Abstract
Introduction
Left ventricular (LV) thrombus is a complication of acute myocardial infarction and is associated with systemic thromboembolism. We describe a trans-aortic endoscopic approach to the removal of an LV thrombus in a patient undergoing concurrent coronary artery bypass grafting and aortic valve replacement.
Case presentation
A 47 year old male presented following an embolic middle cerebral artery stroke and underwent transthoracic echocardiography demonstrating a mobile LV thrombus. Additional investigation revealed a moderately stenosed bicispid aortic valve, two vessel coronary artery disease and ischemic cardiomyopathy. The patient underwent early surgery to reduce the risk of further embolic episodes. A trans-aortic approach was utilized with videoscopy and single shafted instrumentation to aide in removal of the thrombus. The patient then underwent aortic valve replacement and coronary artery bypass grafting.
Conclusion
We report an alternative technique for the removal of a left ventricular thrombus in a patient undergoing concurrent coronary and aortic valve surgery. The transaortic video-assisted approach provided excellent visualisation of the apex and near complete removal of the thrombus without damaging the surrounding trabeculae. The main benefit of this technique is sparing of LV tissue, thereby preserving left ventricular function.
Similar content being viewed by others
Introduction
Left ventricular (LV) thrombus is a complication of acute myocardial infarction (MI) and is associated with systemic thromboembolism [1, 2]. It occurs in up to 15% of patients following an ST elevation myocardial infarction (STEMI) and up to 25% of patients following an anterior MI [1]. Systemic thromboembolisation is a feared complication following the development of an LV thrombus and may occur in up to 25% of cases [3]. Stroke may be the first presentation of patients with a LV thrombus. Documented risk factors for the development of LV thrombus following an infarct include large infarct size, severe apical akinesis, LV aneurysm, and anterior MI, which predispose to stasis in the infarcted segment [4].
We describe a patient with severe two vessel disease, ischemic cardiomyopathy and moderate bicuspid aortic stenosis diagnosed after an admission with a left middle cerebral artery (MCA) stroke. Transthoracic echocardiography demonstrated a mobile thrombus. Given the high risk of further embolic sequalae, the patient underwent surgical removal at the time of aortic valve replacement and coronary artery bypass grafting to reduce the patients risk of further stroke.
Case presentation
Background
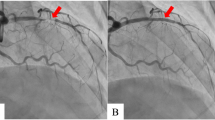
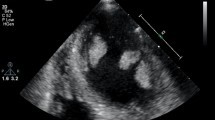
A 47-year-old male presented following acute onset right upper limb weakness and expressive dysphasia. The past medical history included well controlled epilepsy and hypertension. There was no history of diabetes mellitus, hypertension or previous smoking. Urgent CT angiography of the head and neck demonstrated a distal left MCA embolic stroke. Transthoracic echocardiography demonstrated left ventricular dilatation with severe systolic dysfunction (left ventricular ejection fraction [LVEF] 25%). Mobile thrombus was noted at the apex of the left ventricle, measuring 14 × 12 mm (Fig. 1). The aortic valve was bicuspid with moderate stenosis and mild valvular regurgitation. The ascending aorta was mildly dilated at less than 40 mm. Subsequent cerebral MR imaging confirmed a left frontal lobe cortical infarct with evidence of focal haemorrhagic transformation and evidence of previous infarction in the MCA territory. The patient was commenced on apixaban and therapy (Bisoprolol and Ramipril) for left ventricular systolic dysfunction. Subsequent angiography demonstrated a significant (80%) proximal LAD stenosis, proximal 90% stenosis of a small non dominant circumflex and 80% stenosis of a large ramus intermediate.
Given the mobile nature of the LV thrombus and the history of stroke, the patient underwent early surgery to reduce the risk of further embolic episodes. The patient proceeded to coronary artery bypass grafting, mechanical aortic valve replacement and resection of the left ventricular thrombus. A repeat CT head scan performed prior to surgery (12 days following the infarction), demonstrated the frontal lobe infarct with no evidence of further haemorrhagic transformation. Apixaban was discontinued and the patient commenced on intravenous heparin in preparation for surgery.
Surgical procedure
The patient underwent surgery via median sternotomy and the left internal mammary artery (LIMA) was harvested as an in-situ pedicle graft. The left radial was harvested as a free graft. Systemic heparinisation commenced and cardiopulmonary bypass was instituted with bi-caval and distal ascending aortic cannulation. The left ventricle was vented via the right superior pulmonary vein. The aorta was cross clamped and the heart arrested via anterograde and retrograde cold blood cardioplegia. This was repeated at regular intervals to provide myocardial protection.
Intra operative transesophageal echocardiography confirmed a bicuspid aortic valve with moderate aortic stenosis, severe left ventricular dysfunction and apical left ventricular thrombus with a mobile component (Fig. 1). The aorta was opened in a transverse fashion and the native aortic valve was excised and the annulus decalcified. A 5 mm zero degree Olympus® Videoscope was introduced into the left ventricle with excellent visibility of the left ventricular thrombus assisted by venting of the left ventricle (Fig. 2). With visualisation of the apex under the 5 mm camera, the thrombi were removed using a single shaft grasper and rongeur (Fig. 3). All fresh thrombi were removed, and a fine tipped suction catheter aided in removal of residual thrombus (Figs. 4 and 5). One component of the apical thrombus was firmly embedded within the trabeculae and was not removed as it was felt unlikely to pose an embolic threat (Fig. 4). The left anterior descending artery was bypassed with the LIMA, and the radial artery conduit was used to bypass the ramus branch. The aortic valve was replaced with a 25 mm St Jude mechanical prosthesis and the aortotomy closed. The patient was weaned off cardiopulmonary bypass on first attempt with inotropic support. The patient was discharged day 7 with no complications and maintained on warfarin (target INR of 2 to 3). The LVEF on echocardiography was 45% on discharge.
On review six weeks following surgery there was no evidence of left ventricular thrombus. The aortic valve appeared well seated and there was mild paravalvular aortic regurgitation. There was no residual sequelae from his previous stroke.
Discussion
Left ventricular thrombus is a recognised complication following acute MI, and can occur as frequently as in 25% of patients following an anterior MI [1]. Transthoracic echocardiography is typically the screening modality of choice and should be done within 24 h of admission for those at high risk of LV thrombus [1]. If the apex is poorly visualised, anterior or apical regional wall abnormalities are present then contrast TTE or cardiac MRI should be considered [1]. Stroke is a recognized complication following LV thrombus and a retrospective study by Leow et al. demonstrated an incidence of 12% [5]. Treatment of LV thrombus revolves around anticoagulation and close follow up with guidelines advocating for 3 months following detection. When a patient is undergoing concurrent cardiac surgery or where the thrombus is at high risk of embolization, surgical removal may be warranted [3, 6]. Warfarin is the mainstay of anticoagulation [7]. Evidence suggests that novel oral anticoagulation is associated with higher rates of stroke and systemic embolization [7].
Removal of these thrombi via a left ventriculotomy is feasible, however cases of this approach have been reserved for patients with a concurrent ventricular aneurysm requiring repair [8]. The concern with a left ventriculotomy is further depression of left ventricular function, friability of tissue following an acute myocardial infarction and arrhythmogenic potential post operatively. These concerns were relevant to our patient given his pre-existing left ventricular dysfunction. An alternate approach via the left atrium has been described by Tanaka et al. and allows extraction of a larger thrombus than the trans-aortic approach [9]. Pitfalls include limited room for manipulation of the thrombus and should be reserved for those which are felt to be loosely connected with a narrow stalk [10].
Case studies report the use of videoscopy to visualise intra-cardiac structures and to assist in excision of left ventricular lesions as a viable alternative to a ventriculotomy. A systematic review by Soylu et al. summarises a total of 34 studies incorporating 54 patients where left ventricular cardiac tumours were removed with the use of videoscopic devices [11]. The majority of these occurred in conjunction with other cardiac surgical procedures, with complete resection achieved in all cases [11]. Takusube et al. first described the transaortic video assisted removal of a left ventricular thrombus via the use of a videoscope, reporting excellent visualisation of thrombus and satisfactory postoperative outcome [12]. Other cases using this approach for a left ventricular thrombus also report excellent visualisation of the apex and excellent postoperative outcomes [13,14,15,16]. Of note Williamson et al. describe a case of a LV thrombus resection in a patient undergoing concurrent cardiac and aortic valve surgery [13]. They used a 5 mm camera through the aortic annulus and described ease of access to the apex of the LV via curved Randall stone forceps [13]. In our case, the 5 mm scope could be placed across the annulus allowing for the passage of single shafted instrumentation. The scope was also useful as a retractor to obtain better access to the thrombus at the LV apex. In cases where the aortic valve is not replaced, it may be feasible to pass the 5 mm scope across the valve without traumatising the valve with the advantage that it allows for room for the passage of further instrumentation.
Conclusion
We report an alternative technique for the removal of a left ventricular thrombus in a patient undergoing concurrent coronary and aortic valve surgery. The transaortic video-assisted approach provided excellent visualisation of the apex and near complete removal of the thrombus without damaging the surrounding trabeculae. The main benefit of this technique is sparing of LV tissue, thereby preserving left ventricular function.
Availability of data and materials
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.
References
McCarthy C, Vaduganthan M, McCarthy K, Januzzi J, Bhatt D, McEvoy J. Left ventricular thrombus after acute myocardial infarction: screening, prevention and treatment. JAMA Cardiol. 2018;3(7):642–9.
Chiarella F, Santoro E, Domenicucci S, et al. Predischarge two-dimensional echocardiographic evaluation of left ventricular thrombosis after acute myocardial infarction in the GISSI-3 study. Am J Cardiol. 1998;81:822–7.
Lee J, Park J, Jung H, Cho Y, Oh I, Yoon C, et al. Left ventricular thrombus and subsequent thromboembolism, comparison of anticoagulation, surgical removal, and antiplatelet agents. J Atheroscler Thromb. 2013;20(1):73–93.
Delewi R, Zijlstra F, Piek J. Left ventricular thrombus formation after acute myocardial infarction. Heart. 2012;98:1743–9.
Leow AST, Sia CH, Tan BYQ, et al. Characterisation of acute ischemic stroke in patients with left ventricular thrombi after myocardial infarction. J Thromb Thrombolysis. 2019;48:158–66.
O’Gara P, Kushner F, Ascheim D, Casey D, Chung M, Lemos J, et al. 2013 ACCF/AHA guideline for the management of St-elevation myocardial infarction a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines. Circulation. 2013;127:362–425.
Robinson A, Trankle C, Eubanks G, Schumann C, Thompson P, Wallace R, et al. Off-label use of direct oral anticoagulants compared with warfarin for left ventricular thrombi. JAMA cardiol. 2020;5(6):685–92.
Nili M, Devin E, Jortner R, Strasberg B, Levy MJ. Surgical removal of a mobile, pedunculated, left ventricular thrombus: report of 4 cases. Ann Thorac Surg. 1988;46:396–400.
DiBernardo LR, Kirshbom PM, Skaryak LA, Quarterman RL, Johnson RL, Davies MJ, et al. Acute functional consequences of left ventriculotomy. Ann Thorac Surg. 1998;66(1):159–65.
Tanaka D, Unai S, Diehl J, Hirose H. Surgical removal of a large mobile left ventricular thrombus via left atriotomy. World J Clin Cases. 2014;2(2):32–5.
Soylu E, Kidher E, Ashrafian H, Stavridis G, Harling L, Athanasiou T. A systematic review of left ventricular cardio-endoscopic surgery. J Cardiothorac Surg. 2017;12:41.
Tsukube T, Okada M, Ootaki Y, Tsuji Y, Yamashita C. Transaortic video-assisted removal a left ventricular thrombus. Ann Thorac Surg. 1999;68:1063–5.
Williamson C, Sheehan L, Venesy D, Agostino R. Transaortic, video-assisted removal of a mobile left ventricular apical thrombus in a patient with aortic stenosis and severe left ventricular dysfunction. J Thorac Cardiovasc Surg. 2016;151:e1-3.
Park H, Ryu S, Cho S, Park S, Lim S. A treatment case of endoscopic removal of left ventricular thrombus, during coronary artery bypass graft. Korean J Thorac Cardiovasc Surg. 2014;47:434–6.
Kikuchi C, Shimada K, Nakayama K, Ohzeki H. Video-assisted transaortic left ventricular thrombectomy and coronary artery bypass grafting. Gen Thorac Cardiovasc Surg. 2009;57(4):208–10.
Duarte I, Fenton K, Morris BW. Video-assisted removal of left ventricular mass. Ann Thorac Surg. 1997;63(3):833–5.
Acknowledgements
Not applicable.
Funding
There are no sources of funding to disclose.
Author information
Authors and Affiliations
Contributions
The authors contributions are as follows: AE (corresponding author) summarized the case and relevant literature, the editorial process of the paper was overseen by CV, PS and NC. The case was performed by PS.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All information pertaining to the patient has been depersonalised. The patient in question has consented for publication.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Eranki, A., Villanueva, C., Collins, N. et al. Video assisted, transaortic removal of left ventricular thrombus during concurrent cardiac surgery: a case report. J Cardiothorac Surg 16, 242 (2021). https://doi.org/10.1186/s13019-021-01626-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13019-021-01626-4