Abstract
Background
Acute Type A aortic dissection (ATAAD) is a life-threatening cardiovascular disease associated with high mortality rates, where surgical intervention remains the primary life-saving treatment. However, the mortality rate for ATAAD operations continues to be alarmingly high. To address this critical issue, our study aimed to assess the correlation between preoperative laboratory examination, clinical imaging data, and postoperative mortality in ATAAD patients. Additionally, we sought to establish a reliable prediction model for evaluating the risk of postoperative death.
Methods
In this study, a total of 384 patients with acute type A aortic dissection (ATAAD) who were admitted to the emergency department for surgical treatment were included. Based on preoperative laboratory examination and clinical imaging data of ATAAD patients, logistic analysis was used to obtain independent risk factors for postoperative in-hospital death. The survival prediction model was based on cox regression analysis and displayed as a nomogram.
Results
Logistic analysis identified several independent risk factors for postoperative in-hospital death, including Marfan syndrome, previous cardiac surgery history, previous renal dialysis history, direct bilirubin, serum phosphorus, D-dimer, white blood cell, multiple aortic ruptures and age. A survival prediction model based on cox regression analysis was established and presented as a nomogram. The model exhibited good discrimination and significantly improved the prediction of death risk in ATAAD patients.
Conclusions
In this study, we developed a novel survival prediction model for acute type A aortic dissection based on preoperative clinical features. The model demonstrated good discriminatory power and improved accuracy in predicting the risk of death in ATAAD patients undergoing open surgery.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Backgroud
Acute type A aortic dissection (ATAAD) is a severe cardiovascular condition characterized by a tear in the inner lining of the aorta, posing a life-threatening risk to patients. Surgical intervention is the primary life-saving treatment approach for ATAAD. However, During the last decade, in-hospital mortality was reported to be 22% according to contemporary studies [1,2,3].
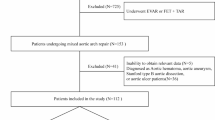
Despite advancements in medical care, several challenges contribute to the elevated mortality rate in ATAAD cases. These challenges include difficulties in timely surgical screening [4], delayed diagnosis, limited access to specialized care, and the intricate nature of surgical procedures. Consequently, there is a pressing need to identify key prognostic factors that are linked to these challenges and develop targeted interventions to address them effectively. Current prognostic studies on mortality in ATAAD patients have demonstrated suboptimal performance and significant variability [5]. This highlights the necessity for significant efforts to enhance the utilization of predictive models and investigations into prognostic factors for this patient population. The flowchart is shown in Fig. 1.
In light of the aforementioned issues, our objective was to conduct a comprehensive analysis incorporating preoperative clinical data, serum markers, and imaging studies [6]. By taking this multifaceted approach, we aimed to identify independent risk factors associated with mortality in hospitalized ATAAD patients. The ultimate goal was to develop a reliable nomogram that can accurately predict survival outcomes in ATAAD patients.
By employing this predictive model, surgeons can significantly improve their ability to assess the risk of early postoperative mortality in patients undergoing acute type A surgery. This, in turn, will aid in formulating effective surgical strategies to enhance patient survival and overall outcomes.
Methods
Patients
This study received approval from the ethical committees of Nanjing Drum Tower Hospital (No.2022–157-01). It was not feasible to obtain informed consent from all patients due to the nature of the study. However, since this study posed no risk to the patients involved, the institutional ethics committee waived the requirement for informed consent. The study adhered strictly to the Declaration of Helsinki (seventh revision, 2013) and was conducted under the supervision of the ethics committee. After obtaining approval from the ethical committees, a review was conducted using hospital medical records, nursing records, laboratory data, and surgical databases. This retrospective study enrolled patients diagnosed with acute aortic dissection (AAD) who underwent open surgery at Nanjing Drum Tower Hospital between March 2019 and March 2022. A total of 384 patients were included in our study. The initial data was screened using exclusion criteria, which consisted of the following: (1) patients diagnosed with type B dissection, (2) patients who did not undergo computed tomography angiography (CTA) before operation, (3) patients undergoing reoperation for recurrent aortic dissection, (4) patients who did not undergo surgical treatment and opted for conservative management or declined treatment,(5) patients died before surgery, (6) patients with a significant proportion of missing clinical characteristic information.
Surgical techniques
After the completion of anesthesia, the thoracic cavity is accessed through a median sternotomy. The axillary artery is then freed, and extracorporeal circulation is established through either the axillary artery or femoral artery, along with the superior and inferior vena cava or unicaval vein. Cardiac arrest is induced. The surgeon examines the aortic root and valves to determine the subsequent aortic root surgery. In our center, we employ the "double vest wrap" technique for root reconstruction surgery [7].
The procedure for aortic root reconstruction is as follows: Firstly, the thrombus in the remaining aortic root dissection is completely removed. A polyester sheet is then cut to match the shape of the dissection and placed between the aortic media and adventitia. Subsequently, a strip-shaped lining, consisting of a Dacron sheet, is positioned on the aortic intima surface. The tape-shaped Dacron sheet, the aortic media, the Dacron sheet within the dissection, and the adventitia are continuously sutured using 5–0 polypropylene sutures to form a new proximal aorta. Next, the avulsed aortic valve is reattached to the wall of the aortic sinus. Finally, the aortic medial layer, the Dacron sheet within the dissection, and the adventitia are reinforced using interrupted sutures of polypropylene sutures along the proximal edge of the dissection.
In patients without root involvement and aortic insufficiency, ascending aortic replacement is typically performed. However, for patients with a dilated aortic arch (≥ 45 mm), a tear located in the aortic arch, or damage to the aortic arch structure, total arch replacement with a frozen elephant trunk technique is generally employed. In cases where the aortic arch is damaged, the options include hemiarch replacement or fenestrated arch stenting [8, 9].
Data collection
Patient laboratory tests, clinical features, and surgical-related information were extracted from our Hospital Information System (HIS). Follow-up data is collected through telephone interviews. The imaging information was obtained from the hospital's imaging system, and the extraction of CTA information was performed by two experienced radiologists. Complete blood count, comprehensive biochemical profile, coagulation function, cardiac enzymes, B-type natriuretic peptide (BNP), and other relevant indicators are obtained through urgent investigations after emergency admission.
The Inflammation Index was calculated using the following formula:
Statistical analysis
Continuous variables were generally described as mean ± SD or median with interquartile ranges (IQR), while discrete variables were expressed as frequencies (n, %). The student t-test was utilized for normally distributed continuous variables, while the Mann–Whitney U nonparametric method was employed for non-normally distributed continuous variables. Categorical data were compared using either the chi-square test or Fisher exact test. Receiver operating characteristic (ROC) curves and Youden indices were used to assess predictive values and cutoff points. Logistic regression and Cox regression analyses were performed to assess the independent risk factors for in-hospital death and postoperative survival, respectively. Statistical significance was considered when the two-tailed p-value was less than 0.05. Postoperative survival analysis was carried out using the Kaplan–Meier (KM) method and the Log rank test. The prediction accuracy of the nomogram was evaluated using the time-dependent receiver operating characteristic curves, with the areas under the curve (AUC) calculated. The calibration curve was utilized to assess the consistency between the predicted survival probability of the nomogram and bootstrap resamples. Additionally, decision curve analysis (DCA) was employed to evaluate the net benefit of the nomogram. We excluded variables with a missing value ratio exceeding 10% and conducted multiple imputation for variables with a missing value ratio below 10%. All statistical analyses were conducted using SPSS version 26.0 (IBM, USA) and R version 4.3.0, with appropriate packages and functions utilized.
Results
A total of 384 patients undergoing open surgery were included in our retrospective study. Among them, 42 (10.9%) patients died within 30 days of hospitalization. The demographic and social data of the two groups revealed that out of the total patients, 307 (79.9%) patients were males, and the average age of the patients was 56.09 ± 13.48 years. A significant portion of the patients, 287 (74.7%) patients had a history of hypertension. Additionally, 141 (36.7%) patients had smoking history, while 21(5.5%) patients had a history of coronary atherosclerotic heart disease. Aortic valve replacement was performed in 75(19.5%) patients, while partial arch replacement of the aorta was performed in 204 (53.1%) patients. 18 (4.7%) patients underwent coronary artery bypass grafting. The group of patients died exhibited a higher frequency of blood transfusions compared to the group of patients who survived {TPT: death group 168.75 [108.12, 238.75] vs. survival group 100.00 [75.00, 144.38], P < 0.001; Platelets: death group 2.00 [1.00, 3.00] vs. survival Group 1.00 [1.00, 2.00], P < 0.001; TTCF: Death group 12.12 [8.94, 15.00] vs. Survival group 9.50 [8.00, 13.00], P = 0.007; TTRBC LR: Death group 15.50 [9.62, 26.12] vs. Survival group 8.50 [6.00, 12.50], P < 0.001;}. Moreover, the death group had a longer postoperative invasive ventilator time compared to the survival group {death group: 103.50 [56.00, 192.75] vs. survival group: 25.00 [16.00, 66.00]}. However, there were no significant differences between the deceased and surviving groups in terms of calculated inflammatory indices (SIRI, NLR, MLR, PLR, SII, SCI). Regarding imaging data, the rate of patients with multiple tears in the survival group was significantly higher than in the death group {survival group: 69 cases (20.2%) vs. death group: 1 case (2.4%), p = 0.009}. Additionally, the death group had a higher incidence of cumulative mesenteric artery involvement in dissection compared to the survival group {survival group: 31 cases (9.1%) vs. death group: 10 cases (23.8%)}. The baseline data characteristics of the two groups of patients are presented in Table 1. The comparison results of the baseline data of two groups of patients over 30 days are shown in the more detail [see Additional file 1].
Short- term and mid-term outcomes
The short-term and mid-term prognosis results of the patients are presented in Table 2. Within 30 days after surgery, 289 patients (75.3%) experienced at least one postoperative complication during hospitalization. The mortality rate due to gastrointestinal bleeding was higher in the death group compared to the survival group (Death group: 10 cases (23.8%) vs. Survival group: 12 cases (3.5%), P < 0.001). The mortality rate after ECMO re-use was higher in the death group compared to the survival group (Death group: 4 patients (9.5%) vs. Survival group: 1 case (0.3%), P < 0.001). The postoperative IABP mortality rate was higher in the death group compared to the survival group (Death group: 2 cases (4.8%) vs. Survival group: 0 patients, P = 0.004). The postoperative CRRT mortality rate was higher in the death group compared to the survival group (Death group: 25 cases (59.5%) vs. Survival group: 46 cases (13.5%)). During the follow-up period, 47 cases (12.2%) of patients required a second surgical intervention. Additionally, 5 patients (1.3%) underwent a third surgical intervention. Among patients with aortic dissection, 42 patients (10.9%) experienced mortality within 30 days of hospitalization. Within 90 days after surgery, 53 patients (13.8%) experienced mortality, while within 1 year after surgery, 59 patients (15.3%) experienced mortality. There were 67 patients (17.4%) who experienced mortality within 4 years after the operation.
Logistic regression analyses and cox regression analyses
Logistic regression analysis was employed to identify independent risk factors associated with in-hospital mortality within 30 days among patients undergoing open surgery. The results of both univariate and multivariate analyses are presented in Table 3. The results of the multicollinearity diagnosis of multivariate logistic regression are shown in Supplementary file [see Additional file 2]. The results of the multicollinearity diagnosis of multivariate Cox regression are shown in Supplementary file [see Additional file 3].
The univariate logistic regression analysis revealed several potential risk factors for in-hospital mortality within 30 days. These factors included a history of renal nephritis with dialysis, Marfan syndrome, age over 58 years, postoperative cerebral hemorrhage, reusing ECMO postoperatively, reintubation postoperatively, postoperative use of continuous renal replacement therapy (CRRT), preoperative white blood cell count (WBC) ≥ 10.45 (× 10^9/L), alanine transaminase (ALT) ≥ 33.5 (U/L), alkaline phosphatase (ALP) ≥ 80 (U/L), lactate dehydrogenase (LDH) ≥ 610 (U/L), total bilirubin (TBIL) ≥ 19 (umol/L), creatinine (CR) ≥ 104 (umol/L), uric acid, phosphorus ≥ 1.4 (mmol/L), glomerular filtration rate (GFR), fibrinogen, D-dimer ≥ 4.4 (mg/L), Dimer l ≥ 5.56, and SCI ≥ 34.
The multivariate logistic regression analysis demonstrated that the following factors exhibited significant associations with in-hospital mortality within 30 days: history of renal nephritis with dialysis (OR: 4.494; 95% CI: 1.136, 17.782; p < 0.05), Marfan syndrome (OR: 14.016; 95% CI: 1.031, 190.492; p < 0.05), postoperative cerebral hemorrhage (OR: 12.167; 95% CI: 1.08, 137.03; p < 0.05), reintubation postoperatively (OR: 6.710; 95% CI: 2.813, 16.007; p < 0.001), postoperative use of CRRT (OR: 4.541; 95% CI: 1.979, 10.421; p < 0.001), preoperative WBC ≥ 10.45 (× 10^9/L) (OR: 3.937; 95% CI: 1.338, 11.579; p < 0.05), ALP ≥ 80 (U/L) (OR: 1.004; 95% CI: 1.001, 1.006; p < 0.05), LDH ≥ 610 (U/L) (OR: 3.552; 95% CI: 1.498, 8.424; p < 0.05), and D-dimer ≥ 4.4 (mg/L) (OR: 3.585; 95% CI: 1.095, 11.738; p < 0.05).
Cox regression analysis was employed to identify independent risk factors associated with long-term survival after open repair of aortic dissection. Variables with p-values less than 0.05 and variables considered clinically significant were included in the multivariate Cox analysis. The results of the multivariate Cox analysis revealed several independent risk factors for survival in patients undergoing open surgery for aortic dissection. These factors included age ≥ 58 years old, history of cardiovascular surgery, Marfan syndrome, previous history of nephritis and dialysis, WBC ≥ 10.45(× 10^9/L), TBIL, phosphorus ≥ 1.4 mmol/L, D-dimer ≥ 4.4 mg/L, and multiple tears in aortic dissection. These findings highlight the significant association between these factors and the long-term survival outcomes of patients who underwent open surgery for aortic dissection. The results of both univariate and multivariate cox analyses are presented in Table 4.
Survival prediction model
We developed a nomogram utilizing the outcomes of multivariate Cox analysis to prognosticate survival in surgically treated patients with acute aortic dissection. The nomogram incorporates nine noteworthy independent factors, with Marfan syndrome exerting the most substantial influence on survival. Other significant factors comprise a history of previous cardiac surgery, prior renal dialysis, direct bilirubin and serum phosphorus, D-dimer, white blood cell counts, multiple dissection breaks, and age. Each variable is assigned a score on the scoring scale, and the scores are summed to derive a total score, which is then plotted on the corresponding scale. The nomogram encompasses scales that estimate the probability of survival at specific time points, including 1 month, 3 months, 1 year, and 4 years. The nomogram is shown in Fig. 2.
In this, we provide a practical case to demonstrate how to use this model. First, find the corresponding points on the point axis according to the patient's characteristics. For example, a patient is 67 years old, the corresponding points on the points axis are 40; the patient has a history of heart surgery, the corresponding points on the points axis are approximately 67; the patient does not have Marfan syndrome, the points obtained on the points axis are 0; the patient does not have a history of Nephritis, the corresponding points on the points axis are 0; the patient's WBC is 12.8 (× 10*9/L), then it belongs to WBC ≥ 10.45, and the corresponding points on the axis are 40; the patient's phosphorus is 0.73 (mmol/L) and belongs to the ≤ 1.4 category, then the points are 0; the patient has ≥ 2 ruptures, then it belongs to the YES category, and the score on the axis is 0; the patient's D dimer value is 23.94 (mg/L), then it belongs to the ≥ 4.4 category, so the points obtained are approximately 45; the patient's TBIL is 14, the corresponding points on the points axis are 14; then add up all the points to get the total points. In this example, the total points are 40 + 65 + 0 + 0 + 40 + 0 + 0 + 45 + 14 = 204. Next, find the point corresponding to 204 on the total points axis, then draw a line down from the total points obtained until it intersects with the survival probability axis. In this example, the final score is 204, the corresponding 1-month survival rate is approximately 76%, the 3-month survival rate is approximately 70%, the 1-year survival rate is approximately 63%, and the 4-year survival rate is approximately 60%.
To assess the predictive capability of the nomogram, we employed time-dependent receiver operating characteristic (ROC) analysis with area under the curve (AUC) (Fig. 3A-D). The results demonstrated the robust predictive power of the nomogram for overall survival at different time points. Specifically, the AUC was 0.849 (95% CI: 0.79–0.91) for the one-month survival probability (Fig. 3A)., 0.833 (95% CI: 0.77–0.89) for the three-month survival probability (Fig. 3B), and 0.849 (95% CI: 0.79–0.90) for the one-year survival probability (Fig. 3C). For the four-year survival probability, the AUC was 0.816 (95% CI: 0.75–0.88) (Fig. 3D). Furthermore, the calibration plot demonstrated excellent concordance between the predictions of the nomogram and the actual observations of overall survival at each time point (Figs. 4A-D and 5). This indicates that the nomogram provides accurate and reliable survival predictions. To evaluate the clinical applic ability of the model, we employed Decision Curve Analysis (DCA) curves. The findings indicate that the model is effective in predicting the one- and three-month as well as the one- and four-year survival probabilities in patients with acute type A aortic dissection (ATAAD) who undergo surgical repair.
Robustness of the final model
The robustness of the final model was examined by repeatedly refitting the model to 300 differently sampled training and test sets (ratio 80:20) via the bootstrap procedure. The mean AUC is 0.783 with a 95% bootstrap CI of 0.783–0.796.
Discussion
In this single-center retrospective study, a predictive model was used by clinicians to identify high-risk patients with acute type A aortic dissection (ATAAD) who were scheduled to undergo surgery. We developed a novel ATAAD survival prediction model based on comprehensive preoperative clinical characteristics. The results showed a significant improvement in predicting the risk of death in patients with ATAAD, demonstrating good discriminative power. This predictive model provides invaluable support for clinicians in identifying high-risk ATAAD patients for planned surgery.
In our constructed prediction model, we included nine significant independent risk factors: Marfan syndrome, previous cardiac surgery history, previous renal dialysis history, direct bilirubin level, serum phosphorus level, D-dimer, white blood cell levels, multiple breaches, and age. We found that using fewer variables in other models resulted in less discriminative models, thus emphasizing the importance of including these variables for better prediction. Our model has several advantages over other predictive models. Firstly, it allows for quick acquisition of information upon admission to the emergency department enabling early identification of patients at high risk of in-hospital death. Additionally, most predictive models are biased towards a single variable, potentially leading to a model biased towards a specific patient type. In contrast, our model encompasses clinical characteristics, serology, and imaging, making it applicable to patients with diverse characteristics. Secondly, while most models primarily focus on short-term survival, our model concentrates on mid- and long-term survival.
Unlike predictive models developed by other researchers, our model highlights the significant contributions of Marfan syndrome, previous history of open heart surgery (except for type A aortic dissection), and previous history of renal dialysis [10, 11]. The logistic regression results also demonstrate that Marfan syndrome and previous history of renal dialysis are independent risk factors for death within 30 days in patients with acute aortic dissection undergoing surgical treatment [12,13,14]. This indicates that surgeons should not solely focus on aortic dissection but also obtain a detailed history of the patient's past medical records.
Inflammation plays a crucial role in the progression of aortic dissection [15,16,17,18,19]. Aortic tissue injury and thrombus formation in the false lumen can trigger an inflammatory response [20]. Previous research found that white blood cells, including neutrophils and macrophages, were found in the torn aortic tissue [21]. Previous studies have indicated that elevated white blood cell counts are associated with increased in-hospital mortality and serve as a risk indicator for adverse events involving the heart, lungs, brain, and systemic conditions [22,23,24]. Our study reinforces these findings by demonstrating that an increase in white blood cell count is an independent risk factor for death. Similarly, elevated D-dimer levels have been previously associated with in-hospital major adverse events, and our results align with those of previous investigations [25,26,27]. We also identified direct bilirubin level and serum phosphorus level as predictor variables in our model, which have received limited attention in prior studies. These findings suggest potential directions for future research. Unfortunately, we did not include calculated inflammatory indicators such as NLR, MLR, PLR, SII, D dimer/l, and SCI in our model [27,28,29]. Although these indicators showed differences in univariate logistic and Cox analyses, they were all excluded in the multivariate analyses, contradicting previous research results.
Our study dedicated considerable effort to analyzing preoperative imaging information of patients with aortic dissection. Unfortunately, we found no significant associations between aortic true-to-false lumen diameter, true-to-false lumen ratio, or anatomically true-to-false lumen ratio and survival. We speculate that this result may be due to the surgical repair of damaged aortic tissue.
However, we did not conduct further investigation into whether the presence of accumulated but unrepaired planes in patients with aortic dissection was associated with subsequent surgical interventions. Looking at the number of breaches in aortic dissection, we discovered that multiple breaches act as a protective factor against the occurrence of the outcome event. This observation is related to the hemodynamics of aortic dissection, as multiple breaches reduce pressure in the true lumen, thereby mitigating tearing and further extension of dissections.
Naturally, this study, like others, has limitations. Firstly, it was a retrospective study, rendering it susceptible to selection bias. Secondly, the study relied on data from a single center, necessitating further testing to determine if the findings are applicable to other centers.
Limitations
Our study has several limitations. First, it was a retrospective analysis conducted at a single center. Second, despite the sufficient power of our study, the sample size was relatively small. Therefore, further research is needed to validate the conclusions of our study. Third, we used the Robustness method for model validation. Future prospective data from other institutions for external validation of the model may help further test the predictive ability of the line graph and enhance its universality. Fourth, we did not specify the exact cause of death in postoperative patients, which prevented us from linking postoperative death with postoperative complications.
Conclusions
We have developed a novel survival prediction model based on comprehensive preoperative clinical characteristics information for acute aortic dissection. This model demonstrates a significant improvement in accurately predicting the risk of death in patients with Type A acute aortic dissection (ATAAD). Furthermore, the model exhibits good discriminatory power, allowing clinicians to effectively identify high-risk ATAAD patients who require immediate surgical intervention.
Availability of data and materials
The datasets generated and/or analysed during the current study are not publicly available due to patients did not signed consents about upload the data but are available from the corresponding author on reasonable request.
Abbreviations
- ATAAD:
-
Acute type A aortic dissection
- AAD:
-
Acute aortic dissection
- CTA:
-
Computed tomography angiography
- HIS:
-
Hospital information system
- BNP:
-
B-type natriuretic peptide
- NLR:
-
Neutrophil-to-lymphocyte ratio
- MLR:
-
Monocyte-to-lymphocyte ratio
- SIRI:
-
Systemic inflammation response index
- PLR:
-
Platelet-to-lymphocyte ratio
- D-dimer/L:
-
D-dimer-to-lymphocyte ratio
- SII:
-
Systemic immune-inflammation index
- SCI:
-
Systemic coagulation-inflammation index
- ECMO:
-
Extracorporeal membrane oxygenation
- CRRT:
-
Continuous renal replacement therapy
References
Zhu Y, Lingala B, Baiocchi M, Tao JJ, Toro Arana V, Khoo JW, Williams KM, Traboulsi AA, Hammond HC, Lee AM, et al. Type A Aortic Dissection-Experience Over 5 Decades: JACC Historical Breakthroughs in Perspective. J Am Coll Cardiol. 2020;76(14):1703–13.
Trimarchi S, Nienaber CA, Rampoldi V, Myrmel T, Suzuki T, Mehta RH, Bossone E, Cooper JV, Smith DE, Menicanti L, et al. Contemporary results of surgery in acute type A aortic dissection: The International Registry of Acute Aortic Dissection experience. J Thorac Cardiovasc Surg. 2005;129(1):112–22.
Pape LA, Awais M, Woznicki EM, Suzuki T, Trimarchi S, Evangelista A, Myrmel T, Larsen M, Harris KM, Greason K, et al. Presentation, Diagnosis, and Outcomes of Acute Aortic Dissection 17 Year Trends From the International Registry of Acute Aortic Dissection. J Am Coll Cardiol. 2015;66(4):350–8.
Ghoreishi M, Wise ES, Croal-Abrahams L, Tran D, Pasrija C, Drucker CB, Griffith BP, Gammie JS, Crawford RS, Taylor BS. A Novel Risk Score Predicts Operative Mortality After Acute Type A Aortic Dissection Repair. Ann Thorac Surg. 2018;106(6):1759–66.
Ren Y, Huang S, Li Q, Liu C, Li L, Tan J, Zou K, Sun X. Prognostic factors and prediction models for acute aortic dissection: a systematic review. BMJ Open. 2021;11(2): e042435.
Donati T, Wilson J, Kölbel T, Clough RE. Modern diagnostics for type B aortic dissection. Gefasschirurgie. 2015;20(6):420–7.
Xue Y, Zhou Q, Pan J, Cao H, Fan F, Zhu X, Wang D. “Double Jacket Wrapping” Root Reconstruction for Acute Type A Aortic Dissection. Ann Thorac Surg. 2020;110(3):1060–2.
Smith HN, Boodhwani M, Ouzounian M, Saczkowski R, Gregory AJ, Herget EJ, Appoo JJ. Classification and outcomes of extended arch repair for acute Type A aortic dissection: a systematic review and meta-analysis. Interact Cardiovasc Thorac Surg. 2017;24(3):450–9.
Permanyer E, Ruyra X, Evangelista A. The aortic arch management for type A aortic dissection: aggressive but experienced. J Thorac Dis. 2020;12(6):3429–32.
de Beaufort HWL, Trimarchi S, Korach A, Di Eusanio M, Gilon D, Montgomery DG, Evangelista A, Braverman AC, Chen EP, Isselbacher EM, et al. Aortic dissection in patients with Marfan syndrome based on the IRAD data. Annals of Cardiothoracic Surgery. 2017;6(6):633–41.
Gott VL, Greene PS, Alejo DE, Cameron DE, Naftel DC, Miller DC, Gillinov AM, Laschinger JC, Pyeritz RE. Replacement of the aortic root in patients with Marfan’s syndrome. N Engl J Med. 1999;340(17):1307–13.
Wang Z, Ge P, Lu L, Ge M, Chen C, Zhang L, Wang D. Surgical outcomes of acute type A aortic dissection in dialysis patients: lessons learned from a single-center’s experience. Sci Rep. 2022;12(1):5372.
Chou AH, Hsieh ML, Lin YS, Chen DY, Chu PH, Chen SW. Long-term outcome of acute type A aortic dissection repair in chronic kidney disease patients. Medicine (Baltimore). 2023;102(19):e33762.
Rahmanian PB, Adams DH, Castillo JG, Vassalotti J, Filsoufi F. Early and late outcome of cardiac surgery in dialysis-dependent patients: single-center experience with 245 consecutive patients. J Thorac Cardiovasc Surg. 2008;135(4):915–22.
Liu H, Luo Z, Liu L, Yang X, Zhuang Y, Tu G, Ma G, Zhang Y, Zheng J, Zhu D, et al. Inflammatory biomarkers to predict adverse outcomes in postoperative patients with acute type A aortic dissection. Scand Cardiovasc J. 2020;54(1):37–46.
Chen X, Zhou J, Fang M, Yang J, Wang X, Wang S, Yang L. Procalcitonin, Interleukin-6 and C-reactive Protein Levels Predict Renal Adverse Outcomes and Mortality in Patients with Acute Type A Aortic Dissection. Front Surg. 2022;9:902108.
Zhou Q, Chai X-P, Fang Z-F, Hu X-Q, Tang L. Association of plasma pentraxin-3 levels on admission with in-hospital mortality in patients with acute type A aortic dissection. Chin Med J. 2016;129(21):2589–95.
He R, Guo DC, Estrera AL, Safi HJ, Huynh TT, Yin Z, Cao SN, Lin J, Kurian T, Buja LM, et al. Characterization of the inflammatory and apoptotic cells in the aortas of patients with ascending thoracic aortic aneurysms and dissections. J Thorac Cardiovasc Surg. 2006;131(3):671–8.
del Porto F, Proietta M, Tritapepe L, Miraldi F, Koverech A, Cardelli P, Tabacco F, de Santis V, Vecchione A, Mitterhofer AP, et al. Inflammation and immune response in acute aortic dissection. Ann Med. 2010;42(8):622–9.
Mack CP. Signaling Mechanisms That Regulate Smooth Muscle Cell Differentiation. Arterioscler Thromb Vasc Biol. 2011;31(7):1495–505.
Xu Y, Ye J, Wang M, Wang Y, Ji Q, Huang Y, Zeng T, Wang Z, Ye D, Jiang H, et al. Increased interleukin-11 levels in thoracic aorta and plasma from patients with acute thoracic aortic dissection. Clin Chim Acta. 2018;481:193–9.
Fan X, Huang B, Lu H, Zhao Z, Lu Z, Yang Y, Zhang S, Hui R. Impact of Admission White Blood Cell Count on Short- and Long-term Mortality in Patients With Type A Acute Aortic Dissection: An Observational Study. Medicine (Baltimore). 2015;94(42):e1761.
Zhang C, Fu Z, Bai H, Lin G, Shi R, Chen X, Xu Q. Admission white blood cell count predicts post-discharge mortality in patients with acute aortic dissection: data from the MIMIC-III database. BMC Cardiovasc Disord. 2021;21(1):462.
Ma M, Shi J, Feng X, Wang J, Liu L, Wei X. The elevated admission white blood cell count relates to adverse surgical outcome of acute Stanford type a aortic dissection. J Cardiothorac Surg. 2020;15(1):48.
Itagaki R, Kimura N, Mieno M, Hori D, Itoh S, Akiyoshi K, et al. Characteristics and treatment outcomes of acute type A aortic dissection with elevated D-dimer concentration. J Am Heart Assoc. 2018;7(14):e009144.
Shimony A, Filion KB, Mottillo S, Dourian T, Eisenberg MJ. Meta-analysis of usefulness of d-dimer to diagnose acute aortic dissection. Am J Cardiol. 2011;107(8):1227–34.
Xu Y, Liang S, Liang Z, Huang C, Luo Y, Liang G, Wang W. Admission D-dimer to lymphocyte counts ratio as a novel biomarker for predicting the in-hospital mortality in patients with acute aortic dissection. BMC Cardiovasc Disord. 2023;23(1):69.
Zhao Y, Jiang J, Yuan Y, Shu X, Wang E, Fu W, Wang L. Prognostic Value of the Systemic Immune Inflammation Index after Thoracic Endovascular Aortic Repair in Patients with Type B Aortic Dissection. Dis Markers. 2023;2023:2126882.
Liu H, Qian SC, Shao YF, Li HY, Zhang HJ. Prognostic Impact of Systemic Coagulation-Inflammation Index in Acute Type A Aortic Dissection Surgery. JACC Asia. 2022;2(6):763–76.
Acknowledgements
Not applicable.
Funding
This work received support from the National Natural Science Foundation of China (to Dong-Jin Wang No.81970401, to Yun-Xing Xue No.82100508), Jiangsu Provincial Key Medical Discipline (to Dong-Jin Wang No. ZDXKA2016019), and The Health Commission of Nanjing (to Tuo Pan No. YKK22096).
Author information
Authors and Affiliations
Contributions
(I) Conception and design: YX-X (II) Administrative support: D-JW, TP (III) Provision of study materials or patients: YX-X, Y-J (IV) Collection and assembly of data: Y-XL, W-ZW and K-Y Z (V) Data analysis and interpretation: Matniyaz Y (VI) Manuscript writing: All authors (VII) Final approval of manuscript: All authors.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study received approval from the ethical committees of Nanjing Drum Tower Hospital (No.2022–157-01) and individual consent for this retrospective analysis was waived.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Matniyaz, Y., Luo, YX., Jiang, Y. et al. Short- and Long-term survival prediction in patients with acute type A aortic dissection undergoing open surgery. J Cardiothorac Surg 19, 171 (2024). https://doi.org/10.1186/s13019-024-02687-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13019-024-02687-x