Abstract
Background
The aim of this study was to identify the risk factors for postoperative delirium (POD) in elderly patients undergoing heart valve surgery with cardiopulmonary bypass (CPB).
Methods
Elderly patients undergoing elective heart valve surgery with CPB in The First Affiliated Hospital of Wenzhou Medical University between March 2022 and March 2023 were selected for this investigation. They were divided into a POD group and a non-POD group. Their baseline information was collected and recorded, and the patients were subjected to neurocognitive function assessment using the Mini-Mental State Examination and the Montreal Cognitive Assessment scales before surgery. We also recorded their intraoperative indicators such as duration of surgery, duration of CPB, duration of aortic cross-clamp, blood transfusion, and postoperative indicators such as duration of mechanical ventilation, postoperative 24-hour drainage volume, and pain score. Regional cerebral oxygen saturation was monitored intraoperatively by near-infrared spectroscopy based INVOS5100C Regional Oximeter. Patients were assessed for the occurrence of POD using Confusion Assessment Method for the Intensive Care Unit, and logistic regression analysis of risk factors for POD was performed.
Results
The study finally included 132 patients, with 47 patients in the POD group and 85 ones in the non-POD group. There were no significant differences in baseline information and preoperative indicators between the two groups. However, marked differences were identified in duration of surgery, duration of CPB, duration of aortic cross-clamp, duration of postoperative mechanical ventilation, postoperative length of stay in cardiac intensive care unit, postoperative length of hospital stay, intraoperative blood transfusion, postoperative pain score, and postoperative 24-hour drainage volume between the two groups (p < 0.05). Additionally, the two groups had significant differences in rScO2 at each intraoperative time point and in the difference of rScO2 from baseline at each intraoperative time point (p < 0.05). Multivariate logistic regression analysis showed that duration of surgery > 285 min (OR, 1.021 [95% CI, 1.008–1.035]; p = 0.002), duration of postoperative mechanical ventilation > 23.5 h (OR, 6.210 [95% CI, 1.619–23.815]; p = 0.008), and postoperative CCU stay > 3.5 d (OR, 3.927 [95% CI, 1.046–14.735]; p = 0.043) were independent risk factors of the occurrence of POD while change of rScO2 at T1>50.5 (OR, 0.832 [95% CI 0.736–0.941]; p = 0.003) was a protective factor for POD.
Conclusion
Duration of surgery duration of postoperative mechanical ventilation and postoperative CCU stay are risk factors for POD while change of rScO2 at T1 is a protective factor for POD in elderly patients undergoing heart valve surgery with CPB.
Similar content being viewed by others
Background
Valve heart disease (VHD) is the most common structural heart disease, referring to anatomical and functional abnormalities of the heart valves or perivalvular tissue caused by rheumatic conditions, degenerative conditions, infections, or congenital malformations [1]. As the population ages, the incidence of VHD is progressively rising. According to the report from American Heart Association, the incidence of moderate to severe VHD can reach 1.8%. More significantly, the incidence increases with patient age, with 4.4% occurring in the population under 65 years of age and 11.7% in the population over 75 years of age [2]. Heart valve replacement or valvuloplasty under cardiopulmonary bypass (CPB) is one of the most common treatments for VHD [3]. However, different postoperative complications can arise as a result of complex surgery, old age, and other complicating factors [4]. Active precautions need to be taken since postoperative complications after heart valve surgery under CPB affect the prognosis and quality of life of patients.
Recent years have seen amazing advancements in cardiac surgery techniques and perioperative management. As a result, in addition to death and serious postoperative complications such vital organ failure, minor or even imperceptible complications, like postoperative delirium (POD), have received increasing attention. POD is a common postoperative complication after general anesthesia and surgery. As an indicator of acute cerebral dysfunction, POD is accompanied by concentration difficulty, disturbed consciousness, declined cognitive ability, or perceptual disorders, especially in the elderly. POD usually develops quickly after surgery (between a few hours and three days) and the condition fluctuates with time [5, 6]. Studies have shown that the incidence of POD in patients undergoing cardiac surgery ranges from 4.1 to 54.9%, with elderly patients experiencing a higher incidence of over 80% [7, 8]. Additionally, POD may lead to long-term postoperative cognitive dysfunction (POCD), necessitating a referral to a rehabilitation facility for continued treatment after discharge. Even worse, POD may increase in-hospital mortality and long-term post-discharge mortality [9]. Melatonin may be effective in lessening the severity of POD after cardiac surgery [10]. However, it is more important to take measures to prevent the occurrence of POD.
The occurrence of POD will increase the difficulty of postoperative care and the workload of nurses. Clinical and nursing staff have begun to realize the importance of delirium management and pay attention to the assessment, prevention and treatment of delirium in recent years [11]. A recent meta-analysis showed that regional cerebral oxygen desaturations were significantly associated with neurological complications after cardiac surgery, and that monitoring cerebral oximetry during cardiac surgery could help lower the incidence of postoperative cognitive dysfunction or stroke [12]. However, this conclusion was drawn based on data from the general population; very few research concentrated on the elderly patients. In this study, we analyzed the risk factors associated with the occurrence of POD in elderly patients undergoing heart valve surgery with CPB. It is anticipated that our findings would provide some theoretical basis for the prevention of POD in clinical practice.
Study subjects and methods
Study subjects
This study was an observational case-control study. Elderly patients who underwent elective heart valve surgery under CPB at The First Affiliated Hospital of Wenzhou Medical University from March 2022 to March 2023 were selected and divided into a POD group and a non-POD group according to whether or not they developed POD. Confusion Assessment Method for the Intensive Care Unit (CAM-ICU) was used for screening and diagnosis of POD. Inclusion criteria were as follows: (1) patients receiving elective cardiac valve surgery under CPB; (2) patients aged ≥ 65 years old; (3) patients or their family member who agreed to sign an informed consent. Exclusion criteria were as follows: (1) patients with hepatic insufficiency or chronic obstructive pulmonary disease; (2) patients with a history of central nervous system diseases such as delirium, cerebrovascular accident, and dementia or a history of psychosis; (3) patients receiving emergency surgery; (4) patients with carotid artery stenosis ≥ 60%; (5) patients receiving concurrent cardiac coronary artery bypass surgery or operation on great vessels; (6) patients who refused to be followed up. This study complied with the Declaration of Helsinki and gained approval from The First Affiliated Hospital of Wenzhou Medical University Ethics Committee (ethics number: KY2021-130). Informed consent forms were signed by the included patients and their families.
Observation indicators
Indicators recorded preoperatively included sex, age, body mass index (BMI), body surface area, and European system for cardiac operative risk evaluation (EuroSCORE), education level (years), medical history (hypertension, diabetes, atrial fibrillation, cerebral infarction), history of adverse habits (smoking, alcohol), Mini-mental state examination (MMSE) score, Montreal Cognitive Assessment (MoCA) score, and history of other surgical procedures. The recorded perioperative blood parameters included hemoglobin, platelets, serum albumin, urea nitrogen, serum creatinine, estimated glomerular filtration rate, serum calcium, total cholesterol, creatine kinase, creatine kinase-MB (CK-MB), and left ventricular ejection fraction (LVEF). We also recorded intraoperative conditions including type of surgery, duration of surgery, duration of CPB, duration of aortic cross-clamp, and blood transfusion, and postoperative conditions including duration of postoperative mechanical ventilation, postoperative 24-hour drainage volume, postoperative pain score, postoperative cardiac ICU (CCU) stay, and postoperative hospital stay.
Neurocognitive function assessment
MMSE and MoCA scales were responsible for preoperative assessment of neurocognitive function. Given their high sensitivity and specificity, the two scales are most widely used for the screening test of cognitive function in China and even in the world [13,14,15]. The test based on MMSE and MoCA aimed to exclude patients with preoperative cognitive dysfunction. The test was completed by one investigator who was instructed by a neurologist with relevant experience and was trained in more than 10 patients during the pre-experimental phase. In consideration of the patients’ biological clock and preoperative preparations, the test was conducted in the wards between 18:00 pm–20:00 pm the night before surgery. The ward environment was kept quiet and free of disturbances, and the help and reminders of patients’ families were avoided during the test. Patients who were unable to communicate verbally before surgery or who were unable to continue the test due to fatigue or other discomfort during the test were excluded from the study. Since the MMSE and MoCA scores were closely related to the level of education, the assessment scores were adjusted according to education level. The MMSE criteria for each education level were as follows: 17 points for the illiterate group (no education), 20 points for the primary school group (≤ 6 years of education), 22 points for the secondary school group, and 24 points for the university group; patients with corresponding education level who scored below these criteria were excluded from the study. One point was added to the MoCA test results for patients with < 12 years of education, and 2 points were added for patients with < 9 years of education, so as to correct the bias of education level [16].
Perioperative management
After the patient was admitted to the operating room, routine anesthesia for cardiac surgery was performed, with the monitoring of electrocardiogram, pulse oxygen saturation, and bispectral index. Additionally, invasive arterial pressure was monitored through the cannulation of left radial artery. After induction of anesthesia with face mask (oxygen flow rate: 3 L/min), rapid sequence intubation was conducted and a transesophageal echocardiographic probe was placed through the mouth. Etomidate (0.3 mg/kg), midazolam (0.03 mg/kg), sufentanil (1–5 µg/kg), and cis-atracurium (0.1–0.2 mg/kg) were administered through peripheral veins for the induction of anesthesia, and meanwhile, dexmedetomidine (0.5 µg/kg/h) was given continuously. 0.6–0.7 MAC sevoflurane was inhaled or 0.02% norepinephrine was injected as needed for depth of anesthesia or hemodynamics adjustment. During maintenance of anesthesia, end-tidal carbon dioxide was maintained at 30–40 mmHg by adjusting the minute ventilation.
Before performing intubation, 4 mg/kg of heparin sodium was given intravenously, and 30 mg of heparin sodium was added to the CPB prime solution to wet the tube. After 5 min of intravenous heparinization, activated clotting time (ACT) was monitored, with ACT > 480 s allowing the start of intubation and extracorporeal circulation. During extracorporeal circulation, ACT was recorded every 60–90 min and maintained at ≥ 480 s; 50–100 mg of heparin sodium was added when ACT < 480 s. For nonpulsatile CPB with mild hypothermia, crystalloid St.Tomas cardioplegic solution was prepared and perfused into the body at a ratio of 1:4 with oxygenated blood, with a total amount of 20 ml/kg for the first perfusion, and with the dose of body surface area (BSA)×2.0-2.5 L/min to keep the O2 flow within 2-2.5 L for maintaining perfusion. The body temperature was monitored by dual channels, and nasopharyngeal and bladder temperatures were both maintained at 32–36 ℃. With bladder temperature as reference, the difference between nasopharyngeal temperature and bladder temperature was maintained at < 2 ℃ during rewarming. CPB flow rate was kept at 2.2–2.8 L/(min·m2). Invasive mean arterial pressure (MAP) of 50–80 mmHg, arterial partial pressure of oxygen of 150–250 mmHg and partial pressure of carbon dioxide of 35–45 mmHg were maintained throughout CPB.
Regional cerebral oxygen saturation monitoring
The device used to monitor regional cerebral oxygen saturation (rScO2) in this study was the near-infrared spectroscopy (NIRS) based INVOS5100C Regional Oximeter (Medtronic, USA). The patient was admitted to the operating room in a supine position on the operating table. Two self-adhesive NIRS sensors were fixed to the forehead (1–2 cm above the eyebrows) after wiping the forehead with an alcohol cotton ball. Then the patient inhaled air in the operating room in a conscious and quiet state, and rScO2 value was observed 1 min later until the value stabilized; the stable rScO2 value was read as the baseline value (T0). Monitoring of rScO2 was stopped at the end of surgery. Four intraoperative times points were selected: start of anesthesia (T1), start of CPB (T2), start of rewarming (T3), and end of CPB (T4). The values of rScO2 at T1, T2, T3, and T4 were recorded. Then the changes of rScO2 at each time point compare with rScO2 at T0 (change of rScO2 at T1 = rScO2 at T1 - rScO2 at T0, change of rScO2 at T1 = rScO2 at T0 - rScO2 at T2, change of rScO2 at T3 = rScO2 at T0 - rScO2 at T3, and change of rScO2 at T4 = rScO2 at T4 - rScO2 at T0) were calculated. When rScO2 falls below absolute values of 50% or less than 20% of the baseline, doctors would directly or indirectly increase cerebral oxygen values by measures such as increasing aortic perfusion flow or increasing oxygen flow or drugs, while the extracorporeal circulation physician needs to take into account the maintenance of vital signs such as creation of a mean arterial pressure of 50–80 mmHg, maintenance of arterial partial pressures of oxygen of 150–250 mmHg and carbon dioxide partial pressures of 35–45 mmHg in a relatively stable manner.
Postoperative delirium assessment
The patient was transferred to the CCU immediately after cardiac surgery. The patient was assessed for POD by a CCU nurse specialist using CAM-ICU. The CAM-ICU defines POD based on four diagnostic features: acute change or fluctuating course of mental status, inattention, disorganized thinking, and altered level of consciousness [17]. Once a patient was admitted to the CCU, POD assessment was initiated using the CAM-ICU and the duration of delirium was recorded. Each patient was scored every 6–8 h before transfer to the general ward or 7 days after surgery. The evaluator was not a member of the study team and did not have access to any other information of this research.
Statistical analysis
SPSS 25.0 was used for statistical analysis. For continuous data, the Kolmogorove Smirnov test was first applied to assess whether the data were normally distributed. If the data of both groups met normality and the variances between the them were equal, the t-test was responsible for comparison between groups and data were expressed as mean ± standard deviation (SD); otherwise the nonparametric Wilcoxon rank sum test was considered. For categorical data, the chi-square test was responsible for comparison between groups and data were expressed as number of cases (percentage) [n (%)]. Additionally, repeated measures analysis of variance was considered for determining whether there were statistical differences of same parameters between the groups and time points, respectively. The interaction terms between groups and time points were analyzed to explore whether there were differences in change trends between the groups. If the interaction terms were statistically significant, it suggested that the change trend over time may differ between groups. Risk factors for POD were analyzed by binary logistic regression. First univariate logistic regression was used for the initial screening of risk factors, and then the factors with p < 0.05 in univariate logistic regression were included in multivariate logistic regression. A p value < 0.05 was considered statistically significant in multivariate logistic regression.
Study results
General information of patients
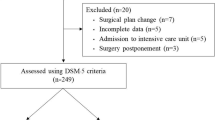
One hundred and fifty-five patients were included in the study based on the inclusion and exclusion criteria. Among them, there were 8 cases with intraoperative changes of surgical methods (combined with coronary artery bypass surgery or operation on great vessels) and 15 cases with missing intraoperative data. Therefore, 132 cases with complete data were finally statistically analyzed (POD group: n = 47; non-POD group: n = 85). Baseline data of patients are shown in Table 1. The POD and non-POD patients had no significant differences in terms of age (70 ± 5 vs. 70 ± 4), sex (M/F: 24/23 vs. 43/42), education level (illiterate/primary/secondary/university: 16/22/5/4 vs. 28/36/15/6), BMI (21.81 ± 3.08 vs. 22.29 ± 2.87), body surface area (1.86 ± 1.97 vs. 1.61 ± 0.14), diabetes mellitus (19.15% vs. 20%), hypertension (38.18% vs. 52.90%), history of smoking (40.43% vs. 47.06), history of alcohol consumption (38.30% vs. 43.53%), history of atrial fibrillation (8.51% vs. 7.06%), history of cerebral infarction (8.51% vs. 4.71%), history of other surgeries (14.89% vs. 14.12%), type of surgery (single-valve/multivalve: 20/27 vs. 50/35), dosage of midazolam (1.83 ± 0.32 vs. 1.77 ± 0.30), mean arterial pressure (76 ± 5.35 vs. 76 ± 6.38), and mean temperature (35.52 ± 0.19 vs. 35.32 ± 0.23).
Preoperative indicators in the POD and non-POD groups
The preoperative indicators of the patients are shown in Table 2. The two groups showed no significant differences in preoperative blood parameters, including hemoglobin (125.85 ± 16.98 vs. 126.35 ± 22.61), platelets (200.69 ± 76.59 vs. 196.70 ± 91.27), albumin (38.26 ± 4.88 vs. 39.76 ± 7.72), urea nitrogen (6.97 ± 3.95 vs. 6.03 ± 1.62), creatinine (80.78 ± 55.12 vs. 76.57 ± 24.57), estimated glomerular filtration rate (90.30 ± 22.61 vs. 133.40 ± 196.57), serum calcium (2.27 ± 0.14 vs. 2.23 ± 0.11), total cholesterol (125.85 ± 16.98 vs. 126.35 ± 22.61), creatine kinase (109.13 ± 181.93 vs. 144.74 ± 242.58), creatine kinase-MB (13.44 ± 24.62 vs. 13.75 ± 14.05), left ventricular ejection fraction (61.42 ± 8.15 vs. 64.80 ± 3.80) (p > 0.05). Additionally, no marked difference was identified between the two groups in terms of preoperative MMSE (21.6 ± 3.5 vs. 21.2 ± 3.1), preoperative MoCA (20.2 ± 3.1 vs. 20.9 ± 2.7), and EuroSCORE (3.26 ± 0.14 vs. 3.12 ± 0.10) (p > 0.05).
Intraoperative regional cerebral oxygen saturation in the POD and non-POD groups
There was no discernible difference in preoperative baseline values of rScO2 (T0) between the two groups (p > 0.05). rScO2 values of POD patients and non-POD patients were recorded continuously during surgery; rScO2 at all intraoperative time points in the POD group were smaller than those in the non-POD group. Repeated measures analysis of variance confirmed that the differences were all considered statistically significant (p < 0.01) (Table 3). Meanwhile, the difference between rScO2 at each intraoperative time point and rScO2 at baseline in the two groups was subjected to t-test. Compared to the non-POD group, the rScO2 values in the POD group showed a smaller increase and a larger decrease from baseline (p < 0.01) (Table 3).
Intraoperative and postoperative indicators in the POD and non-POD groups
Significant differences were found in duration of surgery (295.8 ± 23.4 vs. 274.7 ± 19.9), duration of CPB time (155.9 ± 17.9 vs. 142.9 ± 16.1), duration of aortic cross-clamp (132.1 ± 57.0 vs. 100.8 ± 38.8), intraoperative blood transfusion (78.8% vs. 48.2%), duration of postoperative mechanical ventilation (26.7 ± 22.5 vs. 18.5 ± 8.9), postoperative CCU stay (5.5 ± 2.1 vs. 4.0 ± 1.2), postoperative hospital stay (26.6 ± 9.9 vs. 19.4 ± 5.6), postoperative pain score (1.4 ± 0.2 vs. 0.9 ± 0.1), and postoperative 24-hour drainage volume (264.6 ± 6.2 vs. 218.1 ± 3.7) between the POD and non-POD groups (p < 0.05) (Table 4).
Risk factor analysis of POD
Risk factors for POD were analyzed using binary logistic regression. First univariate logistic regression analysis showed that duration of surgery > 285 min (OR, 1.015 [95% CI, 1.005–1.025]; p = 0.003), duration of CPB > 156 min (OR, 1.018 [95% CI, 1.010–1.027]; p = 0.001), duration of aortic cross-clamp > 133.5 min (OR, 1.020 [95% CI, 1.011–1.029]; p = 0.001), duration of postoperative mechanical ventilation > 23.5 h (OR, 1.053 [95% CI, 1.025–1.082]; p = 0.001), postoperative CCU stay > 3.5 d (OR, 1.486 [95% CI, 1.209–1.827]; p = 0.001), Postoperative hospital stay > 24.5 d (OR, 1.088 [95% CI, 1.033–1.146]; p = 0.001), Postoperative pain score > 1.5 (OR, 1.333 [95% CI, 1.003–1.772]; p = 0.048), Change of rScO2 at T1 > 50.5 (OR, 0.870 [95% CI, 0.804–0.940]; p = 0.001), Change of rScO2 at T2 > 39.25 (OR, 1.097 [95% CI, 1.011–1.189]; p = 0.026), Change of rScO2 at T3 > 37.5 (OR, 1.099 [95% CI, 1.018–1.186]; p = 0.015) and change of rScO2 at T4 > 51 (OR, 0.865 [95% CI, 0.795–0.942]; p = 0.001) were possible predictors of the occurrence of POD. The above indicators were included in a multivariate regression model. Multivariate regression analysis demonstrated that duration of surgery (OR, 1.021 [95% CI, 1.008–1.035]; p = 0.002), duration of postoperative mechanical ventilation > 23.5 h (OR, 6.210 [95% CI, 1.619–23.815]; p = 0.008), and postoperative CCU stay > 3.5 d (OR, 3.927 [95% CI, 1.046–14.735]; p = 0.043) were independent risk factors of the occurrence of POD while change of rScO2 at T1 > 50.5 (OR, 0.832 [95% CI 0.736–0.941]; p = 0.003) was a protective factor of POD (Table 5).
Discussion
The nomenclature of postoperative neurocognitive disorders is consistent with the Diagnostic and Statistical Manual of Mental Disorders, fifth edition (DSM-5), as per the latest published recommendations for the nomenclature of cognitive change associated with anaesthesia and surgery. In DSM-5, postoperative neurocognitive disorder is divided into two phases: the acute phase (from hours after surgery to hospital discharge) called POD and the chronic phase (30 days to 5 years after surgery or death) called POCD [18]. Therefore, the main observation period of POD in this study was from the transfer to the CCU to discharge. The uniform observation period and definition of POD make the study results more comparable and facilitate the interpretation of the study results. A total of 132 elderly patients undergoing heart valve surgery under CPB were included in this study, and the incidence of POD in CCU was 35.6%. MMSE and MoCA scales were used in this study to exclude patients with preoperative cognitive dysfunction. The occurrence of POD was significantly associated with duration of surgery, duration of CPB, duration of aortic cross-clamp, duration of postoperative mechanical ventilation, postoperative CCU stay, postoperative hospital stay, intraoperative blood transfusion, postoperative pain score, and postoperative 24-hour drainage volume. POD patients and non-POD patients showed significant differences in T1–T4 and change of rScO2 at T1-T4. Further duration of surgery, duration of postoperative mechanical ventilation and postoperative CCU stay were confirmed as independent risk factors for POD and change of rScO2 at T1 was a protective factor for POD.
Previous studies have different definitions of POD and different ranges of low rScO2. For example, in a randomized controlled trial by Lei et al., a lower incidence of POD was found in patients receiving a ScO2 monitoring and an intervention for intraoperative rScO2 below 75% of the baseline value than the controls with no rScO2 monitoring and no intervention for rScO2 [19]. Schoen et al. conducted an observational study of 231 patients, and the results showed a 4-fold increase in the rate of POD in patients with rScO2 ≤ 50% compared to those with rScO2 > 50%. They concluded that preoperative rScO2 < 59.5% was a high risk factor for POD after cardiac surgery [20]. In the study of Hong et al., patients undergoing elective valvular heart surgery under CPB were assessed for neurocognitive function using MMSE 1 day before and 7 days after surgery. As a result, they observed that no association existed between the decrease in intraoperative rScO2 and occurrence of POCD, and that patients with reduced ScO2 had a much longer postoperative hospital stay [21]. The difference in preoperative rScO2 baseline values between the two groups was not statistically significant in our study, but during surgery, the absolute value of lowest rScO2 was around 45% in POD patients and above 50% in non-POD patients. It is clear that our findings are consistent with other previous studies [22, 23].
Intraoperative rScO2 values obtained from NIRS-based monitoring continuously change with body temperature and surgical procedures at different time periods. Accordingly, trends in rScO2 over time must be taken into consideration in addition to a single intraoperative rScO2 value or preoperative baseline value in order to accurately predict the incidence of POD. A recent study demonstrated that significant changes in rScO2 during the rewarming phase of CPB were strongly associated with the postoperative neurocognitive recovery [24]. Jufar et al. reported that during CPB cardiac surgery, patients were vulnerable to neurological damage caused by microembolism and hypoperfusion and consequently developed impaired cerebral autoregulation [25]. Also, Joshi et al. showed that the impairment degree of cerebral autoregulation peaked during the rewarming phase of CPB [26]. Therefore, in this study, rScO2 T0–T4 were recorded, and based on these five values, the trend of intraoperative rScO2 and increase/decrease of intraoperative rScO2 were observed and analyzed. Further analysis in this study proved that rScO2 ΔT1 was a risk factor for POD, which supports the idea that the cooling phase of CPB may cause damage to cerebral blood flow autoregulation and put patients at risk of POD. Additionally, the decrease and increase of rScO2 in the POD group were significantly different from those in the non-POD group. The reason may be put down to that the cooling and rewarming phases of CPB impaired cerebral autoregulation, resulting in smaller magnitude of rScO2 increase and larger magnitude of rScO2 decrease. In contrast, rScO2 was monitored from 1 day before surgery to 72 h after surgery in Eertmans et al.‘s study, and no significant relationship was found between POD and intraoperative ScO2. However, there was a significant relationship between POD and a decrease in the relative or absolute value of postoperative ScO2 [27]. The limitation of this study is that each CAM-ICU assessment was performed at the end of an 8-hour shift, making it challenging to pinpoint when the decrease in rScO2 occurred-before, during, or after POD.
It is well known that short duration of surgery leads to relatively less anesthetic drug dosage, less surgical traum weaker and stress response in the brain. Conversely, lengthy surgeries are generally more traumatic, resulting in more blood loss, higher probability of blood transfusion, higher incidence of postoperative complications and higher difficulty of postoperative care. In our study, the POD group had longer duration of surgery and CPB, and aortic cross-clamp and postoperative mechanical ventilation and postoperative CCU stay than the non-POD group.First univariate logistic regression analysis showed that duration of surgery > 285 min and duration of CPB > 156 min and duration of aortic cross-clamp > 133.5 min and Change of rScO2 at T1 > 50.5 and Change of rScO2 at T2 > 39.25 and Change of rScO2 at T3 > 37.5 and change of rScO2 at T4 > 51 were possible predictors of the occurrence of POD (Table 5). For medical staff in the CCU, the occurrence of any one or several of the following situations during surgery in cardiac patients should be taken seriously as indicators that the patient may develop postoperative delirium. Furthermore, the multivariate logistic regression analysis showed that duration of surgery , duration of postoperative mechanical ventilation > 23.5 h (OR, 6.210 [95% CI, 1.619–23.815]; p = 0.008), and postoperative CCU stay > 3.5 d (OR, 3.927 [95% CI, 1.046–14.735]; p = 0.043) were independent risk factors of the occurrence of POD while change of rScO2 at T1 > 50.5 (OR, 0.832 [95% CI 0.736–0.941]; p = 0.003) was a protective factor of POD (Table 5). In order to minimize the risk of neurological complications following surgery, it is imperative that the CPB perfusionist has a thorough understanding of the surgical procedure, prevents patients from becoming too cold or too hot to shorten the rewarming phase, and avoids excessive temperature difference between the poikilothermia water tank and body temperature during the rewarming phase. Besides, medical staff are recommended to improve the efficiency of team communication, surgical skills and teamwork, so as to minimize the duration of surgery (< 285 min) and postoperative mechanical ventilation (< 23.5) h and postoperative CCU stay (< 3.5 d) and reduce POD incidence (Table 5). Some studies have shown that intraoperative blood transfusion, especially intraoperative plasma or platelet transfusion, is a high risk factor for POD [28, 29], similar to the results of our study. The CPB perfusionist can prepare autologous blood for transfusion before surgery or reduce the amount of CPB prime solution. Additionally, controlling intraoperative blood component loss can reduce the probability of intraoperative transfusion and thus lower the incidence of POD.
Innovativeness and limitations
This study is innovative in the following ways. First, variability of rScO2 over time was taken into account for predicting the occurrence of POD requires in addition to a single intraoperative rScO2 value or preoperative baseline value. In this study, in addition to preoperative baseline, four intraoperative times points were selected: start of anesthesia (T1), start of CPB (T2), start of rewarming (T3), and end of CPB (T4). Second, NIRS-based INVOS5100C Regional Oximeter was used to automatically record rScO2 every 5 s and the data were stored offline in real time, allowing the extraction of rScO2 T0–T4. Finally, based on the above five values, the difference between the intraoperative ScO2 at each time point and the baseline value was determined. Therefore, it is possible to observe and analyze the trend of intraoperative rScO2, the magnitude of the increase and decrease of rScO2, and their correlation with POD.
However, this study still has several limitations. First, the change of postoperative neurocognitive function is a long-term postoperative manifestation, but this study lacks a long-term follow-up [30]. Second, this is a single-center observational study with small sample size, so our findings need to be further confirmed in a multicenter study with large sample size. Third, we included only patients with heart valve surgery under CPB, and future studies need to include patients with different surgical procedures or from specific age groups. Fourth, only one method (CAM-ICU) was used for the assessment of POD, and the three daily CAM-ICU assessments were performed at the end of an 8-hour shift. This assessment method and time may cause missed diagnosis of POD cases because delirium is a fluctuating syndrome. Fifth, we did not take into account intraoperative factors (e.g., hypo- or hyper-capnia, inflammatory factors) that may affect rScO2 and the risk of delirium. Finally, our ability to adjust confounding factors was limited, and some other variables that may influence the primary outcome were not analyzed in this study.
Conclusion
Risk factors for POD in patients undergoing heart valve surgery under CPB include duration of surgery duration of postoperative mechanical ventilation and postoperative CCU stay. Besides, change of rScO2 at T1 was a protective factor for POD. Therefore, medical staff in CCU should pertinently manage POD for patients undergoing cardiac surgery depending on these risk factors.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Mrsic Z, Hopkins SP, Antevil JL, et al. Valvular Heart Disease Prim Care. 2018;45:81–94.
Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart Disease and Stroke Statistics—2017 update: a Report from. Am Heart Association. 2017;131:e29.
Writing Committee M, Otto CM, Nishimura RA, et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice guidelines. J Thorac Cardiovasc Surg. 2021;162:e183–e353.
Samiei N, Hakimi MR, Mirmesdagh Y, et al. Surgical outcomes of heart valves replacement: a study of tertiary specialied cardiac center. ARYA Atheroscler. 2014;10:233–7.
Oh ST, Park JY. Postoperative delirium. Korean J Anesthesiol. 2019;72:4–12.
Vlisides P, Avidan M. Recent advances in preventing and managing postoperative delirium. F1000Res 2019;8.
Ordonez-Velasco LM, Hernandez-Leiva E. Factors associated with delirium after cardiac surgery: a prospective cohort study. Ann Card Anaesth. 2021;24:183–9.
Chen H, Mo L, Hu H, et al. Risk factors of postoperative delirium after cardiac surgery: a meta-analysis. J Cardiothorac Surg. 2021;16:113.
Migirov A, Chahar P, Maheshwari K. Postoperative delirium and neurocognitive disorders. Curr Opin Crit Care. 2021;27:686–93.
Javaherforoosh Zadeh F, Janatmakan F, Shafaeebejestan E, Jorairahmadi S. Effect of melatonin on Delirium after on-pump coronary artery bypass graft surgery: a Randomized Clinical Trial. Iran J Med Sci. 2021;46(2):120–7.
Aldecoa C, Bettelli G, Bilotta F, et al. European Society of Anaesthesiology evidence-based and consensus-based guideline on postoperative delirium. Eur J Anaesthesiol. 2017;34:192–214.
Semrau JS, Motamed M, Ross-White A, Boyd JG. Cerebral oximetry and preventing neurological complication post-cardiac surgery: a systematic review. Eur J Cardiothorac Surg. 2021;59(6):1144–54.
Liu Z-L, Zhu X-L, Li A-M. Cut-off values of Beijing version Montreal cognitive scale for screening mild cognitive impairment in the Baiyin city Chinese. J Gerontol. 2019;39(17):4271–4.
Hong H-L, et al. Application of MoCA and MMSE scales in screening of mild cognitive impariment. Chin J Gerontol. 2018;38(19):4815–7.
Pei F, Meng T, Zhang K-X. Value of MMSE vs MoCA in screening cognitive dysfunction in elderly. Chin Remedies Clin. 2020;20(11):1771–4.
Jo YY, Shim JK, Soh S et al. Association between Cerebral Oxygen Saturation with Outcome in Cardiac surgery: brain as an Index Organ. J Clin Med 2020;9.
Ely EW, Inouye SK, Bernard GR, et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU). JAMA. 2001;286:2703–10.
Evered L, Silbert B, Knopman DS, et al. Recommendations for the nomenclature of cognitive change associated with anaesthesia and surgery-2018. Br J Anaesth. 2018;121:1005–12.
Lei L, Katznelson R, Fedorko L, et al. Cerebral oximetry and postoperative delirium after cardiac surgery: a randomised, controlled trial. Anaesthesia. 2017;72:1456–66.
Schoen J, Meyerrose J, Paarmann H, et al. Preoperative regional cerebral oxygen saturation is a predictor of postoperative delirium in on-pump cardiac surgery patients: a prospective observational trial. Crit Care. 2011;15:R218.
Hong SW, Shim JK, Choi YS, et al. Prediction of cognitive dysfunction and patients’ outcome following valvular heart surgery and the role of cerebral oximetry. Eur J Cardiothorac Surg. 2008;33:560–5.
Kotfis K, Szylinska A, Listewnik M, et al. Early delirium after cardiac surgery: an analysis of incidence and risk factors in elderly (>/=65 years) and very elderly (>/=80 years) patients. Clin Interv Aging. 2018;13:1061–70.
Brooks K, Anwar S, Stacey S. Cerebral oximetry and postoperative delirium after cardiac surgery. Anaesthesia. 2018;73:647–8.
Liang J, Chen CX, Zhang WH. Effects of CGA comprehensive assessment and intervention model on constipation and nutritional status of elderly cerebral hemorrhage patients. J HeBei United University(Health Sciences). 2021;01:27–32.
Jufar AH, Lankadeva YR, May CN, et al. Renal and cerebral hypoxia and inflammation during cardiopulmonary bypass. Compr Physiol. 2021;12:2799–834.
Joshi B, Brady K, Lee J, et al. Impaired autoregulation of cerebral blood flow during rewarming from hypothermic cardiopulmonary bypass and its potential association with stroke. Anesth Analg. 2010;110:321–8.
Eertmans W, De Deyne C, Genbrugge C, et al. Association between postoperative delirium and postoperative cerebral oxygen desaturation in older patients after cardiac surgery. Br J Anaesth. 2020;124:146–53.
Ellis L, Murphy GJ, Culliford L, et al. The effect of patient-specific cerebral oxygenation monitoring on postoperative cognitive function: a Multicenter Randomized Controlled Trial. JMIR Res Protoc. 2015;4:e137.
Gosselt AN, Slooter AJ, Boere PR, et al. Risk factors for delirium after on-pump cardiac surgery: a systematic review. Crit Care. 2015;19:346.
Inouye SK, Marcantonio ER, Kosar CM, et al. The short-term and long-term relationship between delirium and cognitive trajectory in older surgical patients. Alzheimers Dement. 2016;12:766–75.
Acknowledgements
Not applicable.
Funding
This study is supported by Wenzhou Science and Technology Bureau (2020Y0615).
Author information
Authors and Affiliations
Contributions
N.C., Y.M. and Q.Z. designed this work. M.X., S.C. and W.G. performed the data extraction and statistical analyses. J.W., X.W. and J.W. wrote this article. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study complied with the Declaration of Helsinki and was approved by The First Affiliated Hospital of Wenzhou Medical University Ethics Committee (ethics number: KY2021-130). Informed consent was signed by the included patients and their families.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Chen, N., Mo, Yc., Xu, M. et al. Risk factors for postoperative delirium in elderly patients undergoing heart valve surgery with cardiopulmonary bypass. J Cardiothorac Surg 19, 106 (2024). https://doi.org/10.1186/s13019-024-02568-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13019-024-02568-3