Abstract
Background
We aimed to summarise the existing knowledge regarding antithrombotic medications following surgical aortic valve replacement (SAVR) using a biological valve prosthesis.
Methods
We performed a meta-analysis of studies that reported the results of using antithrombotic medication to prevent thromboembolic events after SAVR using a biological aortic valve prosthesis and recorded the outcomes 12 months after surgery. Since no randomised controlled trials were identified, observational studies were included. The analyses were conducted separately for periods of 0–12 months and 3–12 months after surgery. A random effects model was used to calculate pooled outcome event rates and 95% confidence intervals (CIs).
Results
The search yielded eight eligible observational studies covering 6727 patients overall. The lowest 0- to 12-month mortality was observed in patients with anticoagulation (2.0%, 95% CI 0.4–9.7%) and anticoagulation combined with antiplatelet therapy (2.2%, 95% CI 0.9–5.5%), and the highest was in patients without antithrombotic medication (7.3%, 95% CI 3.6–14.2%). Three months after surgery, mortality was lower in anticoagulant patients (0.5%, 95% CI 0.1–2.6%) than in antiplatelet patients (3.0%, 95% CI 1.2–7.4%) and those without antithrombotics (3.5%, 95% CI 1.3–9.3%). There was no eligible evidence of differences in stroke rates observed among medication strategies. At 0- to 12-month follow-up, all antithrombotic treatment regimens resulted in an increased bleeding rate (antiplatelet 4.2%, 95% CI 2.9–6.1%; anticoagulation 7.5%, 95% CI 3.8–14.4%; anticoagulation combined with antiplatelet therapy 8.3%, 95% CI 5.7–11.8%) compared to no antithrombotic medication (1.1%, 95% CI 0.4–3.4%). At 3- to 12-month follow-up, there was up to an eight-fold increase in the bleeding rate in patients with anticoagulation combined with antiplatelet therapy when compared to those with no antithrombotic medication. Overall, the evidence certainty was ranked as very low.
Conclusion
Although this meta-analysis reveals that anticoagulation therapy has a beneficial tendency in terms of mortality at 1 year after biological SAVR and suggests potential advantages in continuing anticoagulation beyond 3 months, it is limited by very low evidence certainty. The imperative for cautious interpretation and the urgent need for more robust randomised research underscore the complexity of determining optimal antithrombotic strategies in this patient population.
Similar content being viewed by others
Introduction
The prevention of stroke and thrombosis following aortic valve bioprosthetic implantation is a critical aspect of patient care that necessitates a careful balance of anticoagulants and antiplatelet agents. While lifelong warfarin therapy is recommended for mechanical valves, the approach for bioprosthetic valves is nuanced. The months following initial surgery carry an elevated risk of blood clot formation due to surgery-induced inflammation and tissue healing [1,2,3,4,5]. During this period, the body’s own endothelial cells gradually adhere to the bioprosthetic valve’s surface, offering protection against blood clot formation [6, 7].
Both the American Heart Association (AHA) and the European Society of Cardiology (ESC) currently recommend a 3-month course of warfarin post-bioprosthetic implantation [8, 9]. Intriguingly, previous studies have suggested the potential efficacy of aspirin (ASA) or even treatment completely without antithrombotic medications as comparable alternatives to warfarin in the initial 3 months for emboli and stroke prevention [10, 11]. However, there remains a scarcity of research beyond this initial period, leaving uncertainties regarding optimal anticoagulation and antiplatelet strategies.
The divergent recommendations between AHA and ESC complicate the decision-making process. While AHA advocates the continued use of ASA, ESC, in its latest guidelines, has retracted its prior endorsement of ASA as a permanent solution after aortic bioprosthetic implantation [8, 9]. This discrepancy underscores the complexity of therapeutic decisions, especially considering the intersection of valve disease management with coexisting conditions, such as atrial fibrillation, warranting anticoagulation, or cardio- and cerebrovascular diseases, where antiplatelet therapy may be preferable.
Considering these complexities, our aim is to provide a comprehensive summary of the existing knowledge regarding antithrombotic medications after the initial 3 months following surgical aortic valve replacement (SAVR) using a biological valve prosthesis. We hypothesise that a more extensive antithrombotic medication regimen would be related to lower mortality and stroke rates at the cost of higher bleeding rates.
Methods
This meta-analysis was conducted according to the guidelines in the Cochrane Handbook and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and the Meta-analysis of Observational Studies in Epidemiology (MOOSE) checklist [12,13,14].
Search and screening process
The search for this systematic review was performed on 19 January 2024. PubMed, Scopus and Web of Science databases were searched from their inception. The search strategy was as follows: ‘biological AND “aortic valve” AND (replacement OR implantation) AND (antithrombotic OR antiplatelet OR anticoagulation)’. We did not use any filters. Abstract and full-report screening were performed independently by two authors (MU and IK). Covidence software was used in the screening process. We did not search grey literature. We searched the reference lists of the included studies by hand to find any missed relevant studies for inclusion.
Inclusion and exclusion criteria
In the initial screening, we included randomised controlled trials (RCTs) that compared medical treatment regimens to prevent thromboembolic events after SAVR using biological aortic valve prosthesis and reported outcomes 12 months after surgery. In the original protocol, we stated that if there were no eligible RCTs, we would include observational studies (both prospective and retrospective cohort and case-control studies). Studies that did not report original data were excluded. Studies were not excluded based on language restrictions. Transcatheter aortic valve implantation (TAVI) procedures were excluded. Concomitant coronary artery bypass grafting surgery was not regarded as a criterion for exclusion. Paediatric patients, pregnant patients and patients with renal insufficiency requiring dialysis were excluded.
Patients
Patients were required to have undergone SAVR using a biological aortic valve prosthesis. Both chronic and acute phase surgery patients were included. SAVR was defined as a procedure in which the diseased native aortic valve is removed and replaced by a biological valve prosthesis in an open-heart surgery operation.
Intervention
We included all medical treatment regimens targeted against thromboembolic events, including agents affecting blood coagulation and thrombus formation. After the screening, we classified medication strategies into the following groups: 1) antiplatelets, including aspirin and P2Y12 inhibitors (clopidogrel, ticagrelor); 2) anticoagulation (vitamin K antagonists, such as warfarin; direct factor Xa inhibitors, such as rivaroxaban, apixaban and edoxaban); 3) a combination of anticoagulation and antiplatelet agents; 4) 3-month anticoagulation postoperatively and antiplatelet thereafter; and 5) no medication against thromboembolic events. In all medication strategies, the treatment was initiated immediately after surgery and continued for at least 1 year postoperatively.
Outcomes
Our main outcome was mortality. The follow-up time was set at 12 months. Secondary outcomes were strokes (haemorrhagic and ischemic, combined) and bleeding complications during the 12-month follow-up period. We used the definitions used by the original studies for all outcomes.
Data extraction
One author extracted the data and another author validated the extracted data to reduce possible errors. The following information was extracted from each study: authors, inclusion and exclusion criteria, study period, country, intervention definition, control definition, outcome definitions, number of included patients, number of events and main outcome measures.
Evidence certainty
Evidence certainty was assessed according to the Grading of Recommendations Assessment, Development and Evaluation (GRADE) framework [15]. Evidence certainty was ranked from very low to high. Within-study bias was assessed according to the Risk of Bias in Non-randomized Studies – of Interventions (ROBINS-I, 2016) assessment tool [16, 17]. Two authors (MU and IK) independently conducted the ROBINS-I assessments, and disagreements were resolved by reaching a mutual consensus. The risk of bias figure was created using the Robvis shiny app [18].
Statistical methods
Statistical analysis was performed using R statistical software (version 4.3.1; R Core Team, 2023; R Foundation for Statistical Computing, Vienna, Austria). A meta-analysis using a random intercept logistic regression model was conducted, and pooled outcome event rates and 95% confidence intervals (CIs) were calculated. The random effects model was used due to the expected high heterogeneity among the studies. Statistical heterogeneity was assessed by calculating I2 values. Inverse variance with logit transformation was used as a meta-analysis method. Odds ratios (ORs) and 95% CIs for outcome events between different medication regimens were calculated. The pooled outcome event rates were calculated using the ‘metaprop’ function from the ‘meta’ package version 5.1 − 1. Furthermore, to focus on outcome events 3 months after surgery, analysis was performed from studies reporting event rates for both 3 months and 1 year. The event rate for the time period from 3 months to 1 year was calculated by subtracting the 3-month event rate from the 1-year event rate. Publication bias was analysed by drawing funnel plots.
Protocol registration
This review has been registered with PROSPERO (ID: CRD42024503612) and can be accessed via https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=503612.
Results
Search results
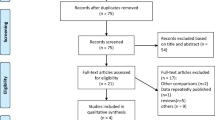
The literature search retrieved 572 abstracts. Since no eligible RCTs were identified in the initial search, the screening was expanded to cover suitable observational studies. After abstract screening, 49 full texts were assessed for eligibility, and eight observational studies were included (Fig. 1) [10, 19,20,21,22,23,24,25].
Study and patient characteristics
Five of the studies were conducted in Europe (two in Germany and one each in Denmark, France and Switzerland) and three in North America (two in Canada and one in the USA) (Table 1). The study periods expanded through a 35-year time span from 1982 to 2017. The number of patients in the medication groups varied from 548 to 2384, resulting in a total of 6727 patients. The mean ages were between 55 and 77 years, and the proportion of female patients was 17–59%. Three of the studies also included patients with a history of atrial fibrillation, with the proportion of these patients being 5–43%. In these studies, the outcome event rates did not show a prominent increase when compared to the other studies. Five studies reported the rate of concomitant coronary artery bypass grafting surgery, and the rate varied from 0% to 36%.
Risk of bias
The overall risk of bias was low in one, moderate in two, and serious in five of the included studies (Supplementary). Most issues were due to confounding factors and possible unrecognised deviations from the intended interventions.
Mortality
Overall mortality during the first year after surgery was assessed in all the included studies (N = 6727 patients). Overall pooled mortality was 4.3% (95% CI 2.7–6.8%; Fig. 2). The lowest mortality rate was observed in patients with anticoagulation (2.0%, 95% CI 0.4–9.7%) and anticoagulation combined with antiplatelet therapy (2.2%, 95% CI 0.9–5.5%), and the highest was in patients without antithrombotic medication (7.3%, 95% CI 3.6–14.2%). Statistical heterogeneity, as shown by I2 values, was high, except in patients with 3-month anticoagulation followed by antiplatelet therapy. According to the funnel plot, there was a suspicion of publication bias, although the bias was balanced (Supplementary). The odds ratios for mortality did not show unequivocal evidence for the superiority of antithrombotic medications compared to no medications (Table 2). In the assessment of mortality from 3 months after surgery, the mortality was lower in the anticoagulant patients (0.5%, 95% CI 0.1–2.6%) than in antiplatelet patients (3.0%, 95% CI 1.2–7.4%) and those without antithrombotics (3.5%, 95% CI 1.3–9.3%; Fig. 3). However, the ORs of anticoagulation for mortality compared to the no medication group suggested only a trend towards a mortality benefit of anticoagulation. Evidence certainty was ranked as very low due to heterogeneity, risk of bias, imprecision and the low number of included studies per medication strategy.
Stroke
The stroke rate at 1 year after surgery was assessed in three studies (N = 1455 patients). The overall pooled stroke rate was 3.1% (95% CI 2.3−6.5%). During the 3–12 months after surgery, the stroke rate was 1.3% (95% CI 0.8–2.1%), with only 15 observed strokes across the studies. The funnel plots did not suggest the existence of publication bias, and heterogeneity was low. However, CIs were wide, and therefore, eligible evidence of differences was not observed between the antithrombotic treatment strategies at either 0- to 12-month or 3- to 12-month follow-up. Evidence certainty was ranked as very low due to risk of bias, imprecision and the low number of included studies per medication strategy.
Bleeding
Bleeding rate was assessed in two of the included studies (N = 1399 patients). The overall pooled bleeding rate was 3.9% (95% CI 2.1–7.2%). There was, at most, a slight risk of publication bias, according to the funnel plots. At 0- to 12-month follow-up, all antithrombotic treatment regimens resulted in an increased bleeding rate compared to antithrombotic medication, with the highest bleeding rate identified in anticoagulation combined with antiplatelet patients. At 3- to 12-month follow-up, the findings were not as equivocal. The point estimates of antiplatelet and anticoagulation patients indicated an increased bleeding rate when compared to patients without medication, but the extremely wide CIs of ORs overlapped, indicating only a trending level difference between these groups. In patients with anticoagulation combined with antiplatelet therapy, there was up to an eight-fold increase in the bleeding rate when compared to patients with no antithrombotics, and this was also shown in the odds ratio (7.76, 95% CI 1.42–42.4). Evidence certainty was ranked as very low due to risk of bias, imprecision and the low number of included studies per medication strategy.
Discussion
In the current meta-analysis, patients on anticoagulation and on anticoagulation combined with antiplatelet therapy showed the lowest mortality rates, while those without antithrombotic medication faced the highest mortality. However, the odds ratios for mortality did not decisively favour any antithrombotic regimen over no medication. After the initial 3 months, anticoagulation suggested a trending level potential mortality benefit compared to antiplatelet use and no medication. However, wide CIs and overlap with the no-medication group imply a tentative rather than conclusive mortality advantage for anticoagulation.
The anticoagulation group consisted mainly of patients with warfarin (99.6%). In this group, mortality was lowest during the 3- to 12-month period. Unexpectedly, the evidence of differences in stroke rates between the groups was uncertain, and the early bleeding rate was substantially higher in the anticoagulation group than in patients without antithrombotic medication. This suggests that although the rates of serious complications related to blood clotting and antithrombotic medication were not lower in patients with anticoagulation, anticoagulation may have a direct beneficial effect on mortality in relation to other medication regimens. It has been reported that the most common causes of death during the first year after SAVR are endocarditis, sepsis, heart failure and sudden cardiac death [26,27,28]. Furthermore, it has been reported that even up to one-quarter of SAVR patients will have myocardial microinfarctions during the 6 months following surgery and that the risk for infarct seems even higher in patients without preoperative coronary artery disease [29]. The resulting subclinical myocardial injury has been shown to increase the risk of sudden cardiac death [30]. It has been proposed that microinfarctions may be caused by microemboli dislodged from the valve prosthesis [31]. The findings of this meta-analysis encourage further investigation of these hypotheses. In addition, data on the outcomes of direct factor Xa inhibitors as an anticoagulative agent are needed, since among patients with bioprosthetic mitral valve, the outcomes have been shown to be non-inferior to warfarin [32].
In contrast, the outcomes in the antiplatelet group, consisting mainly of patients using ASA (97.7%), were unexpectedly unfavourable, as no evident benefit on mortality or stroke rate was observed, even when compared to patients without antithrombotic medication. However, the early bleeding rate seemed to be even higher. This is, at least to some extent, contradictory to prospective studies reporting equal outcomes with ASA and warfarin at 3–6 months after SAVR [33,34,35]. In this meta-analysis, 53% of deaths during the first year after SAVR in the antiplatelet group occurred after the first 3 months, whereas in the anticoagulation group, the respective proportion was 27%, suggesting that after the early postoperative period, mortality remains higher without proper anticoagulation. These findings suggest that ASA as monotherapy may not be an advisable antithrombotic treatment strategy after SAVR using a biological prosthesis.
With regard to stroke rates, wide CIs precluded the observation of eligible evidence for differences between antithrombotic treatment strategies at 0–12 and 3–12 months after surgery. Surprisingly, the point estimate of the stroke rate was lowest in the no antithrombotic medication group, which may be seen as controversial in light of the intended effect of antithrombotic medications in preventing prosthesis valve thrombosis formation and thereby preventing stroke. The stroke rate in patients with no antithrombotic medication was derived from the results of a single study by Gryaznov et al. (2020), in which there was a 43% rate of atrial fibrillation history prior to surgery and thereby an indication for anticoagulation even before surgery [19]. The low stroke rate in patients without antithrombotic medication may reflect a lower risk of haemorrhagic stroke [36, 37]. When comparing the haemorrhagic stroke rates, which were reported by Gryaznov et al. (2020) separately from ischemic strokes, no significant differences were observed between the treatment groups, with a rate of less than 1% in all treatment groups [19]. Thus, there might have been other underlying comorbidities predisposing to stroke rather than the effect of antithrombotic medication falling short of expectations.
At 0- to 12-month follow-up, higher bleeding rates were observed in all antithrombotic regimens compared to those without medication, peaking in anticoagulation combined with antiplatelet therapy. At 3- to 12-month follow-up, the findings were less definitive, with trends of increased bleeding in the antiplatelet and anticoagulation groups, but the wide CIs suggested only a potential difference. However, patients on anticoagulation combined with antiplatelet therapy exhibited up to an eight-fold increase in bleeding rates compared to those without antithrombotics.
Overall, the evidence certainty was very low, which can be attributed to methodological factors and retrospective data. Along with uncontrolled confounding and implicit multidimensionality behind thromboembolic events after biological SAVR, the results of individual studies held a considerable risk of bias. These factors inevitably affected the results and their interpretation in this meta-analysis. With these considerations in mind, the future implications of this meta-analysis include the following. First, anticoagulation therapy using warfarin showed a tendency towards a benefit in mortality at 1 year after surgery when compared to antiplatelet therapy or no antithrombotic medication patients. Second, anticoagulation combined with antiplatelet therapy, antiplatelet therapy with ASA alone and no antithrombotic medication resulted in inferior overall outcomes. Therefore, according to this meta-analysis of observational studies, these antithrombotic medication strategies may not be advisable after SAVR using a biological prosthesis. Third, there seemed to be a higher rate of bleeding events among anticoagulant patients than among antiplatelet patients and no antithrombotic medication patients during the 0- to 12-month follow-up, but at the 3- to 12-month follow-up, the difference was not as clear. This suggests a higher bleeding rate in anticoagulation patients during the first 3 months after surgery, after which the rate seems to even out.
In summary, the results of this meta-analysis cautiously suggest that continuing with anticoagulative medication as the antithrombotic medication strategy may be beneficial 3 months after SAVR using a biological prosthesis. However, attributable to methodological factors, the very low certainty of evidence emphasises the need for cautious interpretation and underscores the imperative for more robust research using randomised data to clarify optimal antithrombotic strategies in this patient population. In addition, there is a lack of data on direct factor Xa inhibitors and P2Y12 inhibitors as antithrombotic medication strategies 3 months after surgery.
Limitations
The absence of RCT data limits the credibility of our findings, as this analysis relies solely on observational studies, including plausible selection bias and potential inaccuracies and deviations from the reported antithrombotic medication regimens. Additionally, the lack or scarcity of information regarding concomitant procedures and comorbidities (especially cerebrovascular disease, separate rates of ischemic and haemorrhagic strokes and arrhythmias, such as atrial fibrillation) and follow-up data on medication usage in the included studies increases uncertainty and adds evidently uncontrolled confounding to our results. Moreover, the variation in prosthesis models and their haematogenic properties among the studies introduces potential heterogeneity. Patient groups, such as paediatric and pregnant patients as well as renal insufficiency patients requiring dialysis, were excluded to avoid special considerations related to antithrombotic treatment in these patient groups. In addition, we did not include TAVI patients; therefore, our results are not generalisable in these patients. These limitations underscore the need for cautious interpretation and highlight areas for future research to enhance our understanding of optimal antithrombotic management in this patient population. A well-designed and appropriately powered RCT is warranted.
Conclusion
In conclusion, despite revealing a tendency towards the benefit of anticoagulation therapy in terms of mortality at 1 year after biological SAVR and suggesting a potential advantage in continuing anticoagulation beyond 3 months, this meta-analysis is limited by very low evidence certainty. The imperative for cautious interpretation and the urgent need for more robust randomised research underscore the complexity of determining optimal antithrombotic strategies in this patient population.
Availability of data and materials
The data supporting the results of our analysis is provided within the manuscript in Table 1.
Abbreviations
- AHA:
-
American Heart Association
- ASA:
-
acetylsalicylic acid
- CI:
-
Confidence interval
- ESC:
-
European Society of Cardiology
- GRADE:
-
Grading of Recommendations Assessment, Development and Evaluation framework
- MOOSE:
-
Meta-analysis of Observational Studies in Epidemiology checklist
- OR:
-
Odds ratio
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-Analysis guidelines
- RCT:
-
Randomised controlled trial
- ROBINS-I:
-
The Risk of Bias in Non-randomized Studies – of Interventions assessment tool
- SAVR:
-
surgical aortic valve replacement
- TAVI:
-
transcatheter aortic valve implantation
References
Paparella D, Yau T, Young E. Cardiopulmonary bypass induced inflammation: pathophysiology and treatment. An update. European journal of cardio-thoracic surgery. Eur J Cardiothorac Surg. 2002;21(2):232–44.
Bronicki RA, Hall M. Cardiopulmonary bypass-induced inflammatory response: pathophysiology and treatment. Pediatr Crit Care Med. 2016;17(8):S272–8.
Wan S, LeClerc J-L, Vincent J-L. Inflammatory response to cardiopulmonary bypass: mechanisms involved and possible therapeutic strategies. Chest. 1997;112(3):676–92.
Nielsen VG, Asmis LM. Hypercoagulability in the perioperative period. Best Pract Res Clin Anaesthesiol. 2010;24(1):133–44.
Rafiq S, Johansson PI, Ostrowski SR, Stissing T, Steinbrüchel DA. Hypercoagulability in patients undergoing coronary artery bypass grafting: prevalence, patient characteristics and postoperative outcome. Eur J Cardiothorac Surg. 2012;41(3):550–5.
Eybl E, Grimm M, Grabenwöger M, Böck P, Müller MM, Wolner E. Endothelial cell lining of bioprosthetic heart valve materials. J Thorac Cardiovasc Surg. 1992;104(3):763–9.
Fischlein T, Lehner G, Lante W, Fittkau M, Murphy J, Weinhold C, et al. Endothelialization of cardiac valve bioprostheses. Int J Artif Organs. 1994;17(6):345–52.
Vahanian A, Beyersdorf F, Praz F, Milojevic M, Baldus S, Bauersachs J, et al. 2021 ESC/EACTS guidelines for the management of valvular heart disease: developed by the Task Force for the Management of Valvular Heart Disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2022;43(7):561–632.
Otto CM, Nishimura RA, Bonow RO, Carabello BA, Erwin JP III, Gentile F, et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2021;77(4):450–500.
Brueck M, Kramer W, Vogt P, Steinert N, Roth P, Görlach G, et al. Antiplatelet therapy early after bioprosthetic aortic valve replacement is unnecessary in patients without thromboembolic risk factors. Eur J Cardiothorac Surg. 2007;32(1):108–12.
Moinuddeen K, Quin J, Shaw R, Dewar M, Tellides G, Kopf G, et al. Anticoagulation is unnecessary after biological aortic valve replacement. Circulation. 1998;98(19 Suppl):II95-8 discussion II8.
Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1–9.
Cumpston M, Li T, Page MJ, Chandler J, Welch VA, Higgins JP, et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev. 2019;2019(10):ED000142.
Brooke BS, Schwartz TA, Pawlik TM. MOOSE reporting guidelines for meta-analyses of observational studies. JAMA Surg. 2021;156(8):787–8.
Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction – GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64(4):383–94.
Sterne JA, Hernán MA, McAleenan A, Reeves BC, Higgins JP. Assessing risk of bias in a non‐randomized study. Cochrane handbook for systematic reviews of interventions. 2019:621–41.
Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919.
McGuinness LA, Higgins JPT. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Res Synthesis Methods. 2021;12(1):55–61.
Gryaznov AA, Saeyeldin A, Abdelbaky M, Zafar MA, Tanweer M, Papanikolaou D, et al. Nonusefulness of antithrombotic therapy after surgical bioprosthetic aortic valve replacement. Am J Cardiol. 2020;129:71–8.
Owais T, Rouman M, Breuer M, Hüter L, Fuchs J, Lauer B, et al. Anticoagulation after biological aortic valve replacement: is there an optimal regimen. J Heart Valve Dis. 2016;25(2):139–44.
Mérie C, Køber L, Olsen PS, Andersson C, Gislason G, Jensen JS, et al. Association of warfarin therapy duration after bioprosthetic aortic valve replacement with risk of mortality, thromboembolic complications, and bleeding. JAMA. 2012;308(20):2118–25.
Al-Atassi T, Lam K, Forgie M, Boodhwani M, Rubens F, Hendry P, et al. Cerebral microembolization after bioprosthetic aortic valve replacement: comparison of warfarin plus aspirin versus aspirin only. Circulation. 2012;126(11_suppl_1):S239–44.
Weber A, Noureddine H, Englberger L, Dick F, Gahl B, Aymard T, et al. Ten-year comparison of pericardial tissue valves versus mechanical prostheses for aortic valve replacement in patients younger than 60 years of age. J Thoracic Cardiovasc Surg. 2012;144(5):1075–83.
Ninet J, Tronc F, Robin J, Curtil A, Aleksic I, Champsaur G. Mechanical versus biological isolated aortic valvular replacement after the age of 70: equivalent long-term results. Eur J Cardiothorac Surg. 1998;13(1):84–9.
Alex S, Hiebert B, Arora R, Menkis A, Shah P. Survival and long-term outcomes of aortic valve replacement in patients aged 55 to 65 years. Thoracic Cardiovasc Surg. 2018;66(04):313–21.
Amrane H, Deeb GM, Popma JJ, Yakubov SJ, Gleason TG, Van Mieghem NM, et al. Causes of death in intermediate-risk patients: the randomized surgical replacement and transcatheter aortic valve implantation trial. J Thoracic Cardiovasc Surg. 2019;158(3):718-28.e3.
Gaudiani V, Deeb GM, Popma JJ, Adams DH, Gleason TG, Conte JV, et al. Causes of death from the randomized CoreValve US pivotal high-risk trial. J Thoracic Cardiovasc Surg. 2017;153(6):1293-301.e1.
Urena M, Webb JG, Eltchaninoff H, Muñoz-García AJ, Bouleti C, Tamburino C, et al. Late cardiac death in patients undergoing transcatheter aortic valve replacement: incidence and predictors of advanced heart failure and sudden cardiac death. J Am Coll Cardiol. 2015;65(5):437–48.
Dobson LE, Musa TA, Uddin A, Fairbairn TA, Swoboda PP, Ripley DP, et al. Post-procedural myocardial infarction following surgical aortic valve replacement and transcatheter aortic valve implantation. EuroIntervention. 2017;13(02):e153–60.
O’Neal WT, Shah AJ, Efird JT, Rautaharju PM, Soliman EZ. Subclinical myocardial injury identified by cardiac infarction/injury score and the risk of mortality in men and women free of cardiovascular disease. Am J Cardiol. 2014;114(7):1018–23.
Levis JT, Schultz G, Lee PC. Acute myocardial infarction due to coronary artery embolism in a patient with a tissue aortic valve replacement. Permanente J. 2011;15(3):82.
Guimarães HP, Lopes RD, De Barros e Silva PG, Liporace IL, Sampaio RO, Tarasoutchi, et al. Rivaroxaban in patients with atrial fibrillation and a bioprosthetic mitral valve. N Eng J Med. 2020;383(22):2117–26.
Rafiq S, Steinbrüchel DA, Lilleør NB, Møller CH, Lund JT, Thiis JJ, et al. Antithrombotic therapy after bioprosthetic aortic valve implantation: warfarin versus aspirin, a randomized controlled trial. Thrombosis Res. 2017;150:104–10.
Gherli T, Colli A, Fragnito C, Nicolini F, Borrello B, Saccani S, et al. Comparing warfarin with aspirin after biological aortic valve replacement: a prospective study. Circulation. 2004;110(5):496–500.
Colli A, Mestres CA, Castella M, Gherli T. Comparing warfarin to aspirin (WoA) after aortic valve replacement with the St. Jude Medical Epic™ heart valve bioprosthesis: results of the WoA Epic pilot trial. J Heart Valve Dis. 2007;16(6):667.
Fitzmaurice DA, Blann AD, Lip GY. Bleeding risks of antithrombotic therapy. BMJ. 2002;325(7368):828–31.
Delaney JA, Opatrny L, Brophy JM, Suissa S. Drug–drug interactions between antithrombotic medications and the risk of gastrointestinal bleeding. CMAJ. 2007;177(4):347–51.
Acknowledgements
None.
Funding
Open access funding provided by Tampere University (including Tampere University Hospital).
Author information
Authors and Affiliations
Contributions
MU: conceptualisation, data curation, formal analysis, investigation, methodology, project administration, software, visualisation and writing – original draft; IK: data curation, writing – review and editing; VP: supervision, methodology, validation, writing – review and editing; AM: validation, clinical consulting, writing – review and editing; MM: validation, clinical consulting, writing – review and editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
13019_2024_2863_MOESM1_ESM.docx
Additional file 1: Supplementary 1.Risk of bias assessment for seven domains and overall for the included studies according to the ROBINS-I (2016) tool. Supplementary 2. Funnel plots showing possible publication bias by antithrombotic treatment groups 0–12 months after surgery. A: mortality, B: strokes, C: bleeding events. Supplementary 3. Funnel plots showing possible publication bias by antithrombotic treatment groups 3–12 months after surgery. A: mortality, B: strokes, C: bleeding events. Supplementary 4. Mortality rates in antithrombotic treatment groups 0–12 months after surgery for each included study. CI = confidence interval. Supplementary 5. Mortality rates in antithrombotic treatment groups 3–12 months after surgery for each included study. CI = confidence interval. Supplementary 6. Stroke rates in antithrombotic treatment groups 0–12 months after surgery for each included study. CI = confidence interval. Supplementary 7. Stroke rates in antithrombotic treatment groups 3–12 months after surgery for each included study. CI = confidence interval. Supplementary 8. Bleeding rates in antithrombotic treatment groups 0–12 months after surgery for each included study. CI = confidence interval. Supplementary 9. Bleeding rates in antithrombotic treatment groups 3–12 months after surgery for each included study. CI = confidence interval.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Uimonen, M., Kuitunen, I., Ponkilainen, V. et al. Antithrombotic management after aortic valve replacement with biological prosthesis: a meta-analysis. J Cardiothorac Surg 19, 385 (2024). https://doi.org/10.1186/s13019-024-02863-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13019-024-02863-z