Abstract
Background
Video-assisted thoracoscopic surgery has been widely used in thoracic surgery worldwide. Our goal was to identify the risk factors for postoperative pneumonia in patients undergoing video-assisted thoracoscopic surgery lobectomy.
Methods
A retrospective analysis of adult patients undergoing video-assisted thoracoscopic surgery lobectomy between 2016 and 05 and 2017–04 was performed. We used univariate analyses and multivariate analyses to examine risk factors for postoperative pneumonia after lobectomy.
Results
The incidence of postoperative pneumonia was 19.7% (n = 143/727). Patients with postoperative pneumonia had a higher postoperative length of stay and total hospital care costs when compared to those without postoperative pneumonia. Multivariate analysis showed that body mass index grading ≥24.0 kg/m2 (vs. <24.0 kg/m2: odds ratio 1.904, 95% confidence interval 1.294–2.802, P = 0.001) and right lung lobe surgery (vs. left lung lobe surgery: odds ratio 1.836, 95% confidence interval 1.216–2.771, P = 0.004) were independent risk factors of postoperative pneumonia. Total intravenous crystalloid infusion grading in the postoperative 24 h ≥ 1500 mL was also identified as the risk factors (vs. 1000 to < 1500 mL: odds ratio 2.060, 95% confidence interval 1.302–3.260, P = 0.002).
Conclusions
Major risk factors for postoperative pneumonia following video-assisted thoracoscopic surgery lobectomy are body mass index grading ≥24.0 kg/m2, right lung lobe surgery and total intravenous crystalloid infusion grading in the postoperative 24 h ≥ 1500 mL.
Similar content being viewed by others
Introduction
Lung resection is the main treatment for benign and malignant pulmonary tumours [1, 2]. Postoperative pneumonia (POP) is one of the most common complications and the main cause of death in patients undergoing lung resection [3,4,5,6]. The incidence of POP after lung surgery has been reported to range from 2.1 to 40.0% and is associated with increased mortality [7]. Several risk factors, such as age, the extent of resection, low forced-expiratory-volume-in-1-s (FEV1), advanced pathologic stage and chronic obstructive pulmonary disease for POP after lung resection have been identified [7, 8]. However, these studies investigating risk factors for POP after lung resection were based on small sample sizes, and it remains difficult to predict who will develop POP after lung resection [7,8,9]. Furthermore, to date, there is scarce literature research on risk factors for POP after video-assisted thoracoscopic surgery (VATS) lobectomy. With the popularity of minimally invasive thoracic surgery, identification of risk factors for POP after VATS lobectomy on a large cohort of patients is warranted. High-risk patients could be identified during the perioperative period, and targeting perioperative interventions in patients at high risk of POP may decrease POP frequency and mortality.
The objectives of this single-centre observational retrospective study were to identify risk factors for POP after VATS lobectomy and provide a reference for clinical prevention of POP.
Materials and methods
Study population and design
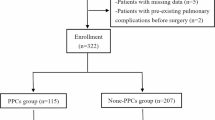
This observational retrospective study included 788 consecutive adult patients who underwent VATS lobectomy between May 2016 and April 2017 at the First Affiliated Hospital, College of Medicine, Zhejiang University (Fig. 1). Patients were considered eligible for inclusion if they were aged over 18 years and were scheduled to undergo VATS lobectomy under general anaesthesia with double-lumen intubation. Lung protective strategy of low tidal volume during single lung ventilation was managed. Excluded from the study were patients undergoing bilobectomy or combined lobectomy and sublobar resection (n = 17) or conversion to thoracotomy (n = 19). Twenty-five patients with missing data of pulmonary function and intraoperative urine output in anaesthesia records were also excluded. Finally, 727 valid cases were included (Fig. 1).
Detailed patient data of the whole cohort are shown in Table 1. Indications for surgical resection were malignant tumour (n = 634), benign tumour (n = 10), benign non-inflammatory disease (n = 7) and inflammatory disease (n = 76). Demographic, intraoperative and outcomes data were extracted from medical records, as described in Table 1. All patients received perioperative antibiotic prophylaxis. Quinolone and clindamycin were used in patients known allergy to cephalosporin. Management of postoperative pain was implemented according to a clinical practice guideline (2016) from the American Pain Society [10]. All patients started oral feedings in the sixth to eighth postoperative hour, unless they were intubated or at risk for aspiration.
Variables and definitions
POP was defined as a new pulmonary infiltrate on chest X-ray (determined independently by two radiologists) with leucocytosis (leukocyte count > 10.0 × 109/L) and fever (ear temperature > 38.0 °C) [11]. Only the first episode of pneumonia diagnosed during the first 7 days after surgery was studied and was defined as POP [12].
According to the Health Industry Standard of China: Adult Weight Determination (WS/T428–2013), we use body mass index (BMI) ≥24.0 kg/m2 as the pre-obesity standard, which is slightly different from the World Health Organization (WHO) standard (Pre-obesity: BMI ≥25.0 kg/m2). Preoperative renal insufficiency was defined as creatinine > 50% the upper limit of the reference range, which is 1.3 and 1.1 mg/dL for men and women, respectively [13]. The amount of total intraoperative fluids was defined as the volumes of crystalloid, colloid and blood products administered between initiation of anaesthesia care and arrival in the postanaesthesia care unit [13]. Total intravenous crystalloid/colloid infusion in the postoperative 24 h was defined as the volumes of intravenous crystalloid/colloid in the postoperative 24 h. Postoperative pathology of malignant tumour included lung cancer, atypical adenomatous hyperplasia, lymphoepithelioma-like carcinoma and lung metastasis. Inflammatory disease included bronchiectasis, tuberculosis, fungal infections, granulomatous inflammation, chronic inflammation and purulent inflammation. A prolonged air leak was defined as leak > 7 days [11]. Postoperative length of stay (PLOS) was defined as the number of hospitalised days after surgery. Hospital costs were the total hospital care costs.
Statistical analysis
Continuous data are presented as mean ± standard deviation and were analysed using one-way variance analysis. If the variance was not homogeneous, a nonparametric test (Kruskal–Wallis H test for multiple independent samples) was used. Categorical variables are expressed as percentages and were compared by the R × C chi-square test or Fisher’s exact test, as appropriate. Binary logistic regression analyses were performed to evaluate the risk factors of POP. We created a multivariate analysis model using significant variables, as determined by the univariate analysis and suggested risk factors of POP. Additional file 1: Table S1 shows the assignment of variables in multivariate analysis. Odds ratios (ORs) were calculated from these models, together with their 95% confidence intervals (CIs). For all tests, a two-tailed P ≤ 0.05 was considered statistically significant. Analyses were conducted using SPSS 25.0 (SPSS, Inc., Chicago, IL, USA) [11, 13, 14].
Results
Patient selection and comparative univariate analysis
A total of 727 patients met our inclusion and exclusion criteria for analysis (Fig. 1). The incidence of POP was 19.7% (143 of 727). No patient died during the period of hospitalisation. The PLOS and total hospital care costs in the POP group were significantly higher than those in the no-POP group (Table 2). Table 1 summarises the perioperative factors and comparative univariate analysis results of the cohort. In univariate analysis, BMI, BMI grading, forced vital capacity (percentage of predicted value), surgical lobe, intraoperative bleeding, total intravenous crystalloid infusion in the postoperative 24 h and total intravenous crystalloid infusion grading in the postoperative 24 h were significantly different between POP and no-POP groups (Tables 1).
Comparative multivariate analysis of the risk factors of postoperative pneumonia
We included statistically significant factors in univariate analysis, namely, BMI grading, forced vital capacity (percentage of predicted value), surgical lobe, intraoperative bleeding, and total intravenous crystalloid infusion grading in the postoperative 24 h, in our multivariate regression model, to evaluate the preoperative predictors of POP. Binary logistics regression analysis demonstrated that BMI grading ≥24.0 kg/m2 (vs. <24.0 kg/m2: OR 1.904, 95% CI 1.294–2.802, P = 0.001), right lung lobe surgery (vs. left lung lobe surgery: OR 1.836, 95% CI 1.216–2.771, P = 0.004) and total intravenous crystalloid infusion grading in the postoperative 24 h ≥ 1500 mL (vs. 1000 to < 1500 mL: OR 2.060, 95% CI 1.302–3.260, P = 0.002) were independent risk factors of POP after VATS lobectomy (Table 3).
Discussion
In our study, POP occurred in 143 (19.7%) of 727 patients who underwent VATS lobectomy (Table 1). Patients with POP had higher PLOS and total hospital care costs than no-POP patients (Table 2). Three independent risk factors for POP after VATS lobectomy were identified: BMI grading ≥24.0 kg/m2, right lung lobe surgery and total intravenous crystalloid infusion grading in the postoperative 24 h ≥ 1500 mL (Table 3).
The incidence of POP after lung resection varies. Simonsen et al. [4] reported frequencies of 3.6% and Lee et al. [9] documented a prevalence of 6.2%, whereas, Arslantas et al. [11] noted that POP occurred in 18.7% patients after lung resection. One of the reasons for this fluctuation is due to the differences in the definitions of POP. In the current study, POP were defined similarly to Arslantas et al. [11] and Allou et al. [12], including a new pulmonary infiltrate on chest X-ray, leukocyte count > 10.0 × 109/L and fever. Our incidence of POP was 19.7%, which was compatible with the reported frequency [11] .
Our study showed that BMI grading ≥24.0 kg/m2 was an independent risk factor for POP after VATS lobectomy. The result of the present study corresponded with the earlier studies, which reported that overweight or obese patients have an increased risk of POP [15,16,17]. Obese patients often have reduced lung volume, altered ventilation pattern, decreased immune function, and comorbid conditions, which are risk factors for intra- and postoperative complications [16,17,18]. Overweight and obesity are spreading worldwide, and thoracic surgeons will encounter more overweight patients in need of surgery in the future [19]. Although a BMI ≥ 24.0 kg/m2 is not a surgical contraindication, it is necessary to pay close attention to overweight patients, and to strengthen respiratory exercise before lobectomy to reduce the risk of POP.
To the best of our knowledge, this is the first report to identify that right lung lobe surgery is an independent risk factor of POP after VATS lobectomy (Table 3). The incidence of POP was 23.0% (103 /448) after right lung lobe surgery and 14.3% (40/279) after left lung lobe surgery, respectively (Table 3). The reasons why right lung lobe surgery has an increasing risk of POP are unclear. The lung volume of right lung is larger than the volume of left lung (right/left lung volume, 1.22 ± 0.14) [20], so right lung lobe surgery has a greater impact on lung function and has a greater trauma than left lung lobe surgery, thus leading to an increasing risk of POP. Therefore, for patients with right lung lobe surgery, it is especially necessary to strengthen the training of respiratory function and cough ability before surgery, and take some measures to prevent lung infection after surgery, to avoid the occurrence of POP.
Finally, our study showed that total intravenous crystalloid infusion grading in the postoperative 24 h ≥ 1500 mL was an independent risk factor of POP after VATS lobectomy. In this study, POPs were all diagnosed 24 h after operation, so POP was not the reason for increased intravenous crystalloid infusion in the postoperative 24 h. Excessive intravenous fluid infusion would cause pulmonary edema and impair gas exchange, thereby placing patients at heightened risk for infection and respiratory failure [11, 13]. Shin et al. found that excessive perioperative fluid is associated with increased risk of postoperative pulmonary complications and increased 30-day mortality [13]. A meta-analysis of several trials suggested that larger fluid volumes increase the chances of postoperative pneumonia and pulmonary edema [21]. The harmful effects of fluid excess are frequently manifested in the lungs, especially after pulmonary lobectomy. Arslantas et al. conducted a study of perioperative fluid administration and the results showed that excessive perioperative infusion fluid during anatomic lung resections could increase postoperative pulmonary complications [11]. Our findings support the view that liberal postoperative fluid infusion has harmful effects on postoperative lung function and adds to the current understanding of the postoperative fluid management in several ways.
Some studies have reported risk factors for POP following lung cancer surgery. Lee noted that age ≥ 70 years, intraoperative red blood cell transfusion and forced expiratory volume in 1 s < 70% were independent risk factors of POP after lung cancer surgery [9]. Simonsen reported that major risk factors for POP following lung cancer surgery are advanced age, obesity, chronic pulmonary disease, alcoholism and atrial fibrillation [4]. The POP risk factors for VATS lobectomy in our study differ from the above studies, thus adding new content to the POP risk factors study.
Our study is one of the few to show risk factors for POP after VATS lobectomy. However, this study has potential limitations. First, it was a single-centre retrospective study. Second, antibiotics were used prophylactically in every patient, thereby masking the discovery of risk factors for POP. Finally, the study population only included adult patients who underwent VATS lobectomy, which limits the generalisability of the findings.
Conclusions
This present study suggested that patients with POP had higher PLOS and total hospital care costs than no-POP patients. The major risk factors for POP following VATS lobectomy included body mass index grading ≥24.0 kg/m2, right lung lobe surgery and total intravenous crystalloid infusion grading in the postoperative 24 h ≥ 1500 mL. Clinicians should remain vigilant in preventing and treating pneumonia and other infections in patients with these risk factors. The next step for future studies is the creation of a clinical scoring system to predict POP.
Availability of data and materials
All data generated or analyzed during this study are included in this published article and its additional file.
Abbreviations
- BMI:
-
Body mass index
- CIs:
-
Confidence intervals
- FEV1:
-
Low forced-expiratory-volume-in-1-s
- ORs:
-
Odds ratios
- PLOS:
-
Postoperative length of stay
- POP:
-
Postoperative pneumonia
- VATS:
-
Video-assisted thoracoscopic surgery
- WHO:
-
World Health Organization
References
Detterbeck FC. Are we there yet?: understanding differences in rates of resection of clinical stage I lung Cancer. Chest. 2019;155(1):7–8.
Paul S, Lee PC, Mao J, Isaacs AJ, Sedrakyan A. Long term survival with stereotactic ablative radiotherapy (SABR) versus thoracoscopic sublobar lung resection in elderly people: national population based study with propensity matched comparative analysis. BMJ. 2016;354:i3570.
Thomas PA, Berbis J, Falcoz PE, Le Pimpec-Barthes F, Bernard A, Jougon J, et al. National perioperative outcomes of pulmonary lobectomy for cancer: the influence of nutritional status. Eur J Cardiothorac Surg. 2014;45(4):652–9 discussion 9.
Simonsen DF, Sogaard M, Bozi I, Horsburgh CR, Thomsen RW. Risk factors for postoperative pneumonia after lung cancer surgery and impact of pneumonia on survival. Respir Med. 2015;109(10):1340–6.
Shiono S, Abiko M, Sato T. Postoperative complications in elderly patients after lung cancer surgery. Interact Cardiovasc Thorac Surg. 2013;16(6):819–23.
Lugg ST, Agostini PJ, Tikka T, Kerr A, Adams K, Bishay E, et al. Long-term impact of developing a postoperative pulmonary complication after lung surgery. Thorax. 2016;71(2):171–6.
Schussler O, Alifano M, Dermine H, Strano S, Casetta A, Sepulveda S, et al. Postoperative pneumonia after major lung resection. Am J Respir Crit Care Med. 2006;173(10):1161–9.
Belda J, Cavalcanti M, Ferrer M, Serra M. Puig de la Bellacasa J, Canalis E, et al. bronchial colonization and postoperative respiratory infections in patients undergoing lung cancer surgery. Chest. 2005;128(3):1571–9.
Lee JY, Jin SM, Lee CH, Lee BJ, Kang CH, Yim JJ, et al. Risk factors of postoperative pneumonia after lung cancer surgery. J Korean Med Sci. 2011;26(8):979–84.
Chou R, Gordon DB, de Leon-Casasola OA, Rosenberg JM, Bickler S, Brennan T, et al. Management of postoperative pain: a clinical practice guideline from the American pain society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists' committee on regional anesthesia, executive committee, and administrative council. J Pain. 2016;17(2):131–57.
Arslantas MK, Kara HV, Tuncer BB, Yildizeli B, Yuksel M, Bostanci K, et al. Effect of the amount of intraoperative fluid administration on postoperative pulmonary complications following anatomic lung resections. J Thorac Cardiovasc Surg. 2015;149(1):314–20 21 e1.
Allou N, Bronchard R, Guglielminotti J, Dilly MP, Provenchere S, Lucet JC, et al. Risk factors for postoperative pneumonia after cardiac surgery and development of a preoperative risk score*. Crit Care Med. 2014;42(5):1150–6.
Shin CH, Long DR, McLean D, Grabitz SD, Ladha K, Timm FP, et al. Effects of intraoperative fluid management on postoperative outcomes: a hospital registry study. Ann Surg. 2018;267(6):1084–92.
Yu D, Deng Q, Wang J, Chang X, Wang S, Yang R, et al. Air pollutants are associated with dry eye disease in urban ophthalmic outpatients: a prevalence study in China. J Transl Med. 2019;17(1):46.
Dancewicz M, Kowalewski J, Peplinski J. Factors associated with perioperative complications after pneumonectomy for primary carcinoma of the lung. Interact Cardiovasc Thorac Surg. 2006;5(2):97–100.
Suemitsu R, Takeo S, Hamatake M, Morokuma A, Suemori Y, Tanaka H. The results of surgery under general anesthesia in patients with lung cancer. Surg Today. 2011;41(1):60–6.
Xia L, Taylor BL, Guzzo TJ. Characteristics and associated factors of postoperative pulmonary complications in patients undergoing radical cystectomy for bladder Cancer: a National Surgical Quality Improvement Program Study. Clin Genitourin Cancer. 2017;15(6):661–9.
Lachmann G, von Haefen C, Kurth J, Yuerek F, Wernecke KD, Spies C. Smoking, gender, and overweight are important influencing factors on Monocytic HLA-DR before and after major Cancer surgery. Biomed Res Int. 2017;2017:5216562.
Wang C, Guo M, Zhang N, Wang G. Association of body mass index and outcomes following lobectomy for non-small-cell lung cancer. World J Surg Oncol. 2018;16(1):90.
Yu CG, Grant CA, Izatt MT, Labrom RD, Askin GN, Adam CJ, et al. Change in lung volume following thoracoscopic anterior spinal fusion surgery: a 3-dimensional computed tomography investigation. Spine (Phila Pa 1976). 2017;42(12):909–16.
Corcoran T, Rhodes JE, Clarke S, Myles PS, Ho KM. Perioperative fluid management strategies in major surgery: a stratified meta-analysis. Anesth Analg. 2012;114(3):640–51.
Acknowledgments
We appreciate the assistance of Large-scale Data Analysis Center of Cancer Precision Medicine-LinkDoc database for clinical and pathological data collected.
Funding
This work was supported by the Natural Science Foundation of Zhejiang Province, China [grant number LQ18H180002]; and the National Natural Science Foundation of China [grant number 31700690].
Author information
Authors and Affiliations
Contributions
YW conceived the idea for the study and managed the project. RY and YW designed the study. RY, CD, JX, LY, and SZ conducted the survey and collected data. RY wrote the manuscript and prepared the figures and tables. All authors interpreted the data and contributed to preparation of the manuscript. No conflicting relationship exists for any author. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The survey was approved by the Medical Ethics Committee of the First Affiliated Hospital, College of Medicine, Zhejiang University, Hangzhou, China (No.2017–58). Because the data were recorded retrospectively and without any specific intervention, the Medical Ethics Committee waived informed consent from the subjected patients. Data were deidentified to protect the privacy and maintain confidentiality of patient information. It was conducted in accordance with the Declaration of Helsinki.
Consent for publication
For this type of study formal consent is not required.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1: Table S1.
Assignment of variables in multivariate analysis.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Yang, R., Du, C., Xu, J. et al. Excessive intravenous crystalloid infusion after video-assisted thoracoscopic surgery lobectomy is associated with postoperative pneumonia. J Cardiothorac Surg 14, 209 (2019). https://doi.org/10.1186/s13019-019-1024-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13019-019-1024-6