Abstract
Background
Splenic abscess is a serious complication associated with infective endocarditis. There is still contradicting evidence regarding the optimal treatment pathway including timing of valve intervention and the approach for managing splenic foci.
Case presentation
We present a case of a hybrid staged approach in which we successfully performed a laparoscopic splenectomy following percutaneous abscess drainage and a delayed aortic valve replacement.
Conclusions
A multidisciplinary teamwork is fundamental in providing optimal care for patients with distant complications associated with infective endocarditis. Our hybrid approach seems safe and feasible.
Similar content being viewed by others
Background
Systemic embolism is a relatively frequent complication of infective endocarditis (IE). It has been estimated that 1 out of 4 patients with IE presents with some degree of systemic embolism, the most common sites being the central nervous system (CNS) and the spleen [1,2,3] However, splenic abscess formation is uncommon, presenting in up to 1% of all cases of IE [1].
The optimal treatment strategy for patients with aortic valve endocarditis with multiple systemic septic embolisms is still a matter of debate as balance between periprocedural risk and removal of septic foci must be obtained to ensure adequate outcomes [1]. Only a few cases or case series describing management of splenic abscess secondary to septic embolism have been published so far [4,5,6,7,8,9,10,11,12,13,14], with varying approaches and outcomes. We present a focused literature review based on a successful case of a multi-stage, hybrid approach for treating a patient with multiple systemic complications including a large splenic abscess secondary to native Aortic Valve Endocarditis.
Case presentation
A 52-year-old male patient was referred to our institution with a diagnosis of a methicillin-susceptible Staphylococcus aureus native aortic valve endocarditis. He had a history of a recent posterior ischemic stroke with hemorrhagic transformation 2 weeks earlier with visual impairment, no motor deficit, and an initial Glasgow scale at 15/15. Upon admission he was in a fair general condition, tachycardic, febrile, without leukocytosis. A grade III diastolic murmur was detected.
Initial transthoracic echocardiogram showed a bicuspid aortic valve and the presence of a pedunculated vegetation which caused a severe aortic stenosis with moderate regurgitation. The left ventricle was mildly dilated with a preserved systolic function (Fig. 1a).
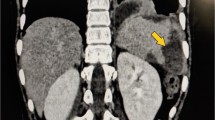
Imaging studies. (a) Transthoracic echocardiogram, showing a large vegetation of the aortic valve (red arrow). (b) Transverse view of brain MRI, showing late subacute infarction with small left occipital hemorrhagic transformation (red arrow). (c) Coronal view of abdominal CT, showing a large unilocular splenic abscess (red arrow)
The patient was started on IV Daptomycin guided by blood cultures.
Due to valvular compromise, a surgical aortic valve replacement was considered, however the procedure was initially deferred given the evidence of recent CNS bleeding on admission MRI (Fig. 1b).
Six days after admission, he presented with new onset fever despite receiving adequate targeted antibiotic treatment, new blood cultures and imaging studies were obtained. Brain angiogram showed no alterations. An abdominal CT revealed a large splenic abscess (Fig. 1c). After multidisciplinary consultation, valve surgery was further deferred aiming to achieve abdominal foci control. Given the radiological characteristics of the abscess, a high surgical risk was considered so a staged hybrid approach was decided. An initial percutaneous drainage of purulent fluid was performed aiming to reduce the size of the abscess and therefore reducing the chances of rupture and abdominal cavity contamination during a subsequent surgical intervention. The procedure was followed by a successful laparoscopic splenectomy three days later, finding an enlarged spleen with necrotic tissue and scarce purulent material. Cultures of the splenic abscess fluid were negative. After guaranteeing an adequate postoperative course, he was taken to a mechanical aortic valve replacement. Intraoperatively, a bicuspid aortic valve (fusion type with Noncoronary-right phenotype) was found with calcifications of the free edges and vegetations on the aortic side of the leaflets. An abscess of the aortic annulus which was extending from the left-right commissure towards the non-coronary-right commissure was found along with a small associated pseudo aneurysm at this level (Fig. 2a). After native valve removal and annulus debridement and decalcification, the abscess was drained. The annulus was sterilized with iodine-based solution and the small remaining cavity was sealed with the pledgeted sutures anchoring the prosthetic valve (Fig. 2b). He required a delayed sternal closure due to operative bleeding secondary to coagulopathy. During immediate postoperative care he required a pericardiocentesis due to cardiac tamponade and he received an implantation of a cardiac resynchronization therapy with a pacemaker (CRT-P) due postoperative grade III AV block. The rest of the postoperative course was uneventful. He was discharged with long term intravenous antibiotics. The patient has continued with strict follow-up, where he has shown no signs of relapse or complications after two-month follow-up. He received standard immunizations for post splenectomy patients which included pneumococcal and meningococcal vaccination along with seasonal influenza.
Schematic representation of intraoperative findings and procedure
(Source – Author AFJO)
(a) A calcified bicuspid valve was found with a large vegetation and an abscess extending through the aortic annulus. (b) Valve implantation technique. The abscess cavity was sealed with pledgeted sutures anchoring the prosthetic valve
Discussion
Systemic septic embolism is a relatively frequent complication of patients with infective endocarditis (IE), with an incidence ranging from 6 to up to 40% of cases [2, 3, 15], and it is a cause of significant morbidity and a mortality rate exceeding 20% [1, 6, 15, 16]. Some studies have reported an incidence of splenic abscess (SA) in this context around 1–8% [6, 10, 17]. Vegetations larger than 20 mm and a preoperative white blood cell count > 12.000 have been identified as risk factors for splenic infarction [1]. According to some studies, less than 5% of these cases will develop a SA, mainly associated with persistent bacteremia [1, 6, 17].
Persistent fever or bacteremia despite adequate antimicrobial therapy has been identified as one of the most common clinical manifestations of SA as other symptoms are nonspecific and infrequent [1, 6]. Therefore, an adequate, timely diagnosis requires a low threshold for imaging screening of septic complications in patients under management of IE. In our case a contrast-enhanced abdominal CT provided useful information allowing targeted decision-making. It has been shown that its sensitivity and specificity range around 90–95% for distinguishing splenic infarction from SA [6, 11, 17] and it has been proposed as the ideal screening study for patients with the previously mentioned high-risk features [15]. Ultrasound, MRI, or PET have also been reported in literature as an adjuvant for adequate diagnosis and surgical planning [1, 13, 18, 19].
Guidelines for treating patients with SA secondary to IE don’t specifically address the optimal management that these patients should receive as there is insufficient high-quality evidence [1]. According to available literature and from our experience, the main factors that must be considered include:
-
1.
Timing of Aortic Valve surgery as compared to splenic interventions.
-
2.
Choosing an adequate approach for controlling the splenic inoculum.
-
3.
Choosing optimal medical therapy.
We provide a proposal of a management pathway for patients with IE and SA (Fig. 3). Several approaches have been proposed regarding timing of valvular and splenic intervention [5,6,7,8, 12, 16, 20,21,22,23]. Table 1 provides a focused summary on the different management pathways that have been used in reports so far.
Timing of aortic valve surgery
There is still controversy around the optimal timing of valve surgery and high-quality studies are lacking [14]. Two factors must be balanced to make adequate clinical decisions: the risk of hemodynamic compromise secondary to valvular damage on one hand, and the risk of prosthetic valve infection if a valvular replacement is undertaken while the patient has active septic foci despite optimal medical therapy (OMT) [6, 16].
Double intervention targeting both the infected valve and the spleen has been reported and it is not a new approach [16, 27] however it is most commonly believed that this operation may represent a significant physiological stress that may put patients at risk [6, 15, 17] so staging the interventions, performing valve surgery after resolution of abdominal foci has been obtained is often preferred [4, 11, 13, 16, 28] although there is contradicting evidence around this topic [7, 8].
This case had a recent embolic stroke with hemorrhagic transformation as a particular complication for deciding the optimal timing for cardiac surgery. Current guidelines recommend deferring surgery for > 1 month after hemorrhagic central nervous system (CNS) events (class IIa) however surgery can be prioritized if a persistent infection, high embolic risk or hemodynamic instability are determined (class IIa) [1]. Surgery was decided after neurology and neurosurgery consultation and CNS imaging. AVR was performed at least 1 month after the detection of the hemorrhagic transformation which occurred before referral to our institution.
Choosing an adequate approach for controlling the splenic inoculum
Complete treatment for SA should be actively seeked as this reduces the risk of valvular prosthesis infection. Performing a splenectomy is not mandatory. Some reports have shown satisfactory results with a conservative treatment consisting of a long intravenous + oral antibiotic regimen [7, 10, 23,24,25,26] or in combination with percutaneous drainage [21]; however, the multidisciplinary group at our institution decided to minimize the risk of complications after aortic valve intervention by performing a full splenectomy as it has been associated with higher survival rates [4, 11, 17, 29, 30].
Percutaneous drainage is a safe approach with minimal morbidity [8, 21]. No clear indications for this procedure have been established so far, however it should be considered in cases of solitary, unilocular SA larger than 3 cm [21].
Both the laparoscopic and open splenectomy have proven effective in treating this group of patients [20, 23, 28]. Our report provides insight on a hybrid, multi-stage approach in which, due to the volume of the SA, we decided to perform a preoperative percutaneous drainage of the SA to minimize blood loss, risk of cavity contamination, SA rupture and overall surgical risk while improving the chance of a successful laparoscopic splenectomy [28] for definitive removal of the infection. In this setting, a minimally invasive laparoscopic approach for the SA has the advantage of allowing a faster recovery for a subsequent valvular intervention [12, 23].
Performing safe laparoscopic surgery in the setting of severe aortic stenosis might be challenging and therefore it is suggested that the anesthesiologists are familiar with intraoperative management of this group of patients.
Choosing optimal medical therapy
This is the cornerstone for IE management. Specific guidelines on choosing and adequate medical therapy have recently been published and are beyond the scope of this manuscript [1, 4, 26]. A management algorithm has been recently published by the ESC regarding medical therapy according to the microbiological profile and choosing an adequate timing of surgery accordingly [1].
Conclusions
Treating patients with splenic septic embolism in the setting of IE is still a clinical and surgical challenge. A wide range of interventions is available and should be chosen on a case-by-case basis. This requires articulation of a multidisciplinary team integrating adequate medical therapy, a timely splenic intervention and delaying aortic valve interventions as much as possible according the hemodynamic and inflammatory state of the patient while allowing adequate control in extracardiac infective foci. Our multi-stage hybrid approach involving optimal antimicrobial therapy, percutaneous drainage, a laparoscopic splenectomy and a delayed AVR guaranteeing an improved clinical condition seems to be feasible and safe.
The current report is aimed at helping guide clinical decision-making by providing a framework for choosing an appropriate treatment pathway. Given the difficulty of implementing large randomized clinical trials in this kind of settings, we believe that clinicians should aim at standardizing and recording multidisciplinary institutional protocols for complex IE cases presenting with systemic embolic complications. Timely report of experiences can improve patient outcomes by providing a strong body of evidence allowing higher-quality information to be developed.
Data availability
No datasets were generated or analysed during the current study.
Abbreviations
- AVR:
-
Aortic valve replacement
- CT:
-
Computed tomography
- CRT:
-
P-cardiac resynchronization therapy with a pacemaker
- IE:
-
Infective endocarditis
- MRI:
-
Magnetic resonance imaging
- PET:
-
Positron emission tomography
- OMT:
-
Optimal medical therapy
- SA:
-
Splenic abscess
References
Delgado V, Ajmone Marsan N, de Waha S, Bonaros N, Brida M, Burri H et al. 2023 ESC Guidelines for the management of endocarditis. Eur Heart J [Internet]. 2023;44(39):3948–4042. https://academic.oup.com/eurheartj/article/44/39/3948/7243107
Habib G, Erba PA, Iung B, Donal E, Cosyns B, Laroche C, et al. Clinical presentation, aetiology and outcome of infective endocarditis. Results of the ESC-EORP EURO-ENDO (European infective endocarditis) registry: a prospective cohort study. Eur Heart J. 2019;40(39):3222–32.
Kildahl HA, Brenne EL, Dalen H, Wahba A. Systemic embolization in infective endocarditis. Indian Journal of Thoracic and Cardiovascular Surgery. Springer; 2023.
Del Perez G, Bakhati B, Ronen JA, Garcia Fernandez A. Double Valve Infective Endocarditis Complicated by Systemic Arterial Embolization. Cureus. 2021.
Magilligan D Jr, Quinn E. The Role of Splenectomy in Endocarditis [Internet]. Vol. 31, Hospital Medical Journal Henry Ford Hospital Medical Journal. 1983. https://scholarlycommons.henryford.com/hfhmedjournal. Available at: https://scholarlycommons.henryford.com/hfhmedjournal/vol31/iss1/11
Alnasser SA, Mindru C, Preventza O, Rosengart T, Cornwell L. Successful conservative management of a large splenic abscess secondary to infective endocarditis. Ann Thorac Surg. 2019;107(4):e235–7.
Akhyari P, Mehrabi A, Adhiwana A, Kamiya H, Nimptsch K, Minol JP, et al. Is simultaneous splenectomy an additive risk factor in surgical treatment for active endocarditis? Langenbecks Arch Surg. 2012;397(8):1261–6.
Saijo F, Funatsu T, Yokoyama J, Ryomoto M, Hayashi K. Percutaneous drainage and staged valve replacement followed by laparoscopic splenectomy in infective endocarditis with splenic abscess. Gen Thorac Cardiovasc Surg. 2022;70(3):285–8.
Wang CC, Lee CH, Chan CY, Chen HW. Splenic infarction and abscess complicating infective endocarditis. Am J Emerg Med. 2009;27(8):1021.e3-1021.e5.
Ulloa N, Cook JM, Smithson S. A Clinical Challenge in the Emergency Department: A Case of Klebsiella Infective Endocarditis Presenting With Splenic Abscess. Cureus. 2020.
Blasi S, De Martino A, Levantino M, Pratali S, Anastasio G, Bortolotti U. Splenectomy and valve replacement in patients with infective endocarditis and splenic abscesses. Ann Thorac Surg. 2016;102(3):e253–5.
Splenic Abscess Complicating Infective Endocarditis. - Three Case Reports.
Elasfar A, AlBaradai A, AlHarfi Z, Alassal M, Ghoneim A, AlGhofaili F. Splenic abscess associated with infective endocarditis; Case series. J Saudi Heart Assoc. 2015;27(3):210–5.
Hasan LZ, Shrestha NK, Dang V, Unai S, Pettersson G, El-Hayek K et al. Surgical infective endocarditis and concurrent splenic abscess requiring splenectomy: a case series and review of the literature. Diagn Microbiol Infect Dis. 2020;97(4).
Aalaei-Andabili SH, Martin T, Hess P, Hoh B, Anderson M, Klodell CT, et al. Management of septic emboli in patients with infectious endocarditis. J Card Surg. 2017;32(5):274–80.
Yoshikai M, Kamachi M, Kobayashi K, Murayama J, Kamohara K, Minematsu N. Splenic Abscess associated With Active Infective Endocarditis.
Naito R, Mitani H, Ishiwata S, Yamaguchi T, Tanaka K, Naruse Y et al. Infective endocarditis complicated with splenic abscess successfully treated with splenectomy followed by double valve replacement. J Cardiol Cases. 2010;2(1).
Groga-Bada P, Mueller II, Foschi F, Gawaz M, Eick C. Mitral Valve Endocarditis due to Lactobacillus. Case Rep Med. 2018;2018.
McOwat L, Fai Wong K, Varughese GI, Nayak AU. Splenic abscess as a potential initial manifestation of quiescent infective endocarditis in a patient with bronchopneumonia.
Simsir SA, Cheeseman SH, Lancey RA, Vander Salm TJ, Gammie JS. Staged laparoscopic splenectomy and valve replacement in splenic abscess and infective endocarditis. Ann Thorac Surg. 2003;75(5):1635–7.
Tsurui T, Lefor T, Nishida A. K. Solitary 15 cm splenic abscess successfully treated with percutaneous drainage. IDCases. 2022;27.
Jolobe OMP, Melnick SC, Sa FCP, Uk MRCP, Phil D. Splenic abscess: successful non-surgical therapy. 59, Postgrad Med J. 1983.
Carbonell AM, Kercher KW, Matthews BD, Joels CS, Sing RF, Todd Heniford B, et al. Laparoscopic splenectomy for splenic abscess. Volume 14. Surg Laparosc Endosc Percutan Tech; 2004.
Park SH, Choi YS, Choi YJ, Cho SH, Yoon HJ. Brucella endocarditis with splenic abscess: a report of the first case diagnosed in Korea. Yonsei Med J. 2009;50(1):142–6.
Nallarajah J, Mujahieth MI. Bacillus cereus Subacute native valve infective endocarditis and its multiple complications. Case Rep Cardiol. 2020;2020:1–4.
Yilmaz MB, Kisacik HL. Persisting fever in a patient with brucella endocarditis: occult splenic abscess [Internet]. Vol. 89, Heart. 2003. http://www.heartjnl.com/cgi/content/full/89/7/e20
McCready RA, Bryant MA, Fehrenbacher JW, Rowe MG. Infected splenic artery aneurysm with associated splenic abscess formation secondary to bacterial endocarditis: case report and review of the literature. J Vasc Surg. 2007;45(5):1066–8.
Lindsey ME, Holley CT, Ramaswamy A, Kelly RF. Infective endocarditis, embolic stroke and splenic abscess: a case report. J Surg Case Rep. 2017;2017(9).
Empyema. and splenic abscess in infective endocarditis.
Young S, Davee T, Fierer J, Morey M. Streptococcus sanguis 11 (Viridans) Prosthetic Valve Endocarditis With Myocardial, Splenic and Cerebral Abscesses. 1987.
Acknowledgements
The multidisciplinary team at Fundación Cardioinfantil-LaCardio (Bogota, Colombia), striving for excellence in patient-centered care.
Funding
The authors received no funding for the generation of the current manuscript.
Author information
Authors and Affiliations
Contributions
NNO, JSL, AFJ: Conceptualization, data gathering, manuscript writing, figures and table preparation, original draft preparation, reviewing and approval of final version. CV, AG, AJP, CFR, PC, JC Supervision, reviewing and approval of final version.
Corresponding author
Ethics declarations
Ethics approval
The current manuscript was reviewed and approved by the institutional Ethics in Research Committee at Fundación Cardioinfantil/LaCardio, resolution number 045-2023.
Consent for publication
Patient consent was obtained prior to publication and will be available upon request.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Nunez-Ordonez, N., Luna, J.S., Mackenzie, J.C. et al. Management of embolic splenic abscess secondary to aortic valve endocarditis – case report and review of literature. J Cardiothorac Surg 19, 220 (2024). https://doi.org/10.1186/s13019-024-02727-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13019-024-02727-6