Abstract
Objectives
Cardiogenic shock (CS) can occur in patients with Takotsubo syndrome (TTS). As TTS has received increasing attention and has been more closely researched, several aspects of the pathogenesis have been identified, particularly that an excessive release of catecholamines plays an important role. Nevertheless, evidence on specific therapy concepts is still lacking. As a result, TTS with severe hemodynamic instability and low cardiac output creates unique challenges, and mechanical circulatory support is needed with as few inotropic drugs as possible.
Methods
We present a 77-year-old female patient who underwent minimally invasive surgical mitral valve replacement. After an uneventful course, the patient developed acute heart failure eleven days after surgery. Transthoracic echocardiography (TTE) revealed a new onset of TTS. The patient needed left ventricular venting and full haemodynamic flow. We successfully implanted a microaxial left ventricular assist device (Impella 5.5) using the transaxillary approach. The haemodynamic situation stabilised immediately. The patient was weaned and the Impella 5.5 was explanted after five days.
Conclusion
We present the first-in-man implantation of a transaxillary Impella 5.5 in a patient with TTS. The patient benefitted from Impella 5.5 therapy with full haemodynamic support and venting of the left ventricle.
Similar content being viewed by others
Backround
It can be assumed that 1–3% [1] of all patients presenting with symptoms which lead to acute coronary syndrome (ACS) have TTS. During the COVID-19 pandemic the incidence of patients presenting with ACS with subsequently confirmed TTS increased to 7.8% [2].
The incidence of cardiogenic shock (CS) in patients with TTS is estimated as ranging between 6 and 20% [3, 4]. Patients with TTS in CS have a high in-hospital mortality of 15% [5]. Both the Mayo Clinic Criteria [6] and the ESC Heart Failure Association criteria [4] are used as instruments for diagnosing TTS. They include echocardiographic evidence of hypo-, a- or dyskinesia extending beyond a single coronary vascular territory, as well as exclusion by coronary angiography of coronary atherosclerosis as a causal agent of regional wall motion abnormality. Physical or emotional triggers may additionally be present. Current evidence suggests that the pathogenesis of TTS is based on an acute surge of catecholamines through activation of the sympathetic nervous system or a result of drug therapy; it occurs in patients with an increased susceptibility of the coronary microcirculation and of cardiac myocytes to stress hormones [7,8,9,10]. The resulting downregulation of myocardial function can be understood as a protective mechanism caused by a severe reduction of perfusion.
Owing to the versatile pathogenesis and triggers of TTS, adequate and evidence-based therapy concepts are lacking; however, there is evidence that the use of catecholamines for circulatory support should be avoided, especially when patients additionally exhibit left ventricular outflow tract obstruction (LVOTO) [11,12,13]. As mechanical circulatory support (MCS) devices are increasingly used in patients with CS and are recommended for SCAI shock stages C, D and E [14], they are also used in patients with TTS with good results [15, 16].
The Impella 5.5 is a transvalvular, microaxial left ventricular assist device (LVAD) designed to provide circulatory support and myocardial unloading. The Impella 5.5, with its high transaortic flow capacity of 5.5 L/min, offers full flow circulatory support and, along with this, sufficient venting of the left ventricle. This generation of Impella can be implanted surgically using a transaxillary approach enabling patient mobilisation and rehabilitation.
Case presentation
Patient details
We present a 77-year-old female patient who was initially hospitalised due to severe primary mitral regurgitation and consequent recurrence of cardiac decompensation. The patient suffered from dyspnoea (NYHA class III). Due to calcification of the anterior and posterior mitral valve leaflet as well as an effective regurgitation orifice area (EROA) of 0.3 cm2 and a regurgitant volume (RV) of 55 mL as evaluated in echocardiography, an indication for minimally invasive mitral valve replacement was established.
The patient had previously suffered three non-ST-elevated myocardial infarctions (NSTEMI) in August 2019, February 2022 and October 2022, all of which were treated with drug-eluting stents (DES), two in the proximal left anterior descending artery (LAD) and one in the first diagonal branch. In addition to cardiovascular risk factors, the patient exhibited chronic heart failure with preserved ejection fraction (HFpEF). Preoperative transoesophageal echocardiography (TEE) confirmed an LVEF of 57%, LVIDd of 4.8 cm and LVIDs of 2.3 cm.
The patient underwent successful minimally invasive mitral valve replacement with a 29 mm biological prosthesis. The patient was extubated six hours after the surgery and was transferred to the normal ward on the first postoperative day (POD). The postoperative course was uneventful, a postoperative TTE confirmed a good LVEF equivalent to the preoperative level and excluded new wall motion abnormalities. On the eleventh POD, TTE showed a reduction of the LVEF to 33% and apical akinesia (Video 1). The papillary muscles of the mitral valve were visualized and found to be intact; there was also no evidence of a systolic anterior motion phenomenon of the anterior mitral valve leaflet. Emergency coronary angiography was performed which ruled out myocardial ischaemia secondary to coronary macrovascular obstruction and confirmed apical ballooning. The patient was haemodynamically impaired and decompensated, necessitating intubation and stabilisation with high-dose inotropes (vasoactive-inotropic score: 19).
The patient’s status was evaluated and the need for full circulatory support and left ventricular (LV) unloading by LV venting was confirmed. Following our evaluated standard operating procedure (SOP) for temporary MCS allocation in CS patients [17], a decision was made to implant an Impella 5.5 using a transaxillary approach within the scope of a bridge-to-recovery concept.
Surgical procedure
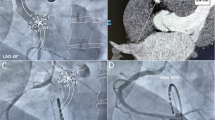
After induction of general anaesthesia, the right axillary artery was exposed through a 6 cm incision of the skin in the infraclavicular fossa. A 10 mm HEMASHIELD vascular graft (Getinge AB, Sweden) was anastomosed to the artery, tunnelled outside the wound and diverted through the skin (Fig. 1). The Impella 5.5 device was passed through the anastomosis into the correct position in the left ventricle under fluoroscopic and echocardiographic guidance (Video 2, 3, 4).
The patient was haemodynamically stabilised and rapidly weaned off inotropic support. The patient was extubated on the first day after implantation. The duration of circulatory support on Impella 5.5 was 5 days and 18 h.
The Impella was weaned successfully. Surgical explantation of the Impella 5.5 was scheduled in the operating room on the fifth day after implantation. After removal of the Impella, the vascular graft through which the Impella was introduced into the axillary artery was shortened, ligated, and sunk under the pectoralis muscle. After three days the patient was transferred to a local hospital. Ten days after the start of MCS, TTE showed an LVEF of 55% and an LVEDD of 36 mm. Due to pre-existing coronary artery disease and to stabilize the restored pump function, heart failure therapy was established in the later course after Impella explantation. The last follow-up before discharge to outpatient follow-up took place 15 days after explantation and still showed recovery of cardiac pump function.
Discussion
TTS is a transient wall motion abnormality. Recovery of apical dyskinesia often appears rapidly and is part of the diagnostic criteria [4].
If TTS as an acute heart failure syndrome leads to CS the patient must be bridged to cardiac recovery, which by definition is the case in all surviving TTS patients.
The challenge of managing CS in the context of TTS is that LV unloading and circulatory support with an increase in LV output should be accomplished with as few inotropic drugs as possible. In this context, MCS is an appropriate and increasingly applied treatment option that yields good results [18]. For TTS-CS patients, the Japanese InterTAK Registry describes a reduced in-hospital mortality in patients supported with MCS versus those not supported with MCS [19]. The indication for MCS in TTS-CS patient is based on the management recommendations for the SCAI Shock Stages [20]. As the complication rates under MCS increase with time on support, MCS support should be kept as short as possible. Owing to the transient character of LV dysfunction in TTS, MCS support duration in this context is relatively short [15, 18, 21]. However, there is some uncertainty as to how long MCS support should, in fact, be.
The pathophysiology of TTS and the mechanisms of different MSC devices can help in selecting the appropriate device; however, evidence for the superiority of one device over other devices is lacking. The use of intra-aortic balloon pumps (IABP) in TTS-CS is decreasing [18] due to the fact that this therapy is only able to increase the cardiac index to a limited extent and can worsen or even cause LVOTO [21]. MCS with veno-arterial extracorporeal membrane oxygenation (v-a ECMO) increases LV afterload as the circulatory support is delivered retrograde, which can cause a further increase in the already elevated LVEDP.
In general, the advantage of the microaxial LVAD is that it provides physiological cardiac support which improves coronary and end-organ perfusion while simultaneously unloading the left ventricle, thereby allowing the initiation of guideline-directed medical therapy. The positive effect of LV unloading becomes clear when comparing patients in CS treated with ECMO alone or with ECMELLA (ECMO plus Impella). LV unloading has been associated with a lower 30-day mortality [22]. MCS with ECMELLA also provides LV unloading and full circulatory support, but evidence suggests that MCS with two devices is also associated with higher complication rates [22]. Recently published case reports and series [15, 23] confirmed the pathophysiological assumed benefit of MCS support via Impella in TTS-CS and showed noteworthy survival rates and excellent myocardial recovery. These publications included cases in which Impella CP, Impella 2.5 and Impella 5.0 (only once) were used.
In our case study we were able to stabilise the patient’s hemodynamics immediately, thereby avoiding further deterioration and end-organ failure. Furthermore, haemodynamic stabilisation and weaning of inotropes prompted rhythm control and respiratory stabilisation (due to volume redistribution to the intravascular system). Our strategy of providing early MCS support with a temporary device to provide full circulatory support and immediate unloading of the impaired LV together with a short duration of MCS enabled a favourable outcome in our patient. It should be noted, however, that MCS therapy is an invasive procedure that can also lead to various complications in the long and short term [24].
Data Availability
All data are available in electronic medical records.
Change history
08 January 2024
A Correction to this paper has been published: https://doi.org/10.1186/s13019-023-02475-z
References
Napp LC, Bauersachs J. Takotsubo syndrome: between evidence, myths, and misunderstandings. Takotsubo-Syndrom: Evidenz, Mythen, Missverständnisse. Herz. 2020;45(3):252–66. https://doi.org/10.1007/s00059-020-04906-2.
Jabri A, Kalra A, Kumar A, Alameh A, Adroja S, Bashir H, Nowacki AS, Shah R, Khubber S, Kanaa’ NA, Hedrick DP, Sleik KM, Mehta N, Chung MK, Khot UN, Kapadia SR, Puri R, Reed G. W. incidence of stress cardiomyopathy during the Coronavirus Disease 2019 Pandemic. JAMA Netw Open. 2020;3(7):e2014780.
Schneider B, Athanasiadis A, Schwab J, et al. Complications in the clinical course of Takotsubo cardiomyopathy. Int J Cardiol. 2014;176:199–205.
Lyon AR, Bossone E, Schneider B, et al. Current state of knowledge on takotsubo syndrome: aposition statement from the task force on Takot-Tsubo syndrome of the Heart Failure Association of the European Society of Cardiology. Eur Heart J Heart Fail. 2016;18:8–27.
Vallabhajosyula S, Dunlay SM, Murphree DH Jr, et al. Cardiogenic shock in Takotsubo Cardiomyopathy Versus Acute Myocardial Infarction: an 8-Year National Perspective on clinical characteristics, management, and outcomes. JACC Heart Fail. 2019;7(6):469–76.
Madhavan M, Rihal CS, Lerman A, Prasad A. Acute Heart Failure in apical ballooning syndrome (TakoTsubo/stress cardiomyopathy): clinical correlates and Mayo Clinic risk score. J Am Coll Cardiol. 2011;57(12):1400–1.
Wittstein IS, Thiemann DR, Lima JA, et al. Neurohumoral features of myocardial stunning due to sudden emotional stress. N Engl J Med. 2005;352(6):539–48.
Wang X, Pei J, Hu X. The brain-heart connection in Takotsubo Syndrome: the Central Nervous System, Sympathetic Nervous System, and Catecholamine Overload. Cardiol Res Pract. 2020;2020:4150291.
Ghadri JR, Wittstein IS, Prasad A, et al. International Expert Consensus Document on Takotsubo Syndrome (Part I): clinical characteristics, Diagnostic Criteria, and pathophysiology. Eur Heart J. 2018;39(22):2032–46.
Nef HM, Möllmann H, Troidl C, et al. Abnormalities in intracellular Ca2 + regulation contribute to the pathomechanism of Tako-Tsubo cardiomyopathy. Eur Heart J. 2009;30(17):2155–64.
Ansari U, El-Battrawy I, Fastner C, et al. Clinical outcomes associated with catecholamine use in patients diagnosed with Takotsubo cardiomyopathy. BMC Cardiovasc Disord. 2018;18(1):54.
Ghadri JR, Wittstein IS, Prasad A, et al. International Expert Consensus Document on Takotsubo Syndrome (Part II): diagnostic workup, outcome, and management. Eur Heart J. 2018;39(22):2047–62.
Bairashevskaia AV, Belogubova SY, Kondratiuk MR, Rudnova DS, Sologova SS, Tereshkina OI, Avakyan EI. Update of Takotsubo cardiomyopathy: Present experience and outlook for the future. Int J Cardiol Heart Vasc. 2022;39:100990.
Baran DA, Grines CL, Bailey S, et al. SCAI clinical expert consensus statement on the classification of cardiogenic shock: this document was endorsed by the American College of Cardiology (ACC), the American Heart Association (AHA), the Society of Critical Care Medicine (SCCM), and the Society of thoracic surgeons (STS) in April 2019. Catheter Cardiovasc Interv. 2019;94(1):29–37.
Napp LC, Westenfeld R, Møller JE, et al. Impella Mechanical Circulatory support for Takotsubo Syndrome with shock: a Retrospective Multicenter Analysis. Cardiovasc Revascularization Medicine: Including Mol Interventions. 2022;40:113–9.
Beneduce A, Fausta Bertoldi L, Melillo F, et al. Mechanical circulatory support with Impella Percutaneous Ventricular assist device as a bridge to recovery in Takotsubo Syndrome complicated by cardiogenic shock and left ventricular outflow tract obstruction. JACC Cardiovasc Intervent. 2019;12(4):e31–2.
Ott S, Lewin D, Nersesian G, Stein J, Just IA, Hommel M, Schoenrath F, Starck CT, O’Brien B, Falk V, Potapov E, Lanmueller P. Improving survival in cardiogenic Shock-A propensity score-matched analysis of the impact of an institutional allocation protocol to short-term mechanical circulatory support. Life (Basel). 2022;12(11):1931.
Mariani S, Richter J, Pappalardo F, et al. Mechanical circulatory support for Takotsubo syndrome: a systematic review and meta-analysis. Int J Cardiol. 2020;316:31–9.
Terasaki S, Kanaoka K, Nakai M, et al. Outcomes of catecholamine and/or mechanical support in Takotsubo syndrome. Heart. 2022;108(18):1467–73. Published 2022 Aug 25.
Napp LC, Medjamia AM, Burkhoff D, Kapur NK, Bauersachs J. The challenge of defining best practice treatment for Takotsubo Syndrome with Shock. Cardiovasc Revasc Med. 2022;42:183–5.
Sangen H, Imori Y, Tara S, Yamamoto T, Takano H, Shimizu W. Haemodynamic deterioration due to intra-aortic balloon counterpulsation in takotsubo cardiomyopathy. Eur Heart J. 2018;39(22):2118.
Schrage B, Becher PM, Bernhardt A, et al. Left ventricular unloading is Associated with Lower Mortality in patients with cardiogenic shock treated with venoarterial extracorporeal membrane oxygenation: results from an International, Multicenter Cohort Study. Circulation. 2020;142(22):2095–106.
Nishikawa R, Nagano N, Kokubu N, et al. Favorable effects of Impella on Takotsubo Syndrome complicated with cardiogenic shock. Int Heart J. 2021;62(6):1430–5.
Nersesian G, Hennig F, Müller M, et al. Temporary mechanical circulatory support for refractory Heart Failure: the German Heart Center Berlin experience. Ann Cardiothorac Surg. 2019;8(1):76–83.
Acknowledgements
None.
Funding
Not applicable.
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
J.K.R.V.M. wrote the original draft, prepared all figures and videos. FS, JK, CTS, EVP, VF edited and reviewed. L.W. conceptualised, edited and reviewed the manuscript.
Corresponding author
Ethics declarations
Ethical approval
All procedures involving the human participant were performed in accordance with the ethical standards of the institutional committee. The authors confirm that written consent for submission and publication of this case report including images and associated text has been obtained from the patient.
Conflict of interest
JKRVM None. AES None. FS Institutional grants from Novartis, Abbott, non-financial support from Medtronic and institutional fees (speaker honoraria) from Orion Pharma outside of the submitted work. JK Grants or contracts from any entity: Edwards, LivaNova. Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events: Edwards, Medtronic, Abbott, LivaNova, CryoLife. Leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid: TC EACTS, ECSC Board, ISMICS Board. CTS Payment to his institution related to his activity as speaker fees, honoraria, consultancy, advisory board fees, investigator, committee member of AngioDynamics, Abiomed, Medtronic, Spectranetics, Biotronik, LivaNova (Sorin) and Cook Medical and departmental or institutional research funding from Cook Medical. EVP Consulting fees: Abbott (institutional grants), Medtronic (institutional grants), Abiomed (institutional grants). Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events: Abbott (institutional grants), Medtronic (institutional grants), Abiomed (institutional grants). Support for attending meetings and/or travel: Abbott (institutional grants), Medtronic (institutional grants), Abiomed (institutional grants). Participation in a Data Safety Monitoring Board or Advisory Board: Abbott, Medtronic. VF Grants or contracts from any entity: Medtronic GmbH, Biotronik SE & Co., Abbott GmbH & Co. KG, Boston Scientific, Edwards Lifesciences, Berlin Heart, Novartis Pharma GmbH, JOTEC/CryoLife GmbH, LivaNova, Zurich Heart. I hereby declare that I have relevant (institutional) financial activities outside the submitted work with the mentioned commercial entities in relation to educational grants (including travel support), fees for lectures and speeches, fees for professional consultation, research and study funds. LW None.
Patient consent
The authors confirm that written consent for submission and publication of this case report including images and associated text was obtained from the patient.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Glossary of Abbreviations
- ECLS
-
extracorporeal life support
- LV
-
left ventricle / left ventricular
- LVAD
-
left ventricular assist device
- LVEF
-
left ventricular ejection fraction
- NYHA
-
New York Heart Association
- TTS
-
Takotsubo syndrome
- CS
-
cardiogenic shock
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
von Mackensen, J.K., Shazly, A.E., Schoenrath, F. et al. Successful treatment of cardiogenic shock due to Takotsubo syndrome with implantation of a temporary microaxial left ventricular assist device in transaxillary approach. J Cardiothorac Surg 18, 343 (2023). https://doi.org/10.1186/s13019-023-02459-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13019-023-02459-z