Abstract
Objective
Esophageal cancer, one of the most common cancers in the upper digestive tract and is one of the leading cancer-related mortality worldwide. Accumulating studies found that Ginsenoside compound K (CK) has significantly anti-tumor effects, especially in the suppression of proliferation, migration, as well as invasion in various human cancers. While the effects of Ginsenoside CK in esophageal cancer have not been well studied. In our present study, we aim to explore the functions and mechanisms of Ginsenoside CK in the progression of esophageal cancer cells (Eca109).
Methods
Cell Counting Kit-8 (CCK-8), wound healing, transwell and flow cytometry assays were applied to analyze the effects of Ginsenoside CK in the progression of Eca109 cell, western blot assay was used to investigate the potential downstream signaling pathway after Ginsenoside CK treatment.
Results
Our study found that Ginsenoside CK can suppress cell proliferation, migration and invasion of Eca109 cell. Furthermore, the flow cytometry showed that Ginsenoside CK increased of apoptosis rates in Eca109 cell. The western blot results indicated that Ginsenoside CK decreased the expression of VEGF-A, P-Pi3k and P-Akt proteins. Moreover, the knockdown of VEGF-A gene could suppress cell proliferation, migration, invasion and induce apoptosis in Eca109 cell, and the expression of P-Pi3k and P-Akt proteins were significantly downregulated.
Conclusions
Our study suggests that Ginsenoside CK inhibits the proliferation, migration, invasion, and induced apoptosis of Eca109 cell by blocking VEGF-A/Pi3k/Akt signaling pathway.
Similar content being viewed by others
Introduction
Esophageal carcinoma (EC) is one of the most malignant tumors worldwide, and present a great threat to the health of society [1, 2]. EC is well known by its high rate of metastasis, aggressive invasion and poor prognosis [3]. Surgery and chemotherapy are effective treatments for EC diagnosed at an early stage, while a lot of EC patients comes to recurrence or metastasis, and eventually develop to advanced stages of cancers, which present poor prognosis [4]. Therefore, it is of great urgent to explore the new and efficient treatment strategies, so as to improve the poor survival status of EC patients.
Ginsenoside CK is the main metabolic component of ginseng in human body [5]. Previous studies confirmed that Ginsenoside CK has anti-tumor, anti-inflammatory, anti-oxidation, liver protection, improving immune function and other effects [6]. Furthermore, the therapeutics values of Ginsenoside CK in tumors have well been studied in bladder cancer, colon cancer, liver cancer which can significantly suppress the proliferation ability of these tumor cells [7,8,9]. Vascular endothelial growth factor-A (VEGF-A), a highly specific pro-vascular endothelial cell growth factor, which can promote extracellular matrix degeneration, vascular permeability, proliferation, migration and angiogenesis of vascular endothelial cells [10, 11]. Study suggested that Ginsenoside CK inhibit tumor angiogenesis by suppressing the proliferation and migration of vascular endothelial cells, inhibiting the activity of VEGF-A and it’s signaling pathway, and the degradation of vascular extracellular matrix in neuroblastoma cells [12]. However, there are few studies focus on the effects of Ginsenoside CK on esophageal cancer cells and its molecular mechanism. As a targeted drug with high efficiency and low toxicity, Ginsenoside CK has great development potential in esophageal cancer.
In our present study, we aim to further explore the effects of Ginsenoside CK on the cell proliferation, migration, invasion, apoptosis and related mechanisms on Eca109 cell.
Materials and methods
Cell line and culture
Human esophageal cancer cell (Eca109) was purchased from the American Type Culture Collection (Manassas, USA). Eca109 cell maintained in RPMI 1640 medium (Gibco, USA) containing with 10% fetal bovine serum (Gibco, USA) and 1% penicillin streptomycin (Gbico, USA) in an atmosphere of 5% CO2 at 37 °C.
Cell proliferation assay
The changes of cell proliferation were evaluated by employing the Cell Counting Kit-8 (CCK-8) assay. Cells (2.5 × 103) per well were cultured in 96-well plate overnight. To investigate the proliferation effect of Ginsenoside CK (MedChemExpress, USA) on Eca109 cell, cells were maintained with different concentrations of Ginsenoside CK for 72 h. Similarly, lentivirus transfection cells were maintained in 96-well plate for 24 h, 48 h, 72 h or 96 h, then added with 10 μl CCK-8 reagent (New Cell & Molecular Biotech, China) to each well, and incubated for 2 h at 37 °C. The absorbance value (OD450) was detected by using the microplate reader (Bio-Tek, USA).
VEGF-A knockdown cell line
Eca109 cells were transfected with knockdown lentivirus sh-VEGF-A, and the corresponding negative control lentivirus sh-NC which were purchased from Hanbio (Shanghai, China). Puromycin was used to screen the stably transfected cells. Western blot analysis was applied to evaluated the efficiency of lentivirus transfection.
Wound healing assay
Eca109 cell (5 × 105) were seeded in 6-well plates for 24 h and scraped by a sterile pipette tip. Cells were cultured with DMSO or Ginsenoside CK in FBS-free medium. sh-NC and sh-VEGF-A cells were maintained with FBS-free medium. The Zen Imaging software (Carl Zeiss, Germany) was applied to observe images at 0 h, 24 h and 48 h. The Image J software (USA) was applied to calculate the scratch area.
Cell migration and invasion assays
The migration and invasion assays were detected by transwell chamber (BD, USA) with the presence of Matrigel (BD, USA) for invasion, and absence of Matrigel for migration. Cells (5 × 104) per well pretreat with Ginsenoside CK or lentivirus were planted into the upper chamber with 100 μl serum-free medium, and the lower chamber with 600 μl complete medium. After incubation at 37 °C for 24 h, the lower chamber cells were fixed with 70% methanol and then stained by crystal violent (Beyotime, China). The migrated or invaded cells were counted.
Flow cytometry
Ec109 cells (1.5 × 105) were maintained in 6-well plates overnight and treated with Ginsenoside CK or DMSO for 48 h. sh-NC and sh-VEGF-A cells (1.5 × 105) were planted in 6-well plates for 48 h. The rates of apoptosis cells were assessed by applying the Annexin V-FITC Apoptosis Detection Kit (Beyotime). The results were detected by the CytoFLEX flow cytometer (Beckman).
Western blot analysis
Membrane and Cytosol Protein Extraction Kit (Beyotime, China) and Bicinchoninic Acid Protein Assay Kit (Beyotime) were used for protein extracted and quantified. Proteins were separated on 10% SDS polyacrylamide gel (Beyotime) and then blotted onto PVDF membranes (Millipore, USA). The PVDF membranes were incubated in 5% skim milk for 2 h. After that, all membranes were cultured with the primary antibodies of anti-Tubulin (1:10,000, Affinity, USA), anti-VEGF-A (1:1000, Affinity), anti-Pi3k (1:1000, Affinity), anti-P-Pi3k (1:1000, Affinity), anti-Akt (1:1000, Affinity) and anti-P-Akt (1:1000, Affinity) overnight at 4 °C. The membranes were then incubated with corresponding secondary antibody (1:3000, Affinity) for 1 h. The protein bands were finally detected by chemiluminescence detection system (ProteinSimple, USA).
Statistical analysis
SPSS 24.0 software (SPSS Inc., Chicago, USA) was applied to data analysis, presented as mean ± SD. The difference between two groups were analyzed by Student’s t-test. P value less than 0.05 was defined as statistically significant.
Results
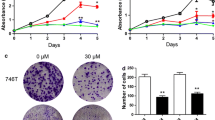
The structure of Ginsenoside CK and Eca109 cell proliferation changes after Ginsenoside CK intervention
The structure of Ginsenoside CK was showed in Fig. 1A, CCK-8 was applied to detected the Eca109 cell proliferation changes after incubation with different concentrations of Ginsenoside CK for 72 h which found that cell proliferation was decreased with the increased concentration of Ginsenoside CK, as shown in Fig. 1B.
Ginsenoside CK suppress cell migration and invasion of Eca109
Wound healing and transwell assays were used to investigated the migration and invasion abilities after Ginsenoside CK intervention, and the results indicated that the migration and invasion abilities were reduced notably in Eca109 cell after the treatment of Ginsenoside CK (Fig. 2A, B).
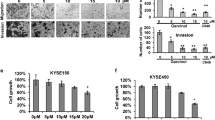
Knockdown of VEGF-A gene suppress cell proliferation, migration and invasion of Eca109
Eca109 cell were transfected with VEGF-A knockdown lentivirus (sh-VEGF-A) or the corresponding negative control lentivirus (sh-NC) (Fig. 3A). The western blot analysis confirmed that Eca109 cell were stably transfected with lentivirus (Fig. 3B). The CCK-8 assay showed that cell proliferation was remarkably reduced after sh-VEGF-A transfection (Fig. 3C). Wound healing and transwell assays further confirmed that VEGF-A gene knockdown suppress the proliferation, migration and invasion of Eca109 cell (Fig. 4A, B).
The knockdown of VEGF-A in Eca109 cell and cell proliferation changes. A Green fluorescent and western bolt analysis showed that Eca109 cell were stably transfected with VEGF-A knockdown gene. B Cell proliferation changes detected by CCK-8 assay showed that cell proliferation decreased after incubation with Ginsenoside CK for 24, 48, 72 and 96 h. ***P < 0.001
Ginsenoside CK intervention and knockdown of VEGF-A gene promote cell apoptosis in Eca109 cell
To investigate the function of Ginsenoside CK intervention and VEGF-A gene knockdown in cell apoptosis, Annexin V-FITC and PI staining was applied. The flow cytometry analysis of apoptosis showed that Ginsenoside CK intervention and VEGF-A gene knockdown promoted the apoptosis rate of Eca109 cell (Fig. 5).
Ginsenoside CK influences Eca109 cell progression via VEGF-A/Pi3k/Akt pathway
It is confirmed that the VEGF-A/ Phosphoinositide 3-kinase (Pi3k)/protein kinase B (Akt) signaling pathway play important roles for the tumor progression on proliferation, migration, and invasion behaviors [13]. The present study detected the decrease expression of VEGF-A, P-Pi3k, and P-Akt proteins after Ginsenoside CK intervention, while the total proteins of Pi3k and Akt were unchanged. The knockdown of VEGF-A inhibits the cell proliferation, migration, invasion and induce apoptosis in Eca109 cell. Moreover, the knockdown of VEGF-A gene lead to the decrease expression of P-Pi3k, and P-Akt proteins (Fig. 6), which suggested that the VEGF-A/Pi3k/Akt pathway may present as downstream of Ginsenoside CK treatment in Eca109 cell.
The involvement of VEGF-A/Pi3k/Akt pathway in Ginsenoside CK intervention and VEGF-A gene knockdown of Eca109 cell. The decreased expressions of VEGF-A, P-Pi3k, P-Akt in Ginsenoside CK intervention (A) and VEGF-A gene knockdown (B) groups, while the total proteins of Pi3k and Akt were unchanged. ***P < 0.001
Discussion
Esophageal cancer is a highly malignant digestive tract tumor, ranking the 5th among the causes of cancer-related death in worldwide [14]. Although the diagnostic techniques and treatment methods for esophageal cancer have been continuously improved recent decade, the overall survival status of esophageal cancer patients is still unsatisfactory due to the characteristics of early invasion and distant metastasis [15]. Ginseng, as a treasure of human pharmaceutical culture, contains a variety of active components, among which ginsenoside is the main bioactive compound in ginseng and has broad implications in human disease [16]. Ginsenoside CK is a natural diol-type ginsenoside with medicinal activity in vivo [17]. Studies found that Ginsenoside CK has inhibiting effects on various tumor cells, containing lung cancer, colon cancer, and liver cancer, as well as bone tumor [18,19,20]. The anti-tumor effects of Ginsenoside CK are mainly reflected in its ability to reduce the proliferation, migration, and invasion in tumor cells [21]. Chen et al. [22] found that Ginsenoside CK can induce cell apoptosis and inhibit the biological activities of human osteosarcoma cells via blocking the Pi3k signaling pathway. Oh et al. [23]revealed that Ginsenoside CK promote cell autophagic and apoptosis which can inhibit human neuroblastoma cells viability both in vitro and vivo. In the present study, we investigated the anti-tumor effects of Ginsenoside CK and its related mechanism in Eca109 cell, which found that Ginsenoside CK can suppress Eca109 cell proliferation, migration, invasion, induce apoptosis and down-regulated the expression of VEGF-A, P-Pi3k, and P-Akt proteins after Ginsenoside CK intervention.
VEGF-A playing as an important regulator of angiogenesis and presenting as the mediator of endothelial cells proliferation [24]. Down-regulating the expression of VEGF-A can suppress tumor progression in gastric cancer [25]. Zhang et al. [26] reported that the blockage of VEGF-A can inhibits the proliferation and invasion of breast cancer cells. Pi3k/Akt signaling pathway can be activated by angiogenesis inducers and growth factors, like angiopoietins and VEGF-A [27]. Some studies found that Pi3k/Akt presented as the main downstream signaling pathway mediating the biological effects of VEGF-A, and the VEGF-A/Pi3k/Akt signaling pathway playing significant roles in proliferation, migration and invasion of various cellular processes [28]. Study confirmed that VEGF-A/Pi3k/Akt signaling pathway present an important role in the proliferation, migration and invasion of renal carcinoma cells [29]. In our study, we observed that Ginsenoside CK intervention decrease the expression of VEGF-A, P-Pi3k, and P-Akt proteins. Therefore, we speculated that the VEGF-A/Pi3k/Akt signaling pathway may present as the downstream in Eca109 cell after Ginsenoside CK intervention. Furthermore, the VEGF-A gene knockdown investigation showed VEGF-A down-regulation inhibit the cell proliferation, migration, invasion, induce apoptosis and decrease expression of P-Pi3k, and P-Akt proteins in Eca109 cell which further supported the hypothesis that Ginsenoside CK suppress the progression of Eca109 cell via affecting VEGF-A/Pi3k/Akt signaling pathway.
Conclusions
In conclusion, our present study found that Ginsenoside CK can suppress the cell proliferation, migration, invasion, and induce apoptosis of Eca109 cell via VEGF-A/Pi3k/Akt signaling pathway, which suggests that Ginsenoside CK may serve as an effective treatment in EC.
Availability of data and materials
The data the support the findings of this study are available on request from corresponding author.
Abbreviations
- EC:
-
Esophageal carcinoma
- CK:
-
Compound K
- VEGF-A:
-
Vascular endothelial growth factor-A
- CCK-8:
-
Cell Counting Kit-8
- Pi3k:
-
Phosphoinositide 3-kinase
- P-Pi3k:
-
Phosphorylated-phosphoinositide 3-kinase
- Akt:
-
Protein kinase B
- P-Akt:
-
Phosphorylated-protein kinase B
References
Li N, Zhao Z, Liu P, et al. Upregulation of deubiquitinase USP7 by transcription factor FOXO6 promotes EC progression via targeting the JMJD3/CLU axis. Mol Ther Oncolytics. 2021;20:583–95.
Zhao Y, Wang Y, Shan L, et al. A network meta-analysis for neoadjuvant and adjuvant treatments for resectable squamous cell carcinoma of esophagus. Sci Rep. 2021;11(1):6800.
Wang Y, Zhang W, Liu W, et al. Long noncoding RNA VESTAR regulates lymphangiogenesis and lymph node metastasis of esophageal squamous cell carcinoma by enhancing VEGFC mRNA stability. Cancer Res. 2021;81(12):3187–99.
Ajani JA, D’Amico TA, Almhanna K, et al. Gastric cancer, version 32016, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2016;14(10):1286–312.
Qi W, Yan X, Xu X, et al. The effects of cytarabine combined with ginsenoside compound K synergistically induce DNA damage in acute myeloid leukemia cells. Biomed Pharmacother. 2020;132: 110812.
Shin KC, Oh HJ, Kim BJ, et al. Complete conversion of major protopanaxadiol ginsenosides to compound K by the combined use of alpha-L-arabinofuranosidase and beta-galactosidase from Caldicellulosiruptor saccharolyticus and beta-glucosidase from Sulfolobus acidocaldarius. J Biotechnol. 2013;167(1):33–40.
Wang H, Jiang D, Liu J, et al. Compound K induces apoptosis of bladder cancer T24 cells via reactive oxygen species-mediated p38 MAPK pathway. Cancer Biother Radiopharm. 2013;28(8):607–14.
Chen L, Meng Y, Sun Q, et al. Ginsenoside compound K sensitizes human colon cancer cells to TRAIL-induced apoptosis via autophagy-dependent and -independent DR5 upregulation. Cell Death Dis. 2016;7(8): e2334.
Zhang J, Jiang Y, Li Y, et al. Micelles modified with a chitosan-derived homing peptide for targeted intracellular delivery of ginsenoside compound K to liver cancer cells. Carbohydr Polym. 2020;230: 115576.
Pan CF, Zhang X, Wang JW, et al. Weichang’an formula inhibits tumor growth in combination with bevacizumab in a murine model of colon cancer-making up for the deficiency of bevacizumab by inhibiting VEGFR-1. Front Pharmacol. 2020;11:512598.
Wang N, Chen Y, Shi C, et al. CREB3L4 promotes angiogenesis and tumor progression in gastric cancer through regulating VEGFA expression. Cancer Gene Ther. 2022;29(2):241–52.
Park D, Yoon M, Compound K. a novel ginsenoside metabolite, inhibits adipocyte differentiation in 3T3-L1 cells: involvement of angiogenesis and MMPs. Biochem Biophys Res Commun. 2012;422(2):263–7.
Wen N, Guo B, Zheng H, et al. Bromodomain inhibitor jq1 induces cell cycle arrest and apoptosis of glioma stem cells through the VEGF/PI3K/AKT signaling pathway. Int J Oncol. 2019;55(4):879–95.
Ding H, Xu J, You J, et al. Effects of enteral nutrition support combined with enhanced recovery after surgery on the nutritional status, immune function, and prognosis of patients with esophageal cancer after Ivor-Lewis operation. J Thorac Dis. 2020;12(12):7337–45.
Jia X, Huang C, Hu Y, et al. Cirsiliol targets tyrosine kinase 2 to inhibit esophageal squamous cell carcinoma growth in vitro and in vivo. J Exp Clin Cancer Res. 2021;40(1):105.
Sarhene M, Ni JY, Duncan ES, et al. Ginsenosides for cardiovascular diseases; update on pre-clinical and clinical evidence, pharmacological effects and the mechanisms of action. Pharmacol Res. 2021;166: 105481.
Guo YP, Shao L, Wang L, et al. Bioconversion variation of ginsenoside CK mediated by human gut microbiota from healthy volunteers and colorectal cancer patients. Chin Med. 2021;16(1):28.
Yang L, Zhang Z, Hou J, et al. Targeted delivery of ginsenoside compound K using TPGS/PEG-PCL mixed micelles for effective treatment of lung cancer. Int J Nanomedicine. 2017;12:7653–67.
Pak JN, Jung JH, Park JE, et al. p53 dependent LGR5 inhibition and caspase 3 activation are critically involved in apoptotic effect of compound K and its combination therapy potential in HCT116 cells. Phytother Res. 2020;34(10):2745–55.
Muthukumar T, Aravinthan A, Sharmila J, et al. Collagen/chitosan porous bone tissue engineering composite scaffold incorporated with Ginseng compound K. Carbohydr Polym. 2016;152:566–74.
Zhang Y, Tong D, Che D, et al. Ascorbyl palmitate/d-alpha-tocopheryl polyethylene glycol 1000 succinate monoester mixed micelles for prolonged circulation and targeted delivery of compound K for antilung cancer therapy in vitro and in vivo. Int J Nanomed. 2017;12:605–14.
Chen K, Jiao J, Xue J, et al. Ginsenoside CK induces apoptosis and suppresses proliferation and invasion of human osteosarcoma cells through the PI3K/mTOR/p70S6K1 pathway. Oncol Rep. 2020;43(3):886–96.
Oh JM, Kim E, Chun S. Ginsenoside compound K induces ros-mediated apoptosis and autophagic inhibition in human neuroblastoma cells in vitro and in vivo. Int J Mol Sci. 2019;20(17):4279.
Vion AC, Perovic T, Petit C, et al. Endothelial cell orientation and polarity are controlled by shear stress and VEGF through distinct signaling pathways. Front Physiol. 2020;11:623769.
Zhang J, Zhang J, Pang X, et al. MiR-205-5p suppresses angiogenesis in gastric cancer by downregulating the expression of VEGFA and FGF1. Exp Cell Res. 2021;404(2):112579.
Zhang Q, Lu S, Li T, et al. ACE2 inhibits breast cancer angiogenesis via suppressing the VEGFa/VEGFR2/ERK pathway. J Exp Clin Cancer Res. 2019;38(1):173.
Peng C, Chen H, Li Y, et al. LRIG3 suppresses angiogenesis by regulating the PI3K/AKT/VEGFA signaling pathway in glioma. Front Oncol. 2021;11: 621154.
Chen CH, Lai JM, Chou TY, et al. VEGFA upregulates FLJ10540 and modulates migration and invasion of lung cancer via PI3K/AKT pathway. PLoS ONE. 2009;4(4): e5052.
Huang J, Wang X, Wen G, et al. miRNA2055p functions as a tumor suppressor by negatively regulating VEGFA and PI3K/Akt/mTOR signaling in renal carcinoma cells. Oncol Rep. 2019;42(5):1677–88.
Acknowledgements
Not applicable.
Funding
This work is supported by Science and technology Bureau of Quanzhou City (2018N052S).
Author information
Authors and Affiliations
Contributions
JHH, XWW and ZQL conducted for conception and design of the work. JHH, DLP and FL contributed to data analysis and manuscript editing. YTH, GH and XWH contributed to data acquisition and statistical analysis. JHH, XWW and ZQL contributed to manuscript revision. All authors approved the revision of the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors consent to manuscript publication.
Competing interests
The authors declare none conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Huang, J., Pan, D., Liu, F. et al. Ginsenoside compound K inhibits the proliferation, migration and invasion of Eca109 cell via VEGF-A/Pi3k/Akt pathway. J Cardiothorac Surg 17, 99 (2022). https://doi.org/10.1186/s13019-022-01846-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13019-022-01846-2