Abstract
Background
Two staging systems, the 8th staging system by the American Joint Committee on Cancer (AJCC) and the 11th Japanese classification by Japan Esophageal Society (JES), are currently applied in the clinic for predicting the prognosis of patients with esophageal squamous cell carcinoma (ESCC). The differences between the two staging systems have been widely researched. However, little studies focus on the differences in specific staging between the two systems. Therefore, we aimed to compare the performance of different staging in predicting overall survival (OS) of Chinese patients with ESCC.
Methods
This retrospective study included 268 patients who underwent radical esophagectomy and mediastinal lymph node dissection for ESCC between January 2008 and December 2013. Patients were staged by the 8th AJCC and 11th JES staging systems. OS was estimated using the Kaplan–Meier method and compared between N stages and between stage groupings using the log-rank test. Cox proportional hazards regression analysis was performed to identify factors independently related to outcome. Further, we compared the concordance indexes (C-indexes) of the two staging systems.
Results
The mean age was 61.25 ± 7.056 years, median follow-up was 44.82 months, and 5-year OS rate was 47%. The OS was well predicted by the 8th AJCC N staging (P < 0.001) and the 11th JES N staging (P < 0.001), with a c-index of 0.638 (95% CI: 0.592–0.683) for AJCC N staging and 0.627 (95% CI: 0.583–0.670) for JES N staging (P = 0.13). In addition, the OS was also well predicted by stage groupings of the 8th AJCC (P < 0.001) and the 11th JES systems (P < 0.001), with a c-index of 0.658 (95% CI: 0.616–0.699) for 8th AJCC stage grouping and 0.629 (95% CI: 0.589–0.668) for the11th JES stage grouping (P = 0.211).
Conclusions
The prognostic effect of 11th JES staging system is comparable with that of AJCC 8th staging system for patients with ESCC. Therefore, both systems are applicable to clinical practice.
Similar content being viewed by others
Background
Esophageal cancer is one of the most common malignant tumors, and the incidence has been gradually growing worldwide [1]. China has the most significant number of cases and deaths of esophageal cancer, especially esophageal squamous cell carcinoma (ESCC). ESCC has the fourth highest mortality rate among the top 10 cancers in China [2]. Given that ESCC is life-threatening, many studies focusing on the therapies of ESCC have been done.
Cancer staging systems are critical for planning treatment and for predicting prognosis for patients with ESCC. The tumor-node-metastasis (TNM) system is the most widely used staging system developed and maintained by the American Joint Committee on Cancer (AJCC) and the International Union for Cancer Control (UICC), herein referred to as the AJCC system. It classifies cancers by the size and direct extent of the primary tumor (T), the involvement of the regional lymph nodes (N), and the presence of distant metastasis (M). The 8th edition of AJCC TNM system for esophageal cancer, based on the Worldwide Esophageal Cancer Collaboration (WECC) database, was published in 2017 [3]. In addition to the AJCC system, a novel staging system for esophageal cancer has been developed by the Japan Esophageal Society (JES). The JES system based on the JES nationwide data registry has been updated to the 11th edition in 2017 [4]. Although both the 8th AJCC and 11th JES classifications are widely applied in clinics, the two show differences in the lymph node maps, N staging, and stage grouping.
The JES system is broadly accepted in Asian countries because squamous cell cancer is the primary pathology in east Asia [5, 6]. It is also extensively used in Europe, mainly because of its detailed classification of lymph node stations [7]. By contrast, the AJCC system is used internationally as a standard scale for the staging of esophageal cancer. A retrospective study by Park et al. [8] demonstrated that the 11th JES and 8th AJCC staging systems carried similar predictive power for disease-free survival. However, Chang et al. [9] found that by comparison with the 8th AJCC staging system, the 11th JES staging system had worse performance in predicting the prognosis of patients with thoracic ESCC. There is still no consensus on which classification system is more useful in assessing patient prognosis. Therefore, in this study, we evaluated the predictive ability of these two staging systems for survival in patients with ESCC.
Methods
Study design and population
A database with 1460 cases of esophageal cancer was reviewed to determine patients who underwent initial surgical treatment for esophageal cancer in the Tumor Hospital of Shandong First Medical University between January 2008 and December 2013. Clinical data of 268 cases who received radical esophagectomy (R0 resection: curative resection, the resection margin was free of cancer cells) with mediastinal and abdominal lymph node dissection and were histologically confirmed to have ESCC, were retrospectively analyzed. Exclusion of patients was based on the following criteria: (1) patients with second primary tumors; (2) patients with other primary esophageal tumors, such as esophageal adenocarcinoma; (3) patients that received preoperative neoadjuvant therapy; (4) patients with incomplete information. For data conformance, only patients with ESCC were analyzed because more than 90% of esophageal cancer is squamous cell carcinoma in east Asia. The flow chart of included patients was shown in Fig. 1.
Preoperative imaging evaluation was carried out, including chest and abdominal computed tomography (CT), positron emission tomography-computed tomography (PET-CT), and esophagogastroduodenoscopy with endoscopic ultrasound. All patients with lymph node metastasis received six cycles of adjuvant chemotherapies (paclitaxel 175 mg/m2 + cisplatin 75 mg/m2, 21 days is one cycle) after surgery. Patients staged as T2 with lymph node metastasis and T3 or T4a were treated with the same adjuvant radiotherapy (the radiotherapy dose is 70 Gy).
Patients were followed up at our outpatient clinic every three months during the first year and annually thereafter. In addition to the physical examination, chest and abdomen CT scans and PET-CT were performed during the follow-up period, and esophagogastroduodenoscopy was conducted annually following the operation. All patients included in the analysis were followed until death or December 2018. This study has been approved by the ethics committee of Tumor Hospital of Shandong First Medical University (2,021,001,008).
Staging and lymph node map
In order to define lymph node stations accurately, lymphatic nodes were collected and classified according to the guidelines of the JES after surgery [3]. The reassessment of pathologic staging based on the 8th AJCC staging system was blinded to patient outcome, and all dissected lymph nodes were reclassified according to the 8th AJCC lymph node map of esophageal cancer [10]. Patients were assigned synchronously to the 8th AJCC staging system and 11th JES staging system according to the results of a lymph node map.
Statistical analysis
Statistical analyses were performed using SPSS (version 22, IBM, Armonk, NY, USA). Numerical data were presented as mean with standard deviation or as median with the range. The endpoints of interest were overall survival (OS) defined as the time from initial surgery to death of any cause or to last follow-up. Survival curves were estimated using the Kaplan–Meier method and compared statistically using the log-rank test. Cox proportional hazard regression results were presented as hazard ratios (HR) with 95% confidence intervals (95% CI). Servcorp packages were responsible for the assessment and calculation of the concordance index (C-index) which was used to compare the prognostic abilities and Cox proportional hazard models of the 8th AJCC staging system and the 11th JES classification. All tests were two-sided, and P-value < 0.05 was considered a significant difference.
Results
Patient demographics and five-year survival rate
The basic patient characteristics were obtained from 268 patients (221 males and 47 females) (Table 1). The mean age was 61.25 ± 7.056 years. The median survival time was 44.82 months, with a 5-year OS at 47% (Fig. 2). Patients were sorted by three main categories, i.e., T, N, and M staging based on the guidelines of the 8th AJCC staging system and the 11th JES staging system. As shown in Table 2, T staging and M staging were similar in both staging systems. However, N staging and stage grouping were different between the two staging systems.
Comparison of survival predication based on N staging between the two staging systems
Since N staging is the main difference between the two staging systems, survival differences were further measured according to N staging. Predication by the 8th AJCC staging system showed that the 5-year OS was 61.4% for N0, 42.5% for N1, 25% for N2, 0% for N3 (P < 0.001) (Fig. 3A). However, according to the 11th JES staging system, the 5-year OS was 61% for N0, 40.3% for N1, 24.1% for N2, 14.3% for N3 (P < 0.001) (Fig. 3B). Therefore, the prognosis of the patients with stage N3 classified by the AJCC staging system was worse than that of the patients with stage N3 classified by the JES staging system. HRs for N staging of both staging systems were calculated using univariate Cox hazards regression analysis; the higher the N stage, the higher the HRs in both of the staging systems (Table 3). Additionally, C-indexes were 0.638 (95% CI: 0.592–0.683) for the 8th AJCC N staging system and 0.627 (95% CI: 0.583–0.670) for the 11th JES N staging system. Compared to the JES N staging, the C-index of AJCC N staging was slightly higher but without significant difference (AJCC 8th vs. JES 11th, P = 0.13).
Comparison of survival predication based on staging grouping between the two staging systems
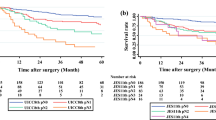
Because of the discrepancy of T, N, and M classification, the stage groupings are different between the two staging systems. Thus, survival differences were further investigated according to stage groupings. The 5-year OS predicated by the 8th AJCC staging system was 100% for stage 0, 78.1% for stage I, 56.6% for stage II, 37.1% for stage III, and 0% for stage IV (P < 0.001) (Fig. 4A). However, according to the 11th JES staging system, the 5-year OS was 87.5% for stage 0, 81% for stage I, 53.8% for stage II, 31.7% for stage III (P < 0.001) (Fig. 4B). In addition, HRs for stage groupings of both staging systems were calculated using univariate Cox hazards regression analysis; the stage groupings were directly proportional to the HRs and this was demonstrated in both of the staging systems (Table 4). C-indexes were 0.658 (95% CI: 0.616–0.699) for the 8th AJCC stage groupings and 0.629 (95% CI: 0.589–0.668) for the 11th JES stage groupings. Compared to the JES stage grouping, the C-index of the AJCC stage grouping was slightly higher but without significant difference (AJCC 8th vs. JES 11th P = 0.211).
Comparison of the prognosis of the two systems according to stage grouping. (A) The 5-year OS according to stage grouping of the AJCC 8th staging; (B) The 5-year OS according to stage grouping of the 11th JES staging. AJCC, the American Joint Committee on Cancer; JES, Japan Esophageal Society; OS, overall survival
Discussion
The occurrence, development, and metastasis of esophageal cancer have certain similarities, but different pathological types have different etiology, epidemiology, and sensitivity to treatment [10,11,12,13,14,15]. The radical treatment of esophageal cancer is still surgical resection [16], but more than 60% of patients cannot receive surgical resection due to local or systemic metastasis [17]. Therefore, reasonable staging is an important way to improve the survival rate and quality of life of patients with esophageal cancer.
Both the pathological staging of surgical resection specimens and the clinical stage before surgery are hot spots of current research on esophageal cancer. The first UICC staging system and the first AJCC staging system for esophageal cancer were proposed in 1968 and in 1977, respectively [18]. However, the uniform and identical definitions and stage groupings for cancers at all anatomical sites have been introduced by AJCC and UICC since 1987 because of the close cooperation between these two organizations. The 8th AJCC/UICC staging system was proposed by WECC, which was founded in 2006 at the request by AJCC [19]. The 8th AJCC staging system was derived from a modern machine-learning analysis and a random forest analysis of data from 22,653 patients from 33 WECC institutions [20, 21]. However, Japanese data was not included in the WECC database because Japanese institutions were not affiliated to WECC [22]. The 1st edition of the Japanese classification for esophageal cancer was published by Japanese institutions in 1969, and the latest 11th edition was released in 2017 [3]. The 11th Japanese classification for esophageal cancer proposed by JES is widely accepted in Asian countries where most of the pathology is squamous cell carcinoma. However, the 8th AJCC staging system is still applied internationally as a common scale.
Each staging system has its characteristics. The most significant difference between UICC/AJCC and JES staging is the definition of N in TNM staging. The former defines N as a regional lymph node and divides N into N0-N3 according to the number of metastatic lymph nodes, while the latter divides N into N0-N4 according to the region of lymph node metastasis. In this study, a total of 268 patients were collected, including 221 men and 47 women. Their average age was 61.25 ± 7.056 years, the median survival time was 44.82 months, and the 5-year survival rate was 47%. According to the 8th AJCC staging system and the 11th JES staging system, patients were divided into three categories, namely T, N and M. The results showed that T staging and M staging were similar in the two staging systems, but differences were observed in N staging and staging grouping between the two systems. N staging of the 8th AJCC staging system based on the number of regional lymph nodes remains problematic in clinical practice. First, the connotation of supraclavicular lymph node metastasis in AJCC and JES systems is mostly different. We evaluated the prognostic significance of the supraclavicular lymph node by three-field lymph node dissection, but this evaluation method has not been adopted by western countries. Therefore, current guidelines for evaluating the prognostic significance of supraclavicular lymph nodes in ESCC are not universally applicable due to inadequate case evidence. Secondly, many metastatic lymph nodes fused are discovered during surgery, which makes the count inaccurate [23]. Thirdly, lymph node metastasis of esophageal cancer has the characteristics of biphasic and cross-cutting transfer [24, 25] and despite the rapid development of surgery, radiotherapy and radical treatment of lymph node metastases, AJCC still defines regional lymph node metastasis as distant metastasis for patients who are not in earlier T stage. Whether such a staging system can provide useful guidance for treatment and prognosis is questionable. Some Asian and western surgeons agree with their Japanese counterparts that the supraclavicular lymph nodes should be considered at least as regional lymph nodes for upper-middle thoracic esophageal cancer [24, 26,27,28]. The 11th JES system classifies supraclavicular nodes as group 3 nodes for lower thoracic esophageal cancer, and group 2 for upper and middle thoracic esophageal cancer. The 8th AJCC system regards patients with supraclavicular lymph node metastasis as distant metastasis requiring no surgical resection.
The selection of the staging system is related to the prediction of survival, the convenience of use, and the selection of surgical indications. Our study further studied the N stage according to the patient’s OS. According to the 8th AJCC staging system, the 5-year OS was 61.4% for N0, 42.5% for N1, 25% for N2, 0% for N3. However, according to the 11th JES staging system, the 5-year OS was 61% for N0, 40.3% for N1, 24.1% for N2, 14.3% for N3. Therefore, the prognosis of the patients with stage N3 classified by the AJCC staging system was worse than that of the patients with stage N3 classified by the JES staging system. Park et al. [7] also found that stage N3 esophageal cancer in the JES staging system had a higher survival rate than that in the AJCC staging system. In addition, univariate Cox risk regression analysis was used to calculate the HRs of N stages of the two staging systems. The results showed that the C index of AJCC N staging was slightly higher than that of JES N staging, but there was no significant difference. HRs for stage groupings of both staging systems were calculated using univariate Cox hazards regression analysis, and the results revealed that the stage groupings were directly proportional to the HRs and this was demonstrated in both of the staging systems. Compared with the JES stage grouping, the C-index of AJCC stage grouping was slightly higher, however, without any significant difference.
This study has several notable limitations. Since this is a 10-year retrospective study, there may be some confounding bias. First, we excluded neoadjuvant therapy and adenocarcinoma patients and analyzed ESCC patients only, so our results should be used cautiously for patients with esophageal adenocarcinoma. Secondly, we have not explained differences in the definition of esophagogastric junction tumors. Thirdly, we have not compared the region and degree of mediastinal and abdominal lymph node dissection. Lastly, the number of patients was relatively small. The AJCC staging system subdivided stages I, II, and III into IA, IB, IIA, IIB, IIIA, IIIB, but due to the small number of patients, we did not calculate survival differences among these subdivided stages.
Conclusions
In conclusion, our study found that the prognostic effect of 11th JES staging system was comparable with that of 8th AJCC staging system in patients with ESCC. Therefore, both systems are applicable to clinical practice.
Data Availability
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AJCC:
-
American Joint Committee on Cancer
- ESCC:
-
esophageal squamous cell carcinoma
- OS:
-
overall survival
- TNM:
-
tumor-node-metastasis
- UICC:
-
International Union for Cancer Control
- WECC:
-
Worldwide Esophageal Cancer Collaboration
- JES:
-
Japan Esophageal Society
- CT:
-
computed tomography
- PET-CT:
-
positron emission tomography-computed tomography
References
Global Burden of Disease Cancer Collaboration. Global, Regional, and National Cancer incidence, mortality, years of Life Lost, Years lived with disability, and disability-adjusted life-years for 29 Cancer groups, 1990 to 2017: a systematic analysis for the global burden of Disease Study. JAMA Oncol. 2019;5:1749–68.
Li S, Chen H, Man J, Blackstone EH, Rusch VW et al. 7th edition of the AJCC Cancer Staging Manual: esophagus and esophagogastric junction. Ann Surg Oncol 2010;17:1721-4.
Rice TW, Blackstone EH, Rusch VW. 7th edition of the AJCC Cancer Staging Manual: esophagus and esophagogastric junction. Ann Surg Oncol 2010;17:1721-4.
Japan Esophageal S. Japanese Classification of Esophageal Cancer, 11th Edition: part I. Esophagus 2017;14:1–36.
Huang W, Huang Y, Sun J, et al. Atlas of the thoracic lymph nodal delineation and recommendations for lymph nodal CTV of esophageal squamous cell cancer in radiation therapy from China. Radiother Oncol. 2015;116:100–6.
Wang F, Zheng Y, Wang Z, et al. Nodal skip metastasis in esophageal squamous cell carcinoma patients undergoing three-field lymphadenectomy. Ann Thorac Surg. 2017;104:1187–93.
Hagens ERC, van Berge Henegouwen MI, Cuesta MA, et al. The extent of lymphadenectomy in esophageal resection for cancer should be standardized. J Thorac Dis. 2017;9:713–S23.
Park SY, Kim DJ, Suh JW, et al. Comparison of the 11(th) japanese classification and the AJCC 7(th) and 8(th) staging systems in esophageal squamous cell carcinoma patients. J Thorac Dis. 2018;10:5039–46.
Chang X, Deng W, Ni W, et al. Comparison of two major Staging Systems in Predicting Survival and recommendation of postoperative Radiotherapy based on the 11th japanese classification for esophageal Carcinoma after curative resection: a propensity score-matched analysis. Ann Surg Oncol. 2021;28:7076–86.
Rice TW, Ishwaran H, Ferguson MK, et al. Cancer of the Esophagus and Esophagogastric Junction: an Eighth Edition staging primer. J Thorac Oncol. 2017;12:36–42.
Song Y, Li L, Ou Y, et al. Identification of genomic alterations in oesophageal squamous cell cancer. Nature. 2014;509:91–5.
Stachler MD, Taylor-Weiner A, Peng S, et al. Paired exome analysis of Barrett’s esophagus and adenocarcinoma. Nat Genet. 2015;47:1047–55.
Hardikar S, Onstad L, Song X, Wei XL, Wang FH, Zhang DS et al. A novel inflammation-based prognostic score in esophageal squamous cell carcinoma: the C-reactive protein/albumin ratio. BMC Cancer 2015;15:350.
Wei XL, Wang FH, Zhang DS, et al. A novel inflammation-based prognostic score in esophageal squamous cell carcinoma: the C-reactive protein/albumin ratio. BMC Cancer 2015;15:350.
Shivappa N, Zucchetto A, Serraino D, et al. Dietary inflammatory index and risk of esophageal squamous cell cancer in a case-control study from Italy. Cancer Causes Control. 2015;26:1439–47.
Katlic MR, Wilkins EW Jr, Grillo HC. Three decades of treatment of esophageal squamous carcinoma at the Massachusetts General Hospital. J Thorac Cardiovasc Surg. 1990;99:929–38.
Shibata Y, Haruki N, Kuwabara Y, et al. Chfr expression is downregulated by CpG island hypermethylation in esophageal cancer. Carcinogenesis. 2002;23:1695–9.
Schroder MS, Culhane AC, Quackenbush J, et al. Survcomp: an R/Bioconductor package for performance assessment and comparison of survival models. Bioinformatics. 2011;27:3206–8.
Rice TW, Blackstone EH. Esophageal cancer staging: past, present, and future. Thorac Surg Clin. 2013;23:461–9.
Rice TW, Apperson-Hansen C, DiPaola LM, et al. Worldwide Esophageal Cancer collaboration: clinical staging data. Dis Esophagus. 2016;29:707–14.
Ishwaran H, Blackstone EH, Apperson-Hansen C, et al. A novel approach to cancer staging: application to esophageal cancer. Biostatistics. 2009;10:603–20.
Ozawa H, Kawakubo H, Takeuchi M, et al. Prognostic significance of the number and extent of metastatic lymph nodes in patients with esophageal Cancer: comparison of the Union for International Cancer Control 8th Edition and Japan Esophageal Society Japanese classification of Esophageal Cancer 11th Edition Classifications for Esophageal Cancer. Ann Surg Oncol. 2021;28:6355–63.
Peng J, Wang WP, Dong T, et al. Refining the nodal staging for esophageal squamous cell Carcinoma based on Lymph Node Stations. Ann Thorac Surg. 2016;101:280–6.
Chen J, Wu S, Zheng X, et al. Cervical lymph node metastasis classified as regional nodal staging in thoracic esophageal squamous cell carcinoma after radical esophagectomy and three-field lymph node dissection. BMC Surg. 2014;14:110.
Cavallin F, Alfieri R, Scarpa M, et al. Nodal skip metastasis in thoracic esophageal squamous cell carcinoma: a cohort study. BMC Surg. 2017;17:49.
Udagawa H, Ueno M, Shinohara H, et al. The importance of grouping of lymph node stations and rationale of three-field lymphoadenectomy for thoracic esophageal cancer. J Surg Oncol. 2012;106:742–7.
Tachimori Y, Ozawa S, Numasaki H, et al. Supraclavicular node metastasis from thoracic esophageal carcinoma: a surgical series from a japanese multi-institutional nationwide registry of esophageal cancer. J Thorac Cardiovasc Surg. 2014;148:1224–9.
Yamasaki M, Miyata H, Miyazaki Y, et al. Evaluation of the nodal status in the 7th edition of the UICC-TNM classification for esophageal squamous cell carcinoma: proposed modifications for improved survival stratification: impact of lymph node metastases on overall survival after esophagectomy. Ann Surg Oncol. 2014;21:2850–6.
Acknowledgements
None.
Funding
This research was supported by the Start-up fund of Shandong Cancer Hospital (2020-27); the Projects of the Medical and Health Technology Development Program in Shandong Province (grant number 202009030676); and the Wu Jieping Medical Foundation (grant number 320.6750.2021-02-42).
Author information
Authors and Affiliations
Contributions
The data for the validation was gathered by Cun-liang Wang and Wan-hu Li. Statistical analyses was performed by Jia-zhen Chen. The manuscript was chiefly written by Chuan-wang Miao and Xu-dong Hu The study was designed by Xi-qin Zhang and Lan-pin Liu. All authors review the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study had been approved by the ethics committee of Tumor Hospital of Shandong First Medical University (2021001008). Informed consent was obtained from all participants. All methods were carried out in accordance with relevant guidelines and regulations.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhang, Xq., Miao, Cw., Liu, Lp. et al. The prognostic value of 11th Japanese classification and 8th AJCC staging systems in Chinese patients with esophageal squamous cell carcinoma. J Cardiothorac Surg 18, 251 (2023). https://doi.org/10.1186/s13019-023-02350-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13019-023-02350-x