H, Cai H. LncRNA DUXAP8 as a prognostic biomarker for various cancers: a meta-analysis and bioinformatics analysis. Front Genet. 2022;13:907774.
Reck M, Remon J, Hellmann MD. First-line immunotherapy for non-small-cell Lung Cancer. J Clin Oncology: Official J Am Soc Clin Oncol. 2022;40(6):586–97.
Osmani L, Askin F, Gabrielson E, Li QK. Current WHO guidelines and the critical role of immunohistochemical markers in the subclassification of non-small cell lung carcinoma (NSCLC): Moving from targeted therapy to immunotherapy, Seminars in cancer biology 52(Pt 1) (2018) 103–109.
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
Wei Fang and Xingqiao Peng conducted the design, study planning, data analysis and data interpretation. Qun Zhou participated in the statistical analyses. Xingqiao Peng wrote and revised the article. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Fang, W., Peng, X. & Zhou, Q. Combination of pemetrexed with bevacizumab for non-small-cell lung cancer: a meta-analysis study. J Cardiothorac Surg 19, 478 (2024). https://doi.org/10.1186/s13019-024-02975-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13019-024-02975-6