Abstract
Objective
This study investigates the efficacy of using a long-acting insulin analog, along with the infusion of regular insulin, in achieving appropriate glycemic control and correcting lactic acidosis in patients post orthotopic heart transplant who demonstrate severe lactic acidosis and insulin resistance.
Methods
This was a retrospective study of two cohorts (IRB FLA 20-003) of patients post orthotopic heart transplant with severe lactic acidosis and insulin resistance who were admitted to a tertiary intensive care unit and treated with (group 1) or without long-acting insulin analog (group 2) within the first 24 h of admission to the intensive care unit. Insulin resistance is defined as the requirement for intravenous regular insulin infusion of more than 20 units/h without the ability to achieve appropriate serum glucose level (120–180 mg /dL). Severe lactic acidosis is defined as arterial lactic acid of more than 10 mmol/L. The following parameters were investigated: time to correct lactic acidosis, duration of postoperative mechanical ventilation, the need for periprocedural mechanical circulatory support, and 28-day mortality.
Results
The 28-day mortality was zero in both groups. Two patients required periprocedural mechanical support in group one, and ten patients required mechanical support in group two (RR = 0.224, 95%, confidence interval 0.052–0.95, Z = 2.029, p = 0.042). Three patients required tracheostomy in group one, and four patients required tracheostomy in group two (RR 0.84, 95 confidence interval 0.20–3.48, Z = 0.23, P = 0.81). Wilcoxon rank-sum test was used to compare time to correct lactic acidosis, with lactic acid resolution being faster in group one (\(\overline{X}\)1 = 19.7 h, SD ± 12.6 h \(\overline{X}\)2 = 29.3 h, SD ± 19.6 h, Z-value − 2.02, p-value 0.043). The duration of mechanical ventilation was less in group one (\(\overline{X}\)1 = 29 h, SD ± 12.7 h, \(\overline{X}\)2 = 55.1 h, SD ± 44.5 h, Z-value: − 1.92, p-value 0.05).
Conclusion
Administration of low-dose long-acting insulin glargine led to the resolution of the lactic acidosis, insulin resistance, and decreased requirements for pressor and inotropic support, which led to decreased need for mechanical circulatory support.
Similar content being viewed by others
Introduction
Glycemic control is an essential component of periprocedural care for patients status post heart transplantation. Both acute and chronic insulin resistance lead to transplant graft malfunction, increased need for pressor and inotropic support and fluid resuscitation. The standard practice in intensive care is to use intravenous infusion of regular insulin. This practice has been promoted for its ease of titration and the short half-life of the regular insulin. Unfortunately, in many situations post heart transplantation, patients develop insulin resistance, lactic acidosis, and high requirements for epinephrine infusion. We reviewed the efficacy of using long-acting insulin analog, along with the infusion of regular insulin, in achieving glycemic control in patients with severe lactic acidosis and insulin resistance. Additionally, we examined if rapid correction of lactic acidosis leads to decreased epinephrine infusion requirement, time to extubation, and 28-day mortality, as well as the need for postoperative mechanical support.
Study design, setting, and participants
This was a retrospective study of two cohorts of patients post orthotopic heart transplant with severe lactic acidosis and insulin resistance who were admitted to a tertiary intensive care unit treated with (33 patients) or without long-acting insulin analog (37 patients) within the first 24 h of intensive care unit admission. There were 57 males and 13 females in this study. The average age was 57.5 years. In group one, 42% of the patients had diagnosis of type II diabetes mellitus, compared to 35% in group two. Insulin resistance was defined as insulin infusion administration at a dose of at least 20 units/h to achieve serum glucose level of 120–180 mg/dL, and severe lactic acidosis was defined as lactic acid of more than 10 mmol/L. All patients were managed based on a standard postoperative protocol with the goal to maintain cardiac index \(\ge\) 3 L/min/ M2, CVP: (8–10) cm.H2O, PADP: (12–16) cm.H2O, SVO2 \(\ge\) 60%. On arterial blood gas analysis, lactic acid was measured every 4 h. Glycemic control was achieved by using an infusion of regular insulin with goal serum glucose 120–180 mg/dl per institutional protocol. The following parameters were analyzed: time to correct lactic acidosis, duration of postoperative mechanical ventilation, the need for periprocedural mechanical support, and 28-day mortality.
Results
Seventy patients were reviewed, all of whom met the criteria for perioperative insulin resistance Table 1. 33 patients were treated with glargine in addition to regular insulin infusion (group one), and 37 were treated with intravenous insulin infusion only (group two). The 28-day mortality was zero in both groups. Two patients required periprocedural mechanical circulatory support in group one, and ten patients required mechanical support in group two (RR = 0.224, 95%, confidence interval 0.052–0.95, Z = 2.029, p = 0.042). Three patients required tracheostomy in group one, and four patients required tracheostomy in group two (RR 0.84, 95 confidence interval 0.20–3.48, Z = 0.23, P = 0.81). Wilcoxon rank-sum test was used to compare time to correct lactic acidosis; lactic acid resolution was faster in group one (\(\overline{X}\)1 = 19.7 h, SD ± 12.6 h, \(\overline{X}\)2 = 29.3 h, SD ± 19.6 h, Z-value − 2.02, p-value 0.043). The duration of mechanical ventilation was less in group one (\(\overline{X}\)1 = 29 h, SD ± 12.7 h, \(\overline{X}\)2 = 55.1 h, SD ± 44.5 h, Z-value: − 1.92, p-value 0.05).
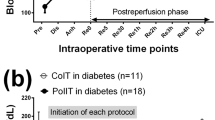
The timeline resolution of insulin resistance after administration of insulin analog in three patients with refractory insulin resistance Fig. 1.
Discussion
Appropriate glycemic control in patients post open heart surgery leads to a lower incidence of sternal wound infection [1] and decreased mortality [2]. Insulin therapy is an essential component of periprocedural care post heart transplantation, as acute and chronic insulin resistance both lead to graft malfunction. Heart failure is known to coexist with type II diabetes mellitus, and this remains true for all heart failure patients including those with low or preserved left ventricular systolic function [3]. Despite the relationship between insulin resistance and heart failure, there currently exists no diagnostic criteria or guidelines for treatment. Moreover, the concept of myocardial insulin resistance is not well studied and difficult to diagnose in clinical practice.
Multiple studies have shown diabetes mellitus often coexists with heart failure, suggesting that these pathologies exacerbate each other clinically. In heart failure patients who receive mechanical circulatory support, insulin resistance improved [4]. It has been shown that activation of B2AR (beta-2 adrenergic receptors) regulates cardiac glucose uptake and promotes insulin resistance, a condition that was reported in heart failure. Insulin stimulation of B2AR promotes coupling with Gi and expression of phosphodiesterase 4 which compromise cardiac contractility [5,6,7]. This cycle in which activation of B2AR leads to insulin resistance and impaired cardiac function increases the requirement for inotropic support [8].
Insulin glargine (Lantus) is a long-acting human insulin analog that is used in patients in type I and type II diabetes. Insulin glargine differs from human insulin in that the amino acid asparagine at A21 is replaced by glycine and two arginines are added to the C-terminus of the B-chain. In our cohort, infusion of large doses of regular insulin did not lead to adequate glycemic control or correction of lactic acidosis. The addition of low dose long acting-insulin analog led to the resolution of the lactic acidosis, significant decrease in the dose of infused regular insulin, as well as decrease of the required epinephrine dose.
The timeline of this resolution matches the timing of long-acting insulin analog administration. The effect starts 4 h post subcutaneous infusion and lasts for 24 h. Within 4 h of administration of insulin analog, insulin requirements begin to decline dramatically to achieve our goal serum glucose of 120–180 mg/dL. Within a few hours, epinephrine infusion was titrated to maintain the standard cardiac index goal, which was associated with normalization of the lactic acid. Numerous etiologies are responsible for the periprocedural lactic acidosis post heart transplant, including circulatory failure, regional tissue ischemia, sepsis with liver being the primary organ responsible for lactate clearance. The resolution of lactic acidosis is dependent on the resolution of the underlying pathology.
In our series the rapid resolution of lactic acidosis was predictable post administration of insulin analog. As per our standard postoperative protocol, patient’s hemodynamics were managed to maintain cardiac index \(\ge\) 3L/min/M2, the inotropic support and volume management are titrated to achieve this goal. Interestingly after the correction of the lactic acidosis the epinephrine infusion requirements decreased significantly, this pattern of recovery indicated that the initial event was the correction of the lactic acidosis by most likely the modulation of beta-adrenergic receptors and that subsequently lead to decrease of epinephrine dose infusion requirements. Figure 1 presents the timeline resolution of lactic acidosis of sample of three patients with insulin resistance and type II diabetes mellitus after administration of long-acting insulin analog post orthotopic heart transplant.
Currently, due to the benefit of adding long-acting insulin analog, it is an accepted practice in our institution to add glargine along with the intravenous insulin infusion to patients with insulin resistance and severe lactic acidosis. In patients without episodes of hypoglycemia, we caution that the required intravenous insulin dose will decrease after the administration of the insulin analog. Based on efficacy compared to regular insulin (one to one), the administration of low dose glargine is not comparable to the very large doses of the infused regular insulin. One possible explanation is that the long-acting insulin analog normalizes the B2AR receptor activation, where regular insulin performs in normal fashion.
Similar observations have been reported in non-critically ill patients with type II diabetes mellitus, in whom the level of HOMA-insulin resistance index (HOMA-IR) in the insulin-glargine group was significantly lower than that observed in the standard-care group [9]. These findings in heart transplant patients are more profound because cardiac denervation, along with the loss of the presynaptic neuronal uptake, leads to hypersensitivity to uptake-1 dependent catecholamines [10]. The preoperative diagnosis of diabetes mellitus type II has been identified as a risk factor for periprocedural insulin resistance and severe lactic acidosis. The difference between the cohort groups could be attributed to the fact that prolonged state of severe lactic acidosis may be subject to more aggressive fluid resuscitation, which could contribute to prolonged post-operative requirements for ventilatory and inotropic support. These findings must be further explored in a randomized trial, as the implications of these findings could affect the management of insulin resistance in patients with HF.
Conclusion
Adding low-dose long-acting insulin glargine to the insulin infusion had facilitated the resolution of the lactic acidosis, insulin resistance and decreased requirements for pressor and inotropic support in heart transplant patients with insulin resistance, which led to the decrease in the periprocedural requirements for mechanical support.
Availability of data and materials
The data is saved on the Cleveland Clinic Computer system.
References
Furnary AP, Zerr KJ, Grunkemeier GL, Starr A. Continuous intravenous insulin infusion reduces the incidence of deep sternal wound infection in diabetic patients after cardiac surgical procedures. Ann Thorac Surg. 1999;67(2):352–60 (discussion 360–362).
Furnary AP, Gao G, Grunkemeier GL, Wu Y, Zerr KJ, Bookin SO, Floten HS, Starr A. Continuous insulin infusion reduces mortality in patients with diabetes undergoing coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2003;125(5):1007–21.
Seferovic PM, Petrie MC, Filippatos GS, Anker SD, Rosano G, Bauersachs J, Paulus WJ, Komajda M, Cosentino F, de Boer RA, et al. Type 2 diabetes mellitus and heart failure: a position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2018;20:853–72.
Uriel N, et al. Improved diabetic control in advanced heart failure patients treated with left ventricular assist devices. Eur J Heart Fail. 2011;13(2):195–9.
Mangmool S, et al. beta-adrenergic receptor and insulin resistance in the heart. Biomol Ther (Seoul). 2017;25(1):44–56.
Ciccarelli M, et al. G protein-coupled receptor kinase 2 activity impairs cardiac glucose uptake and promotes insulin resistance after myocardial ischemia. Circulation. 2011;123(18):1953–62.
Fu Q, et al. Insulin inhibits cardiac contractility by inducing a Gi-biased beta2-adrenergic signaling in hearts. Diabetes. 2014;63(8):2676–89.
Cook SA, Varela-Carver A, Mongillo M, Kleinert C, Khan MT, Leccisotti L, Strickland N, Matsui T, Das S, Rosenzweig A, et al. Abnormal myocardial insulin signalling in type 2 diabetes and left-ventricular dysfunction. Eur Heart J. 2010;31:100–11.
Li J, Feng Z, Li Q, He Y, Zhao C, He J. Insulin glargine effectively achieves glycemic control and improves insulin resistance in patients with early type 2 diabetes that exhibit a high risk for cardiovascular disease. Exp Ther Med. 2014;8(1):147–52. https://doi.org/10.3892/etm.2014.1688.
Koglin J, Gross T, Uberfuhr P, von Scheidt W. Time-dependent decrease of presynaptic inotropic supersensitivity: physiological evidence of sympathetic reinnervation after heart transplantation. J Heart Lung Transplant. 1997;16:621–8.
Acknowledgements
The retrospective review, data collection and analysis were approved by the internal review board at Cleveland Clinic Florida, protocol (FL20-003).
Funding
No funding was received for the creation of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the creation of the manuscript. RD is the lead author; EC created the graph.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Darwish, R., Chen, E., Minear, S. et al. Resolution of insulin resistance, lactic acidosis, and decrease in mechanical support requirements in patients post orthotopic heart transplant with the use of long-acting insulin glargine. J Cardiothorac Surg 19, 99 (2024). https://doi.org/10.1186/s13019-024-02543-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13019-024-02543-y