Abstract
Background
Pectum excavatum is a congenital thoracic alteration that can present important physiological alterations depending on the severity of the case. The Nuss procedure is a minimally invasive technique for managing chest wall deformity, in which there is a risk of perioperative complications.
Case presentation
This article presents the case of a 16-year-old patient who underwent placement of a Nuss bar and suffered intraoperative and postoperative cardiorespiratory arrest.
Conclusions
it is important to consider the possible early and late complications scenarios as well as their treatment in patients with pectum excavatum scheduled for a Nuss procedure.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Background
Pectum excavatum (PE) is a congenital thoracic disorder with an incidence of 1 per 400 births and a prevalence of 2.6% in children aged 7 to 14 years [1]. This deformity predominantly affects men, at a ratio of 5:1 with women [2]. Depending on its severity, it can generate some physiological changes, such as decreased cardiac function, restriction of lung function, and psychological sequelae due to the physical appearance of the patient [3]. Different techniques have been developed to repair this pathology. One of them, the Nuss procedure, was described for the first time in 1998 as a minimally invasive technique that consists of the placement of a metal bar underneath the sternum to reshape the anterior chest wall [4]. Perioperative complications of the Nuss procedure are rare but can threaten the life of the patient [5,6,7,8]. It is not clear how cardiopulmonary resuscitation (CPR) should be performed in patients with a Nuss bar, since the bar can generate technical difficulties for chest compressions and defibrillation [9,10,11].
We present the case of a 16-year-old patient who had intraoperative cardiorespiratory arrest during a Nuss procedure and postoperatively in the intensive care unit.
Case presentation
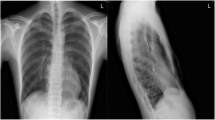
A 16-year-old male patient with Eagle–Barrett syndrome and congenital pectum excavatum was admitted for surgical correction of chest deformity. The preoperative chest X-ray (Fig. 1) showed a Haller index of 5.8 and an electrocardiogram with sinus rhythm and right bundle branch block. The estimated functional capacity of the patient was greater than 6 metabolic equivalents (METs).
On September 6, 2018, a videothoracoscopy-guided Nuss procedure was performed. It was monitored according to the recommendations of the American Society of Anesthesiologists, and a thoracic epidural catheter was placed at the T4-T5 level for postoperative analgesia without complications. Intravenous anesthesia and placement of a left double-lumen endotracheal tube No. 35 followed.
During the passage of the Nuss bar, the patient presented episodes of ventricular tachycardia with a pulse that subsided when the surgical stimulus was suspended. The passage of the bar was continued, and after turning it to correct the defect and leaving it in position, the patient presented severe bradycardia that improved with atropine. Two minutes after having the bar fully in position and before starting to fix it with the stabilizer, he presented ventricular fibrillation. Cardiopulmonary resuscitation was initiated with chest compressions in the lower half of the sternum, placing the hands below the Nuss bar. Three external defibrillations were performed at 200 joules with the defibrillator paddles placed in a conventional manner (right infraclavicular area and cardiac apex). Additionally, two 1-mg intravenous boluses of epinephrine were administered 5 min apart.
Ten minutes after arrest, the patient returned to spontaneous circulation. The fibrillation presented was not continuous but came in two episodes, and in between them, we removed the Nuss bar. After the removal of the rod and with the patient in sinus rhythm, a left radial artery and a right internal jugular central venous catheter were channeled, guided by ultrasound, without complications. The patient continued to have hemodynamic instability, so norepinephrine infusion was started at 0.1 µg/kg/min, with an adequate response. The thoracoscope was reintroduced, and the heart was checked for structural lesions.
A transesophageal echocardiography probe was inserted to verify that there were no cardiac lesions and was used as a guide for volume resuscitation. Blood samples were taken to check the metabolic status of the patient and look for other causes that explained his cardiac arrest.
Since the patient presented significant improvement with a decrease in the use of vasopressors and persistence of sinus rhythm, it was decided to pass the Nuss bar again without finding any alteration of the heart rhythm or hemodynamic changes. The procedure was completed without any other complications. Chest X-ray (Fig. 2) showed a small right pneumothorax without any indication for invasive management.
At the end of the surgery, the neuromuscular blockade was reversed with sugammadex and was confirmed with a train of four test result > 95%. The patient was extubated in the operating room to assess his neurological status. He could move all four limbs, had symmetrical 3-mm pupils with adequate reactivity to light, was alert, and obeyed orders. He was transferred to the pediatric intensive care unit, where he was given oxygen through a 50% Venturi mask.
Hours later, in the intensive care unit, a new chest X-ray showed significant bilateral pneumothorax, for which they performed bilateral thoracostomies. A transthoracic echocardiogram did not show structural cardiac damage, but there was dysfunction of the left ventricle with decreased segmental and global contractility and a left ventricular ejection fraction of 45%, without other abnormalities.
During the first postoperative day, the patient persisted with hemodynamic instability that required vasoactive and inotropic support. At approximately 20 h postoperatively, he presented cardiorespiratory arrest with pulseless electrical activity on two occasions for 10 min each. Chest compressions were performed on the lower half of the sternum, and intravenous calcium gluconate, sodium bicarbonate, and epinephrine were administered. The patient continued with hemodynamic instability and the need for vasoactive and inotropic support in increasing doses.
The medical team decided to remove the Nuss bar, which was achieved without complications and with good postoperative evolution. The control echocardiogram the day after the rod was removed showed adequate biventricular function and a left ventricular ejection fraction of 80%. The next day, he underwent successful extubation. After progressive withdrawal of hemodynamic support, he was discharged from the hospital 12 days after surgery. The patient has been evaluated with ambulatory controls without any alterations of note. One year after this complication, he presented good and stable functional and psychological capacity.
Discussion
Performing the Nuss procedure has become the most widely used technique for the correction of PE in pediatric and adult populations [3, 4]. This procedure brings the risk of lethal complications, although rare, such as lung, heart, or great vessel injury [12, 13]. Major and minor complications have been reported with an incidence that varies between 2% and 20%, although the true incidence is unknown due to the underreporting of these cases [7, 8, 12, 13].
In the literature, almost all published Nuss procedure related cardiac arrests are caused by cardiac or vascular injury. Interestingly in this case the cardiac arrest was not caused by physical heart or vessel damage. There are two possible mechanisms for this rare complication reported in the literature [8]: the elevation of the sternum may have caused slightly rotation of the heart and the great vessels with myocardial ischemia and consequent ventricular fibrillation. And the sudden enlargement of the retrosternal space may have caused a nerve stretch reflex similar to the trigeminocardiac reflex which might have upset the balance between vagal and sympathetic innervation and triggered inhibition of cardiac function and consequent arrest [8].
In patients who present complications during the Nuss procedure that lead to cardiac arrest, advanced CPR is a source of controversy and a challenge for surgical teams since the placement of the Nuss bar makes it much harder to perform chest compressions. The Nuss bar impairs the excursion of the anterior chest wall, which is necessary during CPR maneuvers to achieve compression of the heart between the sternum and the spine. The guidelines of the American Heart Association (AHA) and the International Liaison Committee on Resuscitation (ILCOR) do not give specific recommendations on CPR in patients with a Nuss bar or PE, and the evidence that exists about CPR maneuvers in these patients is scant. In the case reports published to date, recommendations are given based on the clinical, biological, physiological, and imaging knowledge of these patients. They recommend applying compressions with a substantial increase in force, which should be greater the more bars the patient has [9]. In addition, in cases of intraoperative CPR, immediate removal of the metal bar and the performance of high-quality chest compressions are recommended [5].
Through an elastic model, the relationship between chest compression forces and chest displacement was simulated to achieve 5 cm of anterior wall mobility (recommendation of the AHA, as an objective parameter of compressions in CPR), and the presence of Nuss bars did not allow effective cardiopulmonary resuscitation [9, 14].
Active abdominal compression and decompression is a novel technique that appears to improve artificial circulation using thoracic, abdominal, and cardiac pumping mechanisms. This relatively new CPR method induces intra-abdominal pressure changes that activate the abdominal pump to transmit pressure to the thoracic cavity, which in turn activates the thoracic and cardiac pumps through the action of the diaphragm [15]. Although there are no reports on the use of active abdominal compression and decompression in CPR after PE repair, the findings suggest that this alternative method of CPR may have potential utility in these types of patients.
It is also important to determine the best places for external defibrillation paddles in a patient with a Nuss bar, taking into account that some complications occur with shockable arrest rhythms, as in the case of our patient. We positioned the defibrillation paddles based on the AHA and ILCOR guidelines. The usual position of the paddles can allow the flow of electricity from one paddle to the other, passing through the Nuss bar and without passing through the myocardium. Therefore, it is recommended to place the paddles as follows: one anterior on the sternal midline and the other posterior, placing it between the scapulae, forcing the current to pass through the heart [8].
Our recommendations to reduce and detect perioperative complications in these patients are as follows:
-
1.
Take an electrocardiogram and echocardiogram before surgery, as proposed by Zou et al. in their review [8]. In some case reports, fatal complications of Nuss bar placement have been associated with structural or functional alterations of the heart.
-
2.
Keep in mind the possibility of cardiac arrhythmia during the placement and removal of the Nuss bar, monitor the progression, and remove the bar promptly when they occur.
-
3.
Perform intraoperative transesophageal echocardiography in cases of suspected complications to ensure that they are not secondary to direct trauma due to the passage of the bar or its compressive effect on cardiac structures.
Conclusions
In patients with PE who are scheduled for a Nuss procedure, it is important to know the possible early and late complications, as well as the management of these according to the scenarios in which they arise. Future research should determine how best to manage chest compressions in patients with metal bars in the chest without the need to remove them.
Data availability
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.
Abbreviations
- PE:
-
Pectum excavatum
- CPR:
-
Cardiopulmonary resuscitation
- METs:
-
Metabolic equivalents
- AHA:
-
American Heart Association
- ILCOR:
-
International Liaison Committee on Resuscitation
References
Abdullah F, Harris J. Pectus excavatum: more than a matter of aesthetics. Pediatr Ann. 2016;45(11):e403–6.
Abid I, Ewais MM, Marranca J, Jaroszewski DE. Pectus excavatum: a review of diagnosis and current treatment options. J Am Osteopath Assoc. 2017;117(2):106–13.
Zhang DK, Tang JM, Ben XS, Xie L, Zhou HY, Ye X, et al. Surgical correction of 639 pectus excavatum cases via the Nuss procedure. J Thorac Dis. 2015;7(9):1595–605.
Nuss D, Kelly R, Croitoru D, Katz M. A 10-Year review of a minimally invasive technique for the correction of Pectus Excavatum. J Pediatr Surg. 1998;33:545–52.
Zoeller GK, Zallen GS, Glick PL. Cardiopulmonary resuscitation in patients with a Nuss bar - a case report and review of the literature. J Pediatr Surg. 2005;40(11):1788–91.
Goretsky MJ, McGuire MM. Complications associated with the minimally invasive repair of pectus excavatum. Semin Pediatr Surg. 2018;27(3):151–5.
Nakahara O, Ohshima S, Baba H. Cardiopulmonary arrest during the Nuss procedure: case report and review. Acute Med Surg. 2015;2(4):250–2.
Zou J, Luo C, Liu Z, Cheng C. Cardiac arrest without physical cardiac injury during Nuss repair of pectus excavatum. J Cardiothorac Surg. 2017;12(1):10–3.
Sterns J, Twaibu J, Kwaku D, Pizziconi V, Abbas J, Gotimukul A, Jaroszewski D. Efficacy of standard ches compressions in patients with Nuss bars. J Thorac Dis. 2020;12:4299–306.
Russo V, Ranno M, Nigro G. Cardiopulmonary resuscitation iin pectus excavatum patients: is it time to say more? Resuscitation. 2015;88:e5–6.
Glithero K, Tackett J, DeMason K, Burnweit C. Succesful cardiopulmonary resuscitation following minimally invasive pectus excavatum repair: A case report. 2019; 65:255–258.
Hebra A, Kelly R, Ferro M, Yüksel M, Campos JR, Nuss D. Life-threatening complications and mortality of minimally invasive pectus surgery. J Pediatr Surg. 2018;53:728–32.
Coughlin A, Ahsanuddin S, Inglesby D, Fox C, Xu H, Margullies LL, Sayegh F, Soudant C, Sacks H, Kaufman A, Taub P. When to nuss? Patient age as a risk factor for complications on minimally invasive repair of pectus excavatum: a systematic review and meta-analysis. Pediatr Surg Int. 2022;38:365–75.
Part 5. Adult Basic Life Support and Cardiopulmonary Resuscitation Quality: 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2015;132:414–35.
Zhang S, Liu Q, Han S, Zhang Z, Zhang Y, Liu Y, Li J, Wang L. Standard versus abdominal lifting and compression CPR. Evid Based Complement Alternat Med 2016; 2016:9416908.
Acknowledgements
Not applicable.
Author information
Authors and Affiliations
Contributions
IC: idea, conception, first drafting, final approval. DT: idea, conception, first drafting, final approval. MV: idea, conception, first drafting, final approval.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This case report was presented to the Fundación Valle del Lili´s local Institutional Review Board (IRB). It was approved 27-october-2021 (Act No.22 of 2021) No. of case report 563.
Consent for publication
The patient was treated at our institution in 2018 and his last control appointment in 2019. Since the patient lives in a rural area. An attempt was made to contact several times to the telephone numbers and emails registered in our database without an answer. However, the report does not use sensitive information that allows the identification of the patient and informed consent was waived by our IRB after its evaluation.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Cujiño-Álvarez, I.F., Torres-Salazar, D. & Velásquez-Galvis, M. Cardiorespiratory arrest during and after nuss procedure: case report. J Cardiothorac Surg 18, 166 (2023). https://doi.org/10.1186/s13019-023-02262-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13019-023-02262-w