Abstract
Midventricular hypertrophic obstructive cardiomyopathy (HOCM) is characterized by hypertrophy of the interventricular septum (IVS) and - in rare cases - of the papillary muscles (PM), which subsequently can cause dynamic left ventricular outflow tract obstruction (LVOTO) and severe heart failure symptoms. We report on a rare case of a 44-year-old patient suffering from midventricular HOCM with hypertrophic anterolateral PM and an additional chorda between the PM and the IVS.
We describe a new surgical approach via right anterolateral thoracotomy in 3-dimensional (3D) video-assisted minimal-invasive technique with resection of hypertrophic PMs as well as the entire mitral valve-apparatus and valve replacement avoiding septal myectomy and potentially associated complications. After an uneventful procedure clinical symptoms improved from NYHA III-IV at baseline to NYHA 0-I postoperatively and remained stable over a follow-up period of 24 months. Therefore, the presented technique may be considered as a new and alternative approach in patients with hypertrophic PMs and hypertrophic IVS as subtype of midventricular HOCM.
Similar content being viewed by others
Background
Midventricular hypertrophic obstructive cardiomyopathy (HOCM) is a subtype of hypertrophic cardiomyopathy, which is characterized by hypertrophy of anterolateral and posteromedial papillary muscles (PM) and the interventricular septum (IVS). Additionally, anomalies of PMs and abnormal chordal attachment of the anterior mitral leaflet (AML) to the IVS can cause systolic anterior motion (SAM) and dynamic left ventricular outflow tract obstruction (LVOTO) with concomitant mitral regurgitation. Common treatment for HOCM is a myectomy of the hypertrophic IVS. However, surgical treatment of midventricular HOCM is usually challenging. The hypertrophic area cannot be reached via a transaortic approach. For that reason, a transapical ventriculostomy has been described as preferred access for surgical correction [1, 2]. In some cases of diffuse HOCM myectomy has been performed via trans-mitral septal myectomy with a video-assisted minimal invasive 2-dimensional (2D) technique [3].
We present a new approach with resection of hypertrophic PMs and MV replacement without septal myectomy in a patient with midventricular HOCM and hypertrophic PMs conducted via right anterolateral thoracotomy in 3-dimensional (3D) video-assisted minimal-invasive technique.
Case presentation
A 44-year-old man with known midventricular HOCM was referred to our center with progressive heart failure symptoms (NYHA III-IV) and atrial fibrillation, despite optimal medical therapy. Transthoracic echocardiography (TTE) revealed severe diastolic dysfunction of the left ventricle with an E-wave-amplitude of 147 cm/s, an E/e’ of 18,5 and a left atrium volume (LAV)-index of 65,4 ml/m2. It also showed a pronounced hypertrophic IVS causing a SAM phenomenon with concomitant mild mitral regurgitation and a peak left ventricular outflow tract (LVOT) gradient of 28mmHg with no aggravation during stress echocardiography. Transesophageal echocardiography (TEE) demonstrated significant hypertrophy of the anterolateral PM and an additional chorda between the PM and the IVS. To clarify the exact anatomy of involved structures cardiac magnetic resonance imaging (cMRI) was performed (Fig. 1 preoperative cMRI). It showed a maximum IVS diameter of 29 mm at midventricular inferoseptal areas and confirmed PM hypertrophy and mild mitral regurgitation. Exercise stress electrocardiography showed horizontal ST-segment depressions in II, III and aVF with 1,3 − 1,5mV at J80-point with no T abnormalities. The septal branch appeared to be not suitable for alcohol ablation in cardiac angiogram.
Short axis and 4-chamber view pre- and postoperative cMRI of the hypertrophic obstructive cardiomyopathy. The volume of the LV-cavity after resection of PMs increased. cMRI- cardiac magnetic resonance imaging, AL – anterolateral papillary muscle, PM – posteromedial papillary muscle, MR – mitral valve replacement
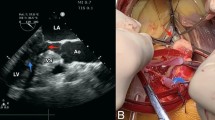
After interdisciplinary heart team discussion surgical treatment was recommended. We performed a right anterolateral mini-thoracotomy after initiating extracorporeal circulation via the femoral vessels. After aortic x-clamping and induction of cardioplegic arrest, the left atrium was opened. In order to obtain optimal view on subvalvular structures and to identify the hypertrophic structures a 3D-Video-system (Endoeye 3D, Olympus Co., Tokyo, Japan) was used. After cryoablation the MV apparatus including chordae tendineae and both hypertrophic PMs was resected (Fig. 2) and a mechanical valve prothesis (St. Jude Medical 31 mm) was implanted. Notably, myectomy of the IVS was not performed. Figure 1postoperative cMRI shows the postoperative result. Comparison between pre- and postoperative MRI (Table 1) found increased indexed LV enddiastolic volume from 45 ml/m² to 49 ml/m² with corresponding increase in LV stroke volume elevation from 79 ml to 84 ml respectively (Fig. 1postoperative cMRI).
The postoperative course was uneventful and the patient was discharged without any residual symptoms. Two years after the operation, the patient remains free from symptoms.
Discussion and conclusion
Midventricular HOCM with severe hypertrophy of PMs is a uncommon subtype of HCM, which can cause dynamic intraventricular obstruction without LVOTO [4]. In our case, TTE showed peak LVOT gradient of only 28mmHg but confirmed severe diastolic dysfunction of the LV with significant hypertrophy of PMs and IVS. The patient suffered from severe heart failure symptoms. This procedure was performed as last remaining option before being listed for heart transplantation.
Despite substantive myectomy, cMRI LV functional parameters only changed minimally, however clinical symptoms alleviated significantly from NYHA III-IV to NYHA 0-I and remained stable 24 months postoperatively. We consider that reduction of midventricular stenosis as shown by cMRI and reduction of diastolic LV dysfunction are the causes of clinical improvement. However, myectomy is reported to improve diastolic function in patients with HOCM [5].
Resection of the hypertrophic IVS in midventricular HOCM is usually challenging as mentioned above. Septal myectomy holds the risk of an iatrogenic ventricular septal defect (VSD) [6]. In comparison with IVS resection, the presented technique was simple, secure and effective avoiding sternotomy. However, to exclude any chance of SAM phenomenon the MV was replaced. 3D scope was helpful to resect papillary muscles precisely up to the level of the apex. Although we did not try additional cryoablation might be effective in this setting.
In conclusion, the presented technique may be considered as new and alternative approach in patients with hypertrophic PMs and hypertrophic IVS as subtype of midventricular HOCM.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AML:
-
anterior mitral leaflet
- HOCM:
-
hypertrophic obstructive cardiomyopathy
- IVS:
-
interventricular septum
- LAV:
-
left atrium volume
- LV:
-
left ventricle
- LVOT:
-
left ventricular outflow tract
- LVOTO:
-
left ventricular outflow tract obstruction
- MV:
-
mitral valve
- cMRI:
-
cardiac magnetic resonance imaging
- PM:
-
papillary muscle
- SAM:
-
systolic anterior motion
- TTE:
-
transthoracic echocardiography
- TEE:
-
transesophageal echocardiography
- VSD:
-
ventricular septal defect
References
Kotkar KD, et al. Hypertrophic obstructive cardiomyopathy: the Mayo Clinic experience. Ann Cardiothorac Surg. 2017;6(4):329–36.
Tang Y, et al. Extended myectomy for hypertrophic obstructive cardiomyopathy patients with midventricular obstruction. Eur J Cardiothorac Surg. 2018;54(5):875–83.
Sakaguchi T, et al. Minimally invasive trans-mitral septal myectomy for diffuse-type hypertrophic obstructive cardiomyopathy. Gen Thorac Cardiovasc Surg. 2018;66(6):321–6.
Slama I, et al. Accessory and solitary main papillary muscle hypertrophy resulting in dynamic mid-left ventricular obstruction: contribution of multimodality imaging in highlighting of dynamic and structural abnormalities. J Cardiol Cases. 2018;18(3):113–7.
Qiulan Y, et al. Impact of septal myectomy on diastolic function in patients with obstructive hypertrophic cardiomyopathy. J Thorac Dis. 2021;13(8):4925–34.
Barioli A, et al. Transcatheter closure of complex iatrogenic ventricular septal defect: a case report. Eur Heart J Case Rep. 2020;4(3):1–5.
Acknowledgements
We acknowledge support by the Open Access Publication Funds of the Ruhr-Universität Bochum.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
J.B. – Conceptualization, Methodology; Writing – original draft.J.E. – VisualizationM.-A.D. – Writing, review & editingJ.F.G. – Writing, review & editingM.H. – Conceptualization; Investigation, Resources, Writing – review & editing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Informed consent is available.
Authors’ information
MH has performed the operation.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Berg, J.J., Eckstein, J., Deutsch, MA. et al. Resection of hypertrophic papillary muscles and mitral valve replacement in a patient with midventricular hypertrophic obstructive cardiomyopathy – a new approach. J Cardiothorac Surg 19, 105 (2024). https://doi.org/10.1186/s13019-024-02529-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13019-024-02529-w