Abstract
Background
A silent left ventricular thrombus is dangerous. The current standard anticoagulation therapy was ineffective in our case or similar, and the outcome was poor.
Case presentation
A 33-year-old man with a silent left ventricular thrombus was detected incidentally by transthoracic echocardiography. After admission, anti-coagulation with low-molecular-weight heparin therapy was carried out. The CAG revealed 70% systolic stenosis in the middle of the right coronary artery along with myocardial bridging. Unfortunately, an acute left temporal embolism emerged 5 days later, then the patient was transferred to the neurology department for further treatment. One month later, the patient underwent left ventricular thrombectomy, ventricular aneurysm resection, and coronary artery bypass grafting (CABG) and was discharged uneventfully after surgery.
Conclusions
Surgical treatment should be a priority for patients with giant or hypermobile left ventricular thrombus or recurrent systemic emboli.
Similar content being viewed by others
Background
Left ventricular thrombus (LVT) is uncommonly seen in unselected patients by echocardiogram. It is, however, more commonly found in patients with heart failure and acute myocardial infarction [1]. Silent LVT detected by transthoracic echocardiography (TTE) is rare and dangerous in those with normal cardiac function without any history of cardiac diseases. The current standard treatment strategy for LVT is anticoagulation therapy, including vitamin K antagonist (VKA), direct oral anticoagulants, low molecular heparin, and intravenous unfractionated heparin [2, 3]. Nevertheless, surgical intervention should be considered if systemic embolism emerged [2].
Case presentation
A 33-year-old man was referred to the cardiac surgery department due to apical space-occupying lesions detected incidentally by TTE during the routine preoperative examination for urinary calculi. In the outpatient department, the patient's blood pressure, heart rate, and percutaneous oxygen saturation were all within normal range, without any signs of acute or chronic cardiac failure. The patient, however, had a more than 10-year history of drinking and smoking.
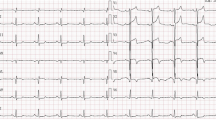
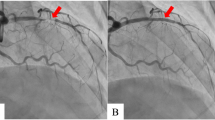
During hospitalization, the electrocardiogram (ECG) suggested a sinus rhythm(Fig. 1). TTE showed an apical space-occupying lesion of about 2*1.5 cm and mild tricuspid regurgitation with a normal range of ejection fraction (EF) at 63%. Coronary angiography (CAG) revealed 30% stenosis in both the middle of the left anterior descending branch and the distal left circumflex branch, and 70% systolic stenosis in the middle of the right coronary artery (RCA) along with myocardial bridging (MB) (Fig. 2).
Besides, Tc-99m myocardial perfusion scintigraphy (resting and activity states) demonstrated that severe myocardial hypoperfusion and hypokinesis in the apex of the left ventricular (LV) were detected. Additionally, the abnormal perfusion myocardium accounted for 19% of the total left ventricular myocardium, with 4% of it being in a hibernated state. Cardiac magnetic resonance imaging (MRI) with delayed enhancement confirmed the formation of the left ventricular aneurysm after myocardial infarction, along with the presence of an apical thrombus (Fig. 3).
Two days after the low molecular weight heparin anticoagulant treatment (Nadroparin calcium, 0.4 ml, subcutaneous injection, every 12 h), the patient developed symptoms consistent with cerebral infarction. MRI examination revealed an acute embolism in the left temporal region. Subsequently, the patient was transferred to the neurology department for further treatment. One month later, after ruling out the possibility of acute cerebral infarction by MRI, cardiac surgery was scheduled for the patient. Median sternotomy, cardiopulmonary bypass with moderate hypothermia, and anterograde and retrograde cold blood cardioplegia were performed. With a 3 cm apical incision, the left ventricular thrombus was fully exposed and completely excised (Fig. 4). Thrombotic debris tissue was carefully cleaned after the thrombectomy. The margin of the aneurysm was carefully inspected, and an intraventricular fresh autologous pericardium patch was utilized to reconstruct the left ventricular morphology. Subsequently, the patch was covered by the aneurysm wall, and the wall itself was closed using continuous sutures with two long gaskets. Finally, aorta-to-right coronary artery bypass grafting was performed through the great saphenous vein. The tracheal tube was removed on the first postoperative day. Seven days later, the patient was discharged uneventfully and prescribed warfarin and aspirin therapy for 1 year. The timeline and major findings are summarized in Fig. 5.
Discussion and conclusions
LVT is mostly found in myocardial infarction, low ejection fraction, LV aneurysms, and ventricular wall akinesis or dyskinesis [4, 5]. The thrombus formation refers to Virchow's triad: blood stasis, endothelial injury, and hypercoagulability [6]. McCarthy et al. reported that the incidence of LVT detected by TTE is only 0.1% in unselected patients. Most LV thrombi are formed within 2 weeks. However, some occur even more later, especially in patients with LV systolic dysfunction [6]. In this case, the patient was unaware of the timing of the left ventricular thrombus formation. It was incidentally discovered during preoperative examination. Echocardiography revealed that the left ventricular thrombus was not a fresh thrombus, indicating that it had been present for a considerable duration of time.
Post-myocardial infarction has been demonstrated as one of the most common risk factors for the development of LVT along with heart failure, alcohol abuse, and tobacco use [1, 7]. In this case, it is worth noting that the patient exhibited three high-risk characteristics including MB in RCA, alcohol abuse, and tobacco abuse, which may be contributed to the development of myocardial infarction. The patient has a history of drinking and smoking for over 10 years, and approximately two years ago, he was transported to the emergency room due to alcohol intoxication. However, the patient has not quit the habit of alcohol abuse and continues to drink excessively on a regular basis. Unfortunately, there has been no improvement in his unhealthy lifestyle. Montone RA et al. reported that coronary spasm with MB is the independent risk factor of myocardial infarction and non-obstructive coronary arteries [8]. During this hospitalization, CAG showed a myocardial bridge in the middle of RCA with 70% systolic stenosis. MRI and myocardial perfusion scintigraphy demonstrated a left ventricular aneurysm with apical thrombus.
Essential thrombocytopenia (ET) is another important incentive that should be considered since it has been demonstrated to attribute to the onset of acute myocardial infarction [9]. To determine if the patient had the ET, complete blood count and gene mutation related to EB were examined. The results showed that the platelet count was 435 × 109 L−1 and there was no mutation in JAK2 V617F, JAK2 exon 12, MPL, and CALR in this patient, which has ruled out the possibility of ET.
LVT remains to be a severe complication associated with a high risk of systemic embolism. According to the latest guidelines [10, 11], several anticoagulation therapies are introduced. The current standard therapy for LVT is chronic warfarin therapy for 3 months at the minimum. In addition, direct oral anticoagulants (DOACs) are recently introduced [3, 12]. The therapeutic dilemmas are: which one is the best? How long the treatment course should take? What's the dose? Several studies have suggested that even following strict anti-coagulant treatment, the prognosis of patients is not satisfactory [13, 14]. Although Lattuca et al. demonstrated that prolonged anticoagulation therapy duration could reduce the occurrence of major adverse cardiovascular events, the bleeding complications were raised [13].
Surgical treatment is another considerable option for patients with LVT, especially for those with giant or hypermobile LVT or recurrent systemic emboli developed undergoing anticoagulant therapy [2]. Lee et al. reported that the operative treatment group tended to have less post-treatment thromboembolism than the anticoagulation and antiplatelet groups [15].
Young patients with MB who have longstanding alcohol abuse and smoking habits have a high chance of sudden MI. Although surgical intervention has some inevitable intrinsic risks, patients would benefit from it. Therefore, we highlight that for patients with giant or hypermobile LVT or recurrent systemic emboli, surgical treatment can be a priority of choice.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Abbreviations
- CABG:
-
Coronary artery bypass grafting
- CAG:
-
Coronary angiography
- ECG:
-
Electrocardiogram
- ET:
-
Essential thrombocytopenia
- LAD:
-
Left anterior descending branch
- LVT:
-
Left ventricular thrombus
- MB:
-
Myocardial bridging
- MRI:
-
Magnetic resonance imaging
- RCA:
-
Right coronary artery
- TTE:
-
Transthoracic echocardiography
- VKA:
-
Vitamin K antagonist
References
McCarthy CP, Murphy S, Venkateswaran RV, Singh A, Chang LL, Joice MG, et al. Left ventricular thrombus: contemporary etiologies, treatment strategies, and outcomes. J Am Coll Cardiol. 2019;73:2007–9.
Demirci G, Guner EG, Corekcioglu B, Guner A, Sen O, Kalkan AK, et al. Left ventricular apical thrombi: silent but terrifying. J Card Surg. 2020;35:3623–5.
Yassin AS, Abubakar H, Mishra T, Adam O, Hartman M, Pahuja M, et al. Rivaroxaban for left ventricular thrombus. Am J Ther. 2019;26:e511–5.
Pasli S, Kamler M, Malik R, Easo J. Complicated massive left ventricular thrombus and surgical treatment. Am J Case Rep. 2022;23:e937341.
Garg P, van der Geest RJ, Swoboda PP, Crandon S, Fent GJ, Foley JRJ, et al. Left ventricular thrombus formation in myocardial infarction is associated with altered left ventricular blood flow energetics. Eur Heart J Cardiovasc Imaging. 2019;20:108–17.
Delewi R, Zijlstra F, Piek JJ. Left ventricular thrombus formation after acute myocardial infarction. Heart. 2012;98:1743–9.
Merkler AE, Alakbarli J, Gialdini G, Navi BB, Murthy SB, Goyal P, et al. Short-term risk of ischemic stroke after detection of left ventricular thrombus on cardiac magnetic resonance imaging. J Stroke Cerebrovasc Dis. 2019;28:1027–31.
Montone RA, Gurgoglione FL, Del Buono MG, Rinaldi R, Meucci MC, Iannaccone G, et al. Interplay between myocardial bridging and coronary spasm in patients with myocardial ischemia and non-obstructive coronary arteries: Pathogenic and prognostic implications. J Am Heart Assoc. 2021;10:e020535.
Singla A, Jagasia D, Garg M, Lowry PA, Stapleton D. Acute st-segment elevation myocardial infarction: a rare initial presentation of previously undiagnosed essential thrombocythemia. Platelets. 2012;23:463–6.
Stevens SM, Woller SC, Kreuziger LB, Bounameaux H, Doerschug K, Geersing GJ, Huisman MV, Kearon C, King CS, Knighton AJ, Lake E, Murin S, Vintch JRE, Wells PS, Moores LK. Antithrombotic therapy for VTE disease: second update of the CHEST guideline and expert panel report. Chest. 2021;160(6):e545–608.
Levine GN, McEvoy JW, Fang JC, Ibeh C, McCarthy CP, Misra A, Shah ZI, Shenoy C, Spinler SA, Vallurupalli S, Lip GYH; American Heart Association Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; and Stroke Council. Management of Patients at Risk for and With Left Ventricular Thrombus: A Scientific Statement From the American Heart Association. Circulation. 2022;146(15):e205–23.
Guddeti RR, Anwar M, Walters RW, Apala D, Pajjuru V, Kousa O, et al. Treatment of left ventricular thrombus with direct oral anticoagulants: a retrospective observational study. Am J Med. 2020;133:1488–91.
Lattuca B, Bouziri N, Kerneis M, Portal JJ, Zhou J, Hauguel-Moreau M, et al. Antithrombotic therapy for patients with left ventricular mural thrombus. J Am Coll Cardiol. 2020;75:1676–85.
Maniwa N, Fujino M, Nakai M, Nishimura K, Miyamoto Y, Kataoka Y, et al. Anticoagulation combined with antiplatelet therapy in patients with left ventricular thrombus after first acute myocardial infarction. Eur Heart J. 2018;39:201–8.
Lee JM, Park JJ, Jung HW, Cho YS, Oh IY, Yoon CH, et al. Left ventricular thrombus and subsequent thromboembolism, comparison of anticoagulation, surgical removal, and antiplatelet agents. J Atheroscler Thromb. 2013;20:73–93.
Acknowledgements
The authors have reported that they had no relationships relevant to the contents of this paper to disclose.
Funding
None.
Author information
Authors and Affiliations
Contributions
LJ finished the surgery for the patient. FH wrote the original manuscript. YJ improved the use of English in the manuscript. They all read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The authors declare that this case report is in agreement with the Ethics Committee of Zhejiang University. Written consent has been obtained for this study.
Consent for publication
We announce that all presentations of this case report have consent to publish.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
He, F., Jiao, Y. & Jiang, L. Case report: hunting the hidden: surgical treatment of chronic silent thrombus in the left ventricle in a young alcoholic patient with myocardial bridging. J Cardiothorac Surg 18, 308 (2023). https://doi.org/10.1186/s13019-023-02414-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13019-023-02414-y