Abstract
Purpose
This study aims to explore whether lobe specific lymph node dissection (LND) is adequate for cN0–1 non-small cell lung cancer (NSCLC) or not.
Methods
Among 5613 cN0–1 NSCLC patients, 394 cases (7.0%) with pN2 were enrolled and the distribution of mediastinal lymph node metastasis was analyzed. The included patients were divided into the non-lobe specific lymph node metastasis (NLSLNM) group and the lobe specific lymph node metastasis (LSLNM) group. The clinicopathological characteristics were compared between two groups and multivariable analysis was performed to find independent factors predicting NLSLNM.
Results
The incidence of pN2 cases deserved serious attention. The proportion of upper zone lymph node metastases was not rare in right (55.0%) and left (35.7%) lower lobe tumors. The proportion of subcarinal zone lymph node involvement was also high in right (21.8%) and left (25.8%) upper lobe tumors. Multivariable analysis showed that elevated carcinoembryonic antigen (CEA) level (P = 0.034), right lower lobe (RLL) tumors (P = 0.022) and station 11 involvement (P = 0.030) were independent risk factors for NLSLNM.
Conclusion
Systematic LND seems to be superior to lobe specific LND in the assessment of lymph node status and high CEA level, RLL tumors and station 11 involvement are predictors for NLSLNM.
Similar content being viewed by others
Introduction
Non-small cell lung cancer (NSCLC) has been one of the most common malignant tumors and the leading cause of cancer-related deaths in the world [1, 2]. Although the treatment methods and prognosis of NSCLC have been improving [3, 4], there are still many debatable problems. In general, surgery is recommended for the patients with resectable NSCLC, and lobectomy combined with systematic lymph node dissection (LND) is the standard surgical procedure [5]. However, this surgical treatment has been questioned with the increasing preference of minimal invasive surgery. The optimal extent of pulmonary resection has been explored by surgeons constantly, as well as the extent of lymphadenectomy. Sublobar resection including segmentectomy and wedge resection has been proven to be appropriate in patients with early stage NSCLC [6, 7]. In patients with T1-2N0–1M0, mediastinal lymph node sampling has shorter operative time, less chest tube drainage and similar survival outcome compared with complete mediastinal LND [8, 9].
In recent years, selective LND has also raised thoracic surgeons’ interest due to the concept of lobe specific lymphatic metastasis. The pattern of lymph node metastasis is thought to be influenced by the location of primary tumors, with tumors in the upper lobe showing a higher incidence of the superior mediastinal lymph nodes involvement than lower lobe tumors which tend to metastasize to the inferior and subcarinal nodes [10,11,12]. However, there are still some studies indicating that complete LND is overwhelmingly superior to lobe specific LND from the oncological point of view [13,14,15]. This study aims to explore whether lobe specific LND is adequate for cN0–1 non-small cell lung cancer (NSCLC) or not.
Materials and methods
Patients selection
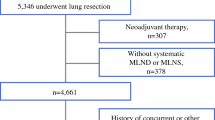
The study protocol was approved by the Institutional Review Board of Hangzhou Red Cross Hospital and the First Affiliated Hospital of Zhejiang University, School of Medicine. From January 2012 to May 2018, a total of 5613 patients with cN0–1 NSCLC had undergone surgery in our institutions. The data of patients were reviewed retrospectively from hospital electronic medical records systems, including demographic data, preoperative investigations and pathological characteristics.
The patients included in the analysis fitted with the following criteria: (1) the disease was diagnosed as cN0–1 preoperatively but was confirmed as pN2 postoperatively; (2) the patient did not have distant metastasis before treatment; (3) the histology was classified as NSCLC; (4) mediastinal LND was performed together with pulmonary resection. And we excluded patients who had multiple tumors in different lobes, those who received induction therapy preoperatively, including chemotherapy or radiotherapy, and those who underwent only biopsy and selective LND. We also excluded patients with tumors invading multiple lobes. Ultimately, 394 patients were included in this study. All enrolled patients were restaged according to the 8th edition of the American Joint Committee on Cancer (AJCC) lung cancer staging classification [16].
Staging policy and lymph node assessment
Preoperative stage evaluations included physical examination, chest CT, abdominal ultrasound, magnetic resonance imaging (MRI) of the brain and fiberoptic bronchoscopy. Bone scintigraphy was only performed in the patients with bone pain and positron-emission tomography (PET) scan was also not routinely performed in early stage NSCLC. Clinical lymph node (LN) status was assessed by CT scan, PET scan and endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA). The lymph node was considered to be positive when its shortest axis was longer than 1 cm on CT scan. PET scan and EBUS-TBNA were not routinely performed unless the patients was highly suspected as N2 disease on CT scan.
The site of mediastinal lymph node was described according to the International Association for the Study of Lung Cancer (IASLC) lymph node map [17], with the upper zone including stations 2, 3 and 4, the aortic-pulmonary zone including stations 5 and 6, the subcarinal zone including station 7 and the lower zone including stations 8 and 9. Lobe specific lymph node stations (LSLNS) depended on the location of primary tumors (stations 2R, 3 and 4R for right upper lobe tumors, stations 4 L, 5 and 6 for left upper lobe tumors, stations 7, 8 and 9 for lower lobe tumors) [18]. Tumors metastasizing to LSLNS was defined as lobe specific lymph node metastasis (LSLNM), while tumors metastasizing beyond LSLNS was defined as non-lobe specific lymph node metastasis (NLSLNM). Skip N2 metastasis was defined as mediastinal lymph node metastasis without N1 metastasis [19].
Surgical procedures
All patients underwent anatomic pulmonary resection combined with mediastinal LND. Mediastinal LND for right side tumors included at least stations 2R, 4R, 7, 8 and 9, while for left side tumors, stations 5, 6, 7, 8 and 9 were required at least. Due to the anatomic limitations, dissection of station 3 and 4 L was not routinely performed unless the lymph nodes metastases were highly suspicious preoperatively or intraoperatively.
Statistical analysis
The measurement data and numeration data were statistically analyzed with t test and χ2 test respectively. If there were clinicopathological characteristics showing significant differences between the NLSLNM group and the LSLNM group, multivariate analysis was performed for those characteristics by the binary logistic regression to identify the factors predicting NLSLNM. Because the lymphatic metastasis patterns of right middle lobe tumors were unclear, the data of those tumors were excluded from the comparison between NLSLNM group and the LSLNM group. All the above analysis was conducted by SPSS software (version 24.0, IBM SPSS Inc. United States). Statistical significance was set at P value < 0.05 (All P values presented were 2-sided).
Results
Distribution of mediastinal lymph node metastasis
Among 5613 cN0–1 NSCLC patients, 394 cases (7.0%) with pN2 were enrolled, and 213 had right NSCLC while the other 181 had left NSCLC. The distributions of mediastinal lymph node metastasis in right and left sides were listed in Table 1 and Table 2, respectively.
Right upper lobe (RUL): lymph nodes in upper zone were involved much more often in the RUL compared with the other two lobes (94.9% vs. 57.1% vs. 55.0%, P < 0.001). Station 4R (80.8%) had the highest proportion to be involved, followed by station 2R (43.6%) and station 3 (41.4%). Station 7 involvement (21.8%) was also occurred with a relatively high proportion. Lymph nodes in station 8 (1.3%) and station 9 (2.6%) were less likely to be involved than other stations.
Right middle lobe (RML): the highest proportion of metastasis was observed in subcarinal zone, station 7 (82.9%). The involvement of upper zone was common as well, with 51.4% in station 4R, 35.7% in station 3 and 37.1% in station 2R. Only 1 patient (2.9%) had positive lymph nodes in the lower zone.
Right lower lobe (RLL): station 7 (86.0%) was also the most common site to be involved in the RLL, follow by station 4R (43.0%). The involvement of station 2R (14.0%) and station 3 (34.9%) was not rare. The metastasis of RLL to the lower zone was more often than the RUL and RML (13.0% vs. 2.6% vs. 2.9%, P = 0.017).
It was notable that 4 patients (5.1%) with RUL tumors had negative LSLNS and 11 patients (11.0%) with RLL tumors had negative LSLNS.
Left upper lobe (LUL): station 5 (73.2%) was the most common site to be involved, followed by station 4 L (48.3%) and 6 (27.8%). The involvement of station 7 (25.8%) was also not rare. As was expected, metastases in the lower zone lymph nodes occupied small percentage, with only 4.1%.
Left lower lobe (LLL): the highest proportion of metastasis was observed in station 7 (67.9%), far more common than the LUL (P < 0.001). It should be noted that the proportion of the upper zone lymph nodes involvement (35.7%) was not far less than that of the lower zone involvement (38.1%).
It should be emphasized that there were 12.4 and 14.3% pN2 patients having negative LSLNS in the LUL and LLL, respectively.
Characteristics of patients with NLSLNM and LSLNM
All enrolled patients except those with RML tumors were divided into the NLSLNM group and LSLNM group on the basis of the lymphatic metastasis pattern, with 129 in NLSLNM group and 230 in LSLNM group. Clinical and pathological characteristics of two groups were shown in Table 3 and Table 4.
There were no statistical differences in sex and age between two groups. In contrast, more patients in LSLNM group had smoking history (50.0% vs. 32.6%, P = 0.001) and hypertension (28.7% vs. 16.3%, P = 0.008) than those in NLSLNM group. Abnormally elevated carcinoembryonic antigen (CEA) level (>5 ng/ml) was detected in more patients in NLSLNM group than LSLNM group (50.4% vs. 34.8%, P = 0.004). The NLSLNM group significantly tended to have RLL tumors while the LSLNM group was more likely to have upper lobe tumors (P < 0.001). The proportion of solid tumors presenting on CT scan was comparable at approximately 95% in both groups. It was worth mentioning that there was one patient in both groups presenting ground-glass opacity (GGO) on CT scan. Clinical T stage and N stage were also similar in two groups.
There were no significant differences in pathological characteristics, except for histology and N1 involvement. Adenocarcinoma was observed in greater percentage of patients in NLSLNM group while squamous cell carcinoma was detected more often in LSLNM group (P = 0.022). Station 10 and 11 lymph nodes were less likely to be involved in LSLNM group compared with those in NLSLNM group (26.5% vs. 39.5%, P = 0.011 and 20.9% vs. 36.4%, P = 0.001, respectively).
Factors predicting NLSLNM
The univariate analysis showed that smoking history, hypertension, CEA level, tumor location, histology, stations 10 and 11 involvement were statistically significant factors influencing the lymphatic metastasis pattern. Multivariate analysis was further performed for these factors (Table 5). The results indicated that hypertension (P = 0.025), CEA level (P = 0.034), tumor location (P = 0.022) and station 11 involvement (P = 0.030) were statistically associated with NLSLNM. High CEA level (OR = 1.684, 95% CI = 1.040–2.725), RLL tumors (OR = 2.111, 95% CI = 1.116–3.992) and station 11 involvement (OR = 1.774, 95% CI = 1.056–2.980) were independent risk factors for NLSLNM, while hypertension (OR = 0.512, 95% CI = 0.286–0.918) was a protective factor for NLSLNM.
Discussion
Although an increasing number of lung cancers are discovered in early stage with the development of lung cancer screening, there have been several studies reporting that unsuspected N2 disease was diagnosed in 4.4–9% of clinical stage I–II NSCLC [15, 20]. The lymph node status including hilar and mediastinal lymph nodes has been one of the most important factors to determine the need for adjuvant therapies and predict the prognosis [21]. Systematic LND that dissects the hilar and mediastinal lymph nodes completely could not only guarantee the accuracy of N stage but also guide the postoperative treatment strategy precisely. As a result, systematic LND has been recommended along with lobectomy, regardless of the tumor stage and location. However, on the basis of lobe specific nodal drainage pattern, lobe specific LND has appealed to some surgeons. Aokage, K. et al. [11] suggested that it was safe to omit station 7 dissection in upper lobe NSCLC patients because subcarinal node metastases from upper lobe NSCLC were rare. Adachi, H. et al. [22] indicated that lobe specific LND might be a standard procedure in surgical treatment for cT1-2 N0–1 M0 NSCLC due to the similar 5-year overall survival between the lobe specific LND group and the systematic dissection group. Also, the most recent and largest retrospective registry study showed that lobe specific LND did not have a negative prognostic impact and it had the potential to be an alternative to systematic nodal dissection for the patients with stage I or II NSCLC [23].
However, several publications suggested that tumor locations were not the predictor of lymphatic drainage pathways [13] and there was a considerable number of pN2 patients having mediastinal lymph node metastasis beyond the lobe specific lymph node stations [15], so systematic LND was still recommended to be performed, even in clinical stage I NSCLC. There are two main reasons for the ambiguous role of lobe specific LND. First, the similar impact on long-term survival between lobe specific LND and systematic LND has not been confirmed by a prospective randomized study. Fortunately, a multi-institutional and randomized Phase III trial (JCOG1413) began in January 2017 to confirm the clinical benefit in terms of survival non-inferiority and less invasiveness of lobe specific LND compared with systematic LND in patients with clinical stage I–II NSCLC [18]. Secondly, the factors predicting NLSLNM or LSLNM has remained unclear.
This study focused on the association between the clinicopathological characteristics and mediastinal lymphatic metastasis pattern in cN0–1 NSCLC. We found that abnormally elevated CEA level (>5 ng/ml), RLL tumors and station 11 involvement were independent risk factors for NLSLNM. This study also explored the distributions of mediastinal lymph node metastasis. Our findings showed that mediastinal lymph node metastasis pattern conformed to “lobe specific” rule to some extent. For example, the upper zone lymph nodes were more likely to be involved in the upper lobe tumors, while the subcarinal zone lymph nodes were more likely to be involved in the lower lobe tumors. However, the proportion of upper zone lymph node metastases was not rare in lower lobe tumors, and similarly, the proportion of subcarinal zone lymph node involvement in upper lobe tumors was also high enough to warrant attention. Furthermore, LSLNS were negative in more than 5% of each lobe tumors and the high proportion required attention in the selection of lymphadenectomy extent. Lobe specific LND should be performed prudently and systematic LND might be a better procedure. These findings were in line with the results of a previous study including a total of 4511 cases [24].
Our study had two main limitations. First, this was a retrospective study, so the selective bias was inevitable. The results should be confirmed by prospective randomized studies in the future. Secondly, dissection of station 3 and 4 L was not routinely performed and the samples were not large. The pattern of station 3 and 4 L metastases needed to be further explored in future studies.
Conclusions
In patients with cN0–1 NSCLC, once mediastinal lymph node metastasis occurs, although different primary tumor locations have a different propensity to be sites of mediastinal lymph node metastasis, each zone and each station have relatively high risk to be involved, so systematic LND should be recommended to guarantee the adequate assessment of mediastinal lymph node status. And abnormally elevated CEA level (>5 ng/ml), RLL tumors and station 11 involvement are independently associated with greater risk of non-lobe specific lymph node metastasis in cN0–1pN2 patients.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- NSCLC:
-
non-small cell lung cancer
- LND:
-
lymph node dissection
- NLSLNM:
-
non-lobe specific lymph node metastasis
- LSLNM:
-
Lobe specific lymph node metastasis
- CEA:
-
Carcinoembryonic antigen
- AJCC:
-
American Joint Committee on Cancer
- MRI:
-
Magnetic resonance imaging
- PET:
-
Positron-emission tomography
- EBUS:
-
TBNA endobronchial ultrasound-guided transbronchial needle aspiration
- IASLC:
-
International Association for the Study of Lung Cancer
- LSLNS:
-
Lobe specific lymph node stations
- RUL:
-
Right upper lobe
- RML:
-
Right middle lobe
- RLL:
-
Right lower lobe
- LUL:
-
Left upper lobe
- LLL:
-
Left lower lobe
- GGO:
-
Ground-glass opacity
- VATS:
-
Video assisted thoracic surgery
- SCC:
-
Squamous cell carcinoma
- NA:
-
Not available
- OR:
-
Odds ratio
- CI:
-
Confidence interval
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30.
Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries [J]. CA Cancer J Clin. 2018;68(6):394–424.
Koike T, Yamato Y, Asamura H, et al. Improvements in surgical results for lung cancer from 1989 to 1999 in Japan. J Thorac Oncol. 2009;4(11):1364–9.
Strand TE, Bartnes K, Rostad H. National trends in lung cancer surgery. Eur J Cardiothorac Surg. 2012;42(2):355–8.
Howington JA, Blum MG, Chang AC, et al. Treatment of stage I and II non-small cell lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines [J]. Chest. 2013;143(5 Suppl):e278S–313S.
Landreneau RJ, Normolle DP, Christie NA, et al. Recurrence and survival outcomes after anatomic segmentectomy versus lobectomy for clinical stage I non-small-cell lung cancer: a propensity-matched analysis. J Clin Oncol. 2014;32(23):2449–55.
Altorki NK, Yip R, Hanaoka T, et al. Sublobar resection is equivalent to lobectomy for clinical stage 1A lung cancer in solid nodules. J Thorac Cardiovasc Surg. 2014;147(2):754–62 Discussion 762-754.
Allen MS, Darling GE, Pechet TT, et al. Morbidity and mortality of major pulmonary resections in patients with early-stage lung cancer: initial results of the randomized, prospective ACOSOG Z0030 trial. Ann Thorac Surg. 2006;81(3):1013–9 discussion 1019-1020.
Darling GE, Allen MS, Decker PA, et al. Randomized trial of mediastinal lymph node sampling versus complete lymphadenectomy during pulmonary resection in the patient with N0 or N1 (less than hilar) non-small cell carcinoma: results of the American college of surgery oncology group Z0030 trial. J Thorac Cardiovasc Surg. 2011;141(3):662–70.
Asamura H, Nakayama H, Kondo H, et al. Lobe-specific extent of systematic lymph node dissection for non-small cell lung carcinomas according to a retrospective study of metastasis and prognosis. J Thorac Cardiovasc Surg. 1999;117(6):1102–11.
Aokage K, Yoshida J, Ishii G, et al. Subcarinal lymph node in upper lobe non-small cell lung cancer patients: is selective lymph node dissection valid? Lung Cancer. 2010;70(2):163–7.
Shimada Y, Saji H, Kakihana M, et al. Retrospective analysis of nodal spread patterns according to tumor location in pathological N2 non-small cell lung cancer. World J Surg. 2012;36(12):2865–71.
Riquet M, Rivera C, Pricopi C, et al. Is the lymphatic drainage of lung cancer lobe-specific? A surgical appraisal. Eur J Cardiothorac Surg. 2015;47(3):543–9.
Rami-Porta R. Leave no lymph nodes behind! Eur J Cardiothorac Surg. 2013;44(1):e64–5.
Bille A, Woo KM, Ahmad U, et al. Incidence of occult pN2 disease following resection and mediastinal lymph node dissection in clinical stage I lung cancer patients. Eur J Cardiothorac Surg. 2017;51(4):674–9.
Rami-Porta R, Asamura H, Travis WD, et al. Lung cancer - major changes in the American joint committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017;67(2):138–55.
Rusch VW, Asamura H, Watanabe H, et al. The IASLC lung cancer staging project: a proposal for a new international lymph node map in the forthcoming seventh edition of the TNM classification for lung cancer. J Thorac Oncol. 2009;4(5):568–77.
Hishida T, Saji H, Watanabe SI, et al. A randomized phase III trial of lobe-specific vs. systematic nodal dissection for clinical stage I-II non-small cell lung cancer (JCOG1413). Jpn J Clin Oncol. 2018;48(2):190–4.
Okada M, Tsubota N, Yoshimura M, et al. Proposal for reasonable mediastinal lymphadenectomy in bronchogenic carcinomas: role of subcarinal nodes in selective dissection. J Thorac Cardiovasc Surg. 1998;116(6):949–53.
Yang CF, Kumar A, Gulack BC, et al. Long-term outcomes after lobectomy for non-small cell lung cancer when unsuspected pN2 disease is found: a National Cancer Data Base analysis. J Thorac Cardiovasc Surg. 2016;151(5):1380–8.
Martini N, Flehinger BJ, Zaman MB, et al. Prospective study of 445 lung carcinomas with mediastinal lymph node metastases. J Thorac Cardiovasc Surg. 1980;80(3):390–9.
Adachi H, Sakamaki K, Nishii T, et al. Lobe-specific lymph node dissection as a standard procedure in surgery for non-small cell lung Cancer: a propensity score matching study. J Thorac Oncol. 2017;12(1):85–93.
Hishida T, Miyaoka E, Yokoi K, et al. Lobe-specific nodal dissection for clinical stage I and II NSCLC: Japanese multi-institutional retrospective study using a propensity score analysis. J Thorac Oncol. 2016;11(9):1529–37.
Liang RB, Yang J, Zeng TS, et al. Incidence and distribution of lobe-specific Mediastinal lymph node metastasis in non-small cell lung Cancer: data from 4511 resected cases. Ann Surg Oncol. 2018;25(11):3300–7.
Acknowledgements
Not applicable.
Funding
This study was funded by Medical Health Science and Technology Project of Zhejiang province (2015KYB310).
Author information
Authors and Affiliations
Contributions
Drs. Likui Fang and Jinming Xu contributed to the conception and design of the work. Dr. Likui Fang and contributed to conception, design, data analysis and editing the manuscript. Drs. Likui Fang, Bo Ye, Guocan Yu and Jun Yang contributed to data acquisition, statistical analysis and interpretation of the data. Drs. Gang Chen and Jinming Xu contributed to the revision of the manuscript. All authors have approved the final draft of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study protocol was approved by the Institutional Review Board of Hangzhou Red Cross Hospital and the First Affiliated Hospital of Zhejiang University, School of Medicine.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Fang, L., Xu, J., Ye, B. et al. Is lobe specific lymph node dissection adequate for cN0–1 non-small cell lung cancer?. J Cardiothorac Surg 15, 46 (2020). https://doi.org/10.1186/s13019-020-1087-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13019-020-1087-4