Abstract
Background
Limited data on the prognostic value of periprocedural changes of plasma N-terminal pro-B-type natriuretic peptide (NT-proBNP) after transcatheter aortic valve replacement (TAVR).
Methods
Data of plasma NT-proBNP were retrospectively collected in 357 patients before TAVR procedure and at discharge from January 1, 2018 to December 31, 2021 in our single center. Patients were grouped as responders and non-responders according to the NT-proBNP ratio (postprocedural NT-proBNP at discharge/ preprocedural NT-proBNP). Responders were defined as NT-proBNP ratio < 1 and non-responders were defined as NT-proBNP ratio ≥ 1. Outcomes were defined according to the Valve Academy Research Consortium (VARC)-3 criteria.
Results
A total of 234 patients (65.5%) and 123 patients (34.5%) were grouped as the responders and the non-responders, respectively. Responders and non-responders were significantly different in both median preprocedural (2103.5 vs. 421.0 pg/ml, p < 0.001) and postprocedural (707.6 vs. 1009.0, p < 0.001) NT-proBNP levels. Patients in the non-responder group were more inclined to have comorbidities of hypertension (73.2% vs. 51.7%, p < 0.001), hyperlipidaemia (46.3% vs. 34.6%, p = 0.031), peripheral vascular disease (20.3% vs. 8.5%, p = 0.001) and pure aortic insufficiency (15.4% vs. 4.3%, p < 0.001). In the contrast, patients in the responder group had higher prevalence of maximum transvalvular velocity (4.6 vs. 4.2 m/s, p < 0.001), reduced left ventricular ejection fraction (58.0% vs. 63.0%, p < 0.001), heart failure (9.4% vs. 2.4%, p = 0.014), mitral regurgitation ≥ moderate (13.7% vs. 4.9%, p = 0.010), tricuspid regurgitation ≥ moderate (12.0% vs. 2.4%, p = 0.002), and pulmonary hypertension (32.9% vs. 13.0%, p < 0.001). Patients in the non-responder group were moderately longer than the responder group in total hospitalization length (14 vs. 12 days, p < 0.001). The non-responder group were significantly associated with cumulative all-cause mortality (p = 0.009) and cardiac mortality (p < 0.001) during the follow-up period.
Conclusions
Periprocedural changes of NT-proBNP is clinically useful for the risk stratification of survival in patients after TAVR.
Similar content being viewed by others
Introduction
Transcatheter aortic valve replacement (TAVR) has emerged as a safe and effective treatment for severe aortic stenosis [1]. A growing number of patients are being referred for TAVR due to the expanding indications for the procedure [2]. It is necessary to develop improved tools for risk stratification of this patient group. Researchers have investigated a variety of biomarkers such as high-sensitive troponin T, GDF15 (growth differentiation factor 15), osteopontin, and soluble ST2 (suppression of tumorigenicity 2) to determine the prognosis in TAVR patients. As a result, more biomarkers are required to predict treatment outcomes and stratify risks.
The N-terminal pro-brain natriuretic peptide (NT-proBNP) is an inactive derivative of cerebral natriuretic peptide (BNP) and has a half-life of 120 min [3, 4]. NT-proBNP is released in response to myocyte stretching following left ventricular hypertrophy [5]. Hence, monitoring NT-proBNP levels may assist in identifying individuals at high risk of adverse cardiovascular events such as left ventricular systolic dysfunction, left ventricular hypertrophy, and heart failure [6,7,8]. Both BNP and NT-proBNP have been identified of predictive value of prognosis in patients undergoing aortic valve replacement. In TAVR patients with aortic stenosis, elevated BNP and NT-proBNP pre- and post-TAVR were associated with clinical outcomes in many studies. However, these studies all excluded patients with pure native aortic regurgitation. The periprocedural changes of plasma NT-proBNP levels and the precise cut points for NT-proBNP at discharge after TAVR are still being clarified.
We therefore investigated the association between NT-proBNP ratio (postprocedural NT-proBNP at discharge/ preprocedural NT-proBNP) and clinical outcomes in TAVR patients, as well as the precise cut points of NT-proBNP at discharge after TAVR in predicting the cumulative late all-cause mortality. These findings may raise concerns about optimizing the timing of TAVR or medical therapy after TAVR to mitigate the pathobiological changes caused by NT-proBNP, thereby improving clinical outcomes.
Materials and methods
Study population
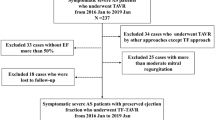
A total of 358 Patients underwent TAVR from January 1, 2018 to December 31, 2021 at our institution and 357 patients were included in the present study (1 patient died the day after TAVR immediately for multiple organ failure). NT-proBNP levels were measured in all patients at baseline before TAVR and at discharge after TAVR. This study was approved by the Medical Ethics Review Committee of Fuwai Hospital and informed consent was waived for the nature of retrospective analysis.
Transcatheter aortic valve replacement procedure
TAVR was performed in a hybrid operating room by the heart team, and patients were under general anesthesia or local anesthesia and monitored by anesthesiologists. All patients underwent TAVR after discussions with the multidisciplinary heart team, and the access site and the type of prosthesis were determined thereafter. Transfemoral access was preferred in all patients with criteria unless prosthesis size, calcification and atheroma of the aorto-iliofemoral artery were considered. All TAVR procedures were performed according to established standards via transfemoral, trans-carotid, trans-subclavian, and contemporary devices of SE-valves (Venus-A [Venus Medtech, Hangzhou, China], VitaFlow [Microport, Shanghai, China], TaurusOne [Peijia Medical, Suzhou, China]) [9,10,11] or balloon expandable (BE) valves (Sapien 3 [Edwards Lifesciences, Irvine, California], Sapien XT [Edwards Lifesciences, Irvine, California]) [12, 13] were implanted.
Data collection
Plasma NT-proBNP have been routinely measured within 48 h before TAVR procedure and 4 to 8 days after TAVR at the date of hospital discharge. Blood samples were drawn from the antecubital or dorsal hand vein and collected in EDTA-containing tubes and immediately processed by the Institute of Clinical Chemistry at Fuwai hospital. NT-proBNP were analyzed from Roche Diagnostics by electro-chemiluminescence immunoassay. The effective measuring range of the assay was 5-35000 pg/ml (a normal value of < 150 pg/ml for the general population). Patients were considered as non-responders if their NT-proBNP value at discharge post-TAVR was equal or greater compared with the baseline value. Baseline characteristics, procedural, and hospitalization data were retrospectively recorded manually from institutional electronic medical record system and entered into a dedicated database. All data were anonymized, systematically collected and assessed for quality.
Follow-up and end points
All endpoints in this study were defined in line with the Valve Academic Research Consortium (VARC)-3 criteria [14]. The primary end point of the study was late cumulative all-cause mortality after TAVR. Patients were followed up till May 31, 2022 by outpatient visits and telephone interview. The median follow-up time was 640.7 days (interquartile range [IQR]:413.7 to 1073.0 days).
Statistical analysis
All continuous variables were tested for normality using the Shapiro-Wilk test. Continuous variables were presented as mean ± standard deviation (SD) and compared using the Student’s t-test, or medians (25th -75th quartile) compared using Mann-Whitney U test. Categorical variables were presented as numbers and percentages and compared using the Chi-square test or Fisher’s exact test. Cumulative clinical outcomes were calculated using the Kaplan-Meier survival analysis, and the log-rank test was used for comparison between the groups. To identify independent predictors of late all-cause mortality, all variables with a p value < 0.05 on univariate analysis were included in a stepwise multivariable Cox regression model. The proportional hazard assumption was confirmed by examination of log (-log [survival]) curves and by testing of partial (Schoenfeld) residuals, and no relevant violations were found. The estimated hazard ratio (HR) with 95% confidence interval (CI) was provided by the Cox regression analysis. To find the cut-off value of NT-proBNP at discharge (1350.5 pg/ml), receiver-operating characteristic (ROC) curve evaluation was performed to assess the area under the curve (AUC) at the 95% confidence interval (CI) in predicting cumulative all-cause mortality. And NT-proBNP ≥ 1350.5 pg/ml was included in the stepwise multivariable Cox regression model. The trend of cumulative all-cause mortality across quartiles of NT-proBNP levels at discharge was analyzed using the Cochrane-Armitage test. To identify the determinants of NT-proBNP non-responders, a multivariable logistic regression analysis was performed using the following covariates selected from factors that were significantly different between NT-proBNP responders and non-responders: hypertension, hyperlipidaemia, NT-proBNP ≥ 1429.60 at baseline (median of NT-proBNP at baseline), peripheral vascular disease, heart failure, pure aortic insufficiency, left ventricular ejection fraction, maximum velocity, mitral regurgitation ≥ moderate, tricuspid regurgitation ≥ moderate, pulmonary hypertension, total length of hospitalization. All tests were 2-sided, and p values < 0.05 were considered to be statistically significant. All statistical analyses were performed using SPSS software version 26.0 (IBM, NY, USA) and R software version 4.1.0 (available at http://www.r-project.org).
Results
Baseline patient characteristics
A total of 357 patients were included for analysis. According to NT-proBNP ratio, 234 patients were divided into responder group for NT-proBNP ratio < 1 and 123 patients were divided into non-responder group for NT-proBNP ratio ≥ 1. The main baseline characteristics of the study population are summarized in Table 1. The two groups did not differ significantly in terms of age (mean: 72.4 years), male sex (57.1%), body mass index (mean: 24.7 kg/m2), New York Heart Association functional class III or IV (70.3%), diabetes mellitus (23.5%), chronic obstructive pulmonary disease (3.6%), chronic kidney disease (3.4%), and coronary artery disease (38.7%). Responders and non-responders were significantly different in both median preprocedural (2103.5 vs. 421.0 pg/ml, p < 0.001) and postprocedural (707.6 vs. 1009.0, p < 0.001) NT-proBNP levels. However, patients in the non-responder group were more inclined to have comorbidities of hypertension (73.2% vs. 51.7%, p < 0.001), hyperlipidaemia (46.3% vs. 34.6%, p = 0.031), peripheral vascular disease (20.3% vs. 8.5%, p = 0.001) and pure aortic insufficiency (15.4% vs. 4.3%, p < 0.001). In the contrast, patients in the responder group showed significantly higher prevalence of maximum velocity (4.6 vs. 4.2 m/s, p < 0.001), reduced left ventricular ejection fraction (58.0% vs. 63.0%, p < 0.001), chronic heart failure (9.4% vs. 2.4%, p = 0.014), mitral regurgitation ≥ moderate (13.7% vs. 4.9%, p = 0.010), tricuspid regurgitation ≥ moderate (12.0% vs. 2.4%, p = 0.002), and pulmonary hypertension (32.9% vs. 13.0%, p < 0.001). Patients in the non-responder group were moderately longer than the responder group in total hospitalization length (14 vs. 12 days, p < 0.001).
Procedural data and results
Procedural data and results are summarized in Table 2. No significant differences were observed between the responders and non-responders in terms of emergency surgery (4.8%), local anesthesia (16.6%), self-expandable valve (91.9%), post-procedure AR grade ≥ moderate (1.4%), conversion to open surgery (1.7%), and second valve implantation (19.6%). Of note, patients in the responder group were treated more frequently through transfemoral access (97.9% vs. 92.7%, p = 0.035).
Clinical outcomes
VARC-3-related clinical outcomes are summarized in Table 3. There were no significant differences regarding 30-day all-cause mortality between the two groups (1.3% vs. 0.8, p = 0.689). However, patients in non-responder group were both significantly higher in cumulative all-cause mortality (20.0% vs. 8.6%, p = 0.009) and cardiac mortality (16.2% vs. 3.9%, p < 0.001) compared with patients in responder group. Kaplan-Meier survival curves stratified by NT-proBNP responders and non-responders are shown in Fig. 1. Rehospitalization for heart failure was no different in the two groups (13.2% vs. 8.7%, p = 0.334). Although all stroke were 19.5% in the responder group and 5.6% in the non-responder group, no significant difference was observed (p = 0.943).
NT-proBNP in mortality prediction
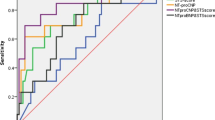
In ROC analysis, NT-proBNP ratio significantly predicted cumulative all-cause mortality (AUC:0.631, 95%CI:0.526–0.736, p = 0.013; sensitivity 54.5%, and specificity 71.3% at a cutoff level of 1.114) and cardiac mortality (AUC:0.710, 95%CI:0.594–0.827, p = 0.001; sensitivity 68.2%, and specificity 71.3% at a cutoff level of 1.114). NT-proBNP at discharge also significantly predicted all-cause mortality (AUC:0.722, 95%CI:0.643–0.802, p < 0.001; sensitivity 72.7%, and specificity 68.5% at a cutoff level of 1350.5 pg/ml) and cardiac mortality (AUC:0.745, 95%CI:0.643–0.847, p < 0.001; sensitivity 81.8%, and specificity 69.3% at a cutoff level of 1396.5 pg/ml). However, NT-proBNP at baseline did not reach statistical significance in predicting cumulative all-cause mortality (AUC:0.569, 95%CI:0.475–0.663, p = 0.192) or cardiac mortality (AUC:0.512, 95%CI:0.398–0.627, p = 0.847).
Multivariable logistic regression analysis revealed that baseline NT-proBNP ≥ 1429.60 pg/ml (odds ratio [OR]: 0.257, 95% CI: 0.136 to 0.487, p < 0.001), peripheral vascular disease (OR: 2.537,95%CI:1.091 to 5.971, p = 0.031), left ventricular ejection fraction (OR: 1.042, 95%CI: 1.010 to 1.075 per 1%, p = 0.011), maximum velocity (OR:0.576, 95%CI: 0.414 to 0.801 per 1 m/s, p = 0.001) were independent determinants of NT-proBNP non-responders (Table 4).
Univariable and multivariable Cox regression analysis was used to identify variables that show statistically significant associations with all-cause mortality during follow-up period. These data are summarized in Table 5. Predictors of all-cause mortality in descending order of HR were NT-proBNP non-responders (HR:4.779, 95%CI:1.815–12.587, p = 0.002), NT-proBNP ≥ 1350.5 pg/ml at discharge (HR:4.218, 95%CI:1.340-13.279, p = 0.014), mitral regurgitation ≥ moderate (HR:3.064, 95%CI:1.046–8.973, p = 0.041), coronary artery disease (HR:2.974, 95%CI:1.131–7.821, p = 0.027), length of intensive care unit (HR:1.051, 95%CI:1.018 to 1.084 per 1 day, p = 0.002) according to multivariable Cox analysis. A univariate Cox regression analysis was conducted to show the significant differences of clinical outcomes among patients with NT-proBNP ≥ 1350.5 pg/ml at discharge according to the NT-ProBNP ratio in Supplementary Table 1. It reveals that NT-proBNP non-responders significantly predicted the late all-cause mortality (HR: 3.061, 95%CI: 1.309–7.154, p = 0.010) and late cardiac mortality (HR: 3.977, 95%CI: 1.417–11.159, p = 0.009).
Kaplan-Meier survival curves stratified by quartiles of NT-proBNP at discharge are shown in Fig. 2. Survival curves of subgroups of NT-proBNP at discharge substantially diverged, illustrating patients with high NT-proBNP levels at discharge (upper quartile) showed the worst survival (log-rank test, p = 0.004). The Cochrane-Armitage test was used to identify the trend of late all-cause mortality across quartiles of NT-proBNP levels at discharge, revealing a significant increase in all-cause mortality as NT-proBNP at discharge increased (p < 0.001).
Discussion
In this study, we examined the association between periprocedural changes of NT-proBNP levels and clinical outcomes after TAVR based on NT-proBNP ratio (postprocedural NT-proBNP at discharge/ preprocedural NT-proBNP). Patients were grouped as responders (NT-proBNP ratio < 1) and non-responders (NT-proBNP ratio ≥ 1). The major findings of the present study were as follows:
-
1.
Median NT-proBNP levels decreased from 1429.6 pg/ml at baseline to 852.0 pg/ml at discharge.
-
2.
34.5% of the study patients were non-responders.
-
3.
Baseline NT-proBNP ≥ 1429.60 pg/ml, peripheral vascular disease, left ventricular ejection fraction, maximum transvalvular velocity were independent determinants of NT-proBNP non-responders.
-
4.
NT-proBNP ratio and NT-proBNP at discharge significantly predicted cumulative all-cause and cardiac mortality after TAVR.
-
5.
NT-proBNP non-responders, NT-proBNP ≥ 1350.5 pg/ml at discharge, baseline mitral regurgitation ≥ moderate, coronary artery disease, and length of intensive care unit were independently associated with lower survival rates during follow-up time.
-
6.
As levels of NT-proBNP at discharge increased, all-cause mortality significantly increased after TAVR.
NT-proBNP has been identified as a diagnostic and prognostic biomarker for a number of cardiovascular diseases [15]. The utility of NT-proBNP levels for risk stratification and as a predictor of adverse clinical outcomes after TAVR has been studied [16, 17]. Studies analyzing the predictive value of periprocedural changes of NT-proBNP in TAVR have yielded conflicting results. In most studies, baseline NT-proBNP levels were identified as predictors for short- and long-term mortality after TAVR [16, 18, 19]. In contrast, other studies found no correlation between baseline NT-proBNP and postprocedural mortality [20]. In our study, no significant association were observed between baseline NT-proBNP levels and all-cause mortality after TAVR. Besides, we found that postprocedural survival rates of NT-proBNP responders and patients with lower NT-proBNP at discharge were better.
Of note, the NT-proBNP responders had higher baseline NT-proBNP levels, lower left ventricular ejection fraction, higher maximum transvalvular velocity and more patients with chronic heart failure. This finding was consistent with previous observation that TAVR in this patient group produced immediate effects in left ventricular unloading and decrease of myocardial stretch, resulting in an acute decrease in circulating NT-proBNP levels after TVAR. An intuitive hypothesis can be made from our findings that patients whose elevated NT-proBNP levels are primarily driven by severe AS and heart failure would benefit most from TAVR.
The association of periprocedural changes of NT-proBNP with survival after TAVR was investigated by Seoudy et al. [21] The study measured NT-proBNP in 704 patients before TAVR and at discharge. The authors demonstrated that highly elevated NT-proBNP levels—both at baseline and at discharge are significantly associated with survival regardless of a postprocedural decrease of NT-proBNP after TAVR. However, NT-proBNP at baseline was not directly associated with all-cause mortality in our study. In our patient population, NT-proBNP at baseline ≥ 1429.60 pg/ml suggested lower non-responder risk, leading to reduce overall and cardiac mortality rates. Unfortunately, end points such as cardiac mortality and rehospitalization for heart failure were not accounted by Seoudy et al. We found that NT-proBNP responders were significantly associated with lower cardiac mortality, but were not associated with rehospitalization for heart failure.
Given the adverse correlation between the increase of NT-proBNP level after TAVR and the poor clinical outcomes, two questions are raised: How to alleviate the increase of NT-proBNP level after TAVR? Will the efforts aimed at pathobiology driving the increase of NT-proBNP after TAVR improve the clinical outcomes? It needs to be clear that the potential therapeutic targets are underlying pathobiology, such as maladaptive hypertrophy, abnormal left ventricular function, increased wall stress, and volume overload, which lead to NT-proBNP release [22]. Optimal medical therapy may mitigate the elevations in NT-proBNP after TAVR. This hypothesis is warranted to confirm in future strategy trials, and whether targeting lower postprocedural NT-proBNP levels may improve clinical outcomes needs to be assessed in future studies.
It is worth using NT-proBNP for risk stratification of TAVR patients, identifying NT-proBNP non-responders earlier, and reducing postprocedural circulating NT-proBNP levels by more optimal timing of TAVR procedure and medical management, which are of great help in improving the prognosis of TAVR patients.
Strengths and limitations
The study is limited inherently by its retrospective single-center design. Data on medical management were not systematically collected in this study. Serial changes of NT-proBNP were not measured in all patients after hospital discharge. The external validation is needed as for the cut-off value of NT-proBNP at baseline or discharge. The present study showed that about 20% of patients experienced second valve implantation mainly due to device migration to left ventricle. This might be explained by the immaturity of early technology, the characteristics of prosthesis, and the cardiac anatomical characteristics of patients. However, our study cohort represents a typical, unselected, real-world TAVR population with a considerable sample size.
Conclusions
Periprocedural changes of NT-proBNP is associated with cumulative all-cause and cardiac mortality after TAVR. NT-proBNP ratio and NT-proBNP at discharge should be carefully monitored to improve postprocedural risk stratification and guide optimal treatment strategy selection.
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Douglas PS, Leon MB, Mack MJ, Svensson LG, Webb JG, Hahn RT, et al. Longitudinal hemodynamics of Transcatheter and Surgical aortic valves in the PARTNER trial. JAMA Cardiol. 2017;2(11):1197–206. PubMed PMID: 28973520. Pubmed Central PMCID: PMC5710363. eng.
Fefer P, Bogdan A, Grossman Y, Berkovitch A, Brodov Y, Kuperstein R et al. Impact of Rapid Ventricular Pacing on Outcome after Transcatheter aortic valve replacement. J Am Heart Assoc. 2018;7(14). PubMed PMID: 29987119. Pubmed Central PMCID: PMC6064853. Epub 20180709. eng.
Wang F, Pan W, Pan S, Wang S, Ge Q, Ge J. Usefulness of N-terminal pro-brain natriuretic peptide and C-reactive protein to predict ICU mortality in unselected medical ICU patients: a prospective, observational study. Crit Care. 2011;15(1):R42. PubMed PMID: 21272380. Pubmed Central PMCID: PMC3221971. Epub 20110128. eng.
Weber M, Hamm C. Role of B-type natriuretic peptide (BNP) and NT-proBNP in clinical routine. Heart. 2006;92(6):843–9. PubMed PMID: 16698841. Pubmed Central PMCID: PMC1860679. eng.
Daniels LB, Maisel AS. Natriuretic peptides. J Am Coll Cardiol. 2007;50(25):2357–68. PubMed PMID: 18154959. eng.
Kara K, Lehmann N, Neumann T, Kälsch H, Möhlenkamp S, Dykun I, et al. NT-proBNP is superior to BNP for predicting first cardiovascular events in the general population: the Heinz Nixdorf Recall Study. Int J Cardiol. 2015;183:155–61. PubMed PMID: 25666125. Epub 20150129. eng.
Hildebrandt P. Natriuretic peptides: prediction of cardiovascular disease in the general population and high risk populations. Dis Markers. 2009;26(5–6):227–33. PubMed PMID: 19773612. Pubmed Central PMCID: PMC3833235. eng.
Taylor CJ, Roalfe AK, Iles R, Hobbs FD. The potential role of NT-proBNP in screening for and predicting prognosis in heart failure: a survival analysis. BMJ Open. 2014;4(4):e004675. PubMed PMID: 24747793. Pubmed Central PMCID: PMC3996821. Epub 20140419. eng.
Liao YB, Zhao ZG, Wei X, Xu YN, Zuo ZL, Li YJ, et al. Transcatheter aortic valve implantation with the self-expandable venus A-Valve and CoreValve devices: preliminary Experiences in China. Catheter Cardiovasc Interv. 2017;89(S1):528–33. PubMed PMID: 28318133. eng.
Wang R, Kawashima H, Gao C, Mou F, Li P, Zhang J, et al. Comparative quantitative Aortographic Assessment of Regurgitation in patients treated with VitaFlow Transcatheter Heart Valve vs. other Self-Expanding Systems. Front Cardiovasc Med. 2021;8:747174. PubMed PMID: 35146004. Pubmed Central PMCID: PMC8821967. Epub 20220125. eng.
Wang M, Song G, Chen M, Feng Y, Wang J, Liu X, et al. Twelve-month outcomes of the TaurusOne valve for transcatheter aortic valve implantation in patients with severe aortic stenosis. EuroIntervention. 2022;17(13):1070–6. PubMed PMID: 34338639. eng.
Webb J, Gerosa G, Lefèvre T, Leipsic J, Spence M, Thomas M, et al. Multicenter evaluation of a next-generation balloon-expandable transcatheter aortic valve. J Am Coll Cardiol. 2014;64(21):2235–43. PubMed PMID: 25456759. Epub 20141124. eng.
Pibarot P, Ternacle J, Jaber WA, Salaun E, Dahou A, Asch FM, et al. Structural deterioration of Transcatheter Versus Surgical aortic valve bioprostheses in the PARTNER-2 trial. J Am Coll Cardiol. 2020;76(16):1830–43. PubMed PMID: 33059828. eng.
Généreux P, Piazza N, Alu MC, Nazif T, Hahn RT, Pibarot P, et al. Valve Academic Research Consortium 3: updated endpoint definitions for aortic valve Clinical Research. J Am Coll Cardiol. 2021;77(21):2717–46. PubMed PMID: 33888385. Epub 20210419. eng.
Richards AM, Nicholls MG, Espiner EA, Lainchbury JG, Troughton RW, Elliott J, et al. B-type natriuretic peptides and ejection fraction for prognosis after myocardial infarction. Circulation. 2003;107(22):2786–92. PubMed PMID: 12771003. Epub 20030527. eng.
Ribeiro HB, Urena M, Le Ven F, Nombela-Franco L, Allende R, Clavel MA, et al. Long-term prognostic value and serial changes of plasma N-terminal prohormone B-type natriuretic peptide in patients undergoing transcatheter aortic valve implantation. Am J Cardiol. 2014;113(5):851–9. PubMed PMID: 24528616. Epub 20131212. eng.
Kaneko H, Hoelschermann F, Tambor G, Okamoto M, Neuss M, Butter C. Impact of N-terminal pro-B-type natriuretic peptide response on long-term prognosis after transcatheter aortic valve implantation for severe aortic stenosis and heart failure. Heart Vessels. 2019;34(5):777–83. PubMed PMID: 30443765. Epub 20181115. eng.
Elhmidi Y, Bleiziffer S, Piazza N, Ruge H, Krane M, Deutsch MA, et al. The evolution and prognostic value of N-terminal brain natriuretic peptide in predicting 1-year mortality in patients following transcatheter aortic valve implantation. J Invasive Cardiol. 2013;25(1):38–44. PubMed PMID: 23293174. eng.
López-Otero D, Trillo-Nouche R, Gude F, Cid-Álvarez B, Ocaranza-Sanchez R, Alvarez MS, et al. Pro B-type natriuretic peptide plasma value: a new criterion for the prediction of short- and long-term outcomes after transcatheter aortic valve implantation. Int J Cardiol. 2013;168(2):1264–8. PubMed PMID: 23280329. Epub 20121230. eng.
Pfister R, Wahlers T, Baer FM, Scherner M, Strauch J, Erdmann E. Utility of NT-pro-BNP in patients undergoing transapical aortic valve replacement. Clin Res Cardiol. 2010;99(5):301–7. PubMed PMID: 20140438. Epub 20100206. eng.
Seoudy H, Frank J, Neu M, Güßefeld N, Klaus Y, Freitag-Wolf S, et al. Periprocedural changes of NT-proBNP are Associated with Survival after Transcatheter aortic valve implantation. J Am Heart Assoc. 2019;8(3):e010876. PubMed PMID: 30686097. Pubmed Central PMCID: PMC6405592. eng.
O’Leary JM, Clavel MA, Chen S, Goel K, O’Neill B, Elmariah S, et al. Association of natriuretic peptide levels after transcatheter aortic valve replacement with subsequent clinical outcomes. JAMA Cardiol. 2020;5(10):1113–23. PubMed PMID: 32667623. Pubmed Central PMCID: PMC7364343. eng.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
Author Jun Yu and author Wei Wang have given substantial contributions to the conception or the design of the manuscript, author Jun Yu to acquisition, analysis and interpretation of the data. All authors have participated to drafting the manuscript, author Wei Wang revised it critically. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethical approval
This study was approved by the Medical Ethics Review Committee of Fuwai Hospital and informed consent was waived for the nature of retrospective analysis.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yu, J., Wang, W. N-terminal pro-B-type natriuretic peptide is associated with clinical outcomes after transcatheter aortic valve replacement. J Cardiothorac Surg 18, 286 (2023). https://doi.org/10.1186/s13019-023-02391-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13019-023-02391-2