Abstract
Background
Histopathological changes in the ascending aorta wall in patients with severe tricuspid aortic valve (TAV) stenosis were graded and correlated to echocardiographic parameters. Objective was to associate threshold echocardiographic values with structural defects in the ascending aorta providing a tool to improve decision-making process in cases when simultaneous aortic valve replacement (AVR) and ascending aorta replacement is considered.
Methods
Biopsies from 108 TAV stenosis patients subjected to AVR were graded into three grades according to severity of aortic wall changes. Echocardiographic parameters obtained preoperatively and correlated to grade, age, gender and risk factors, were diameters of ventriculo-aortic junction (AA), sinus Valsalva (SV), sinotubular junction (STJ), the largest diameter of the visualized ascending aorta (AscA) as well as indexes: sinus Valsalva (SVI), sinotubular junction (STJI), AscA/AA and STJ/AA.
Results
Two echocardiographic parameters portrayed grades with statistical significance: STJ (F = 5.417; p = 0.006 (p < 0.05)) and AscA (F = 3.924; p = 0.023 (p < 0.05)). By using multiple predictors in the setting of Regression analysis, statistically significant differences among grades were reached for AA, SV, STJ, AscA and SVI. With further ROC curves analysis, threshold values for different grades were recognized. Grade 2 is identified in patients with AscA > 3.3 cm, while Grade 3 is identified in patients with values of AscA > 3.5 cm, STJ > 2.9 cm and STJI > 1.
Conclusions
Hemodynamic stress induced by TAV stenosis leads to elastic lamellae disruption in the aortic wall. Those changes could be graded and correlated with echocardiographic parameters of the aortic root and ascending aorta, providing a tool for decision to replace ascending aorta concomitantly with AVR.
Similar content being viewed by others
Background
In the present study, we investigated the spectrum of structural changes in the ascending aortic wall in patients with severe degenerative, calcific aortic stenosis of the tricuspid aortic valve (TAV), and correlated them to echocardiographic parameters. It is important to understand the evolution of aortic wall changes due to aortic stenosis, for tailoring guidelines for surgical treatment of aortic stenosis. Current guidelines recommend that simultaneous surgery of the aortic root and ascending aorta should be considered in patients with degenerative TAV stenosis, when maximal ascending aortic diameter is ≥55 mm. The main goal of our study is to determine when is the replacement of the ascending aorta warranted simultaneously with the aortic valve replacement (AVR), from the histological perspective.
We addressed several issues in this paper. The influence of severe TAV stenosis on structural changes in the wall of ascending thoracic aorta. Design and application of the grading system that identifies gradual progression of aortic wall changes caused by hemodynamic disturbances in the setting of the aortic stenosis. Definition of irreversible changes in the ascending aorta wall in patients with severe aortic stenosis, Correlation of histological grades with echocardiographic parameters in order to obtain reliable insight into the aortic wall structure by means of non-invasive diagnostics.
The answers to issues are particularly complex because aortic stenosis and hemodynamic derangement that it causes, is not the only factor influencing the structure of the ascending aorta. Other factors including aging, arterial hypertension, atherosclerosis and diabetes mellitus may act synergistically resulting in definitive changes [1,2,3]. Finally, we analyzed are there any gender-related differences in the remodeling process.
We focused exclusively on the severe TAV stenosis and its influence on the ascending aorta wall. The chosen method was to compare grades of elastic skeleton defects, assessed by light microscopy, with the echocardiographic parameters. Grading of the structural changes was done according to accepted grading systems.
Methods
Overall patients data
We performed analysis of wall segments of the ascending aorta of 108 patients who were undergoing AVR because of severe, symptomatic TAV stenosis. All patients were operated at Dedinje Cardiovascular Institute. There were 56 (51.9%) males and 52 (48.1%) female patients. The mean age of patients was 67.56 ± 8.23 years. The mean age of male patients was 67.23 ± 8.49 years (median 68.5 (60–74)), while for the female patients it was 67.92 ± 8.01 years (median 70 (59.75–74)). There was no statistical significance in the mean age of male and female patients in the aortic stenosis group (Mann-Whitney U test: 1403.500; p = 0.747 (> 0.05)).
Diameter of the ascending aorta was <5 cm in all patients, with the mean value of 3.33 ± 0.54 cm. The minimal diameter was 2.2 cm and the maximal diameter 4.7 cm.
Excluded from this study group were (1) patients with moderate or severe aortic regurgitation, (2) patients with aortic stenosis and acute or chronic aortic dissection, (3) patients who had had a previous cardiac operation and (4) patients who had had aortic stenosis combined with a connective tissue disorder, bicuspid or congenitally malformed aortic valve.
Intraoperative Aortic Wall sampling
The diagnosis of a severe TAV stenosis was established by preoperative echocardiography. Transverse aortotomy was made approximately 1 cm above the take-off of the right coronary artery, slightly above the level of the sinotubular junction. The aortic wall specimens were taken from the distal lip of the incision at the convexity of the ascending aorta, 2 to 4 cm above the level of the aortic valve annulus [4]. Samples of the aortic wall with the minimal dimensions 1 mm × 9 mm and maximal dimensions 5 mm × 20 mm were excised, immediately fixed in 4% neutral buffered formaldehyde by the immersion procedure, and subsequently processed for the morphological and morphometric analysis.
Echocardiographic parameters
Echocardiographic parameters of the aortic root and the ascending aorta were determined preoperatively from parasternal longitudinal section with standard 2D procedure. Diameters at the level of ventriculo-aortic junction (AA), sinus Valsalva (SV), sinotubular junction (STJ) and the largest diameter of visualized ascending aorta (AscA) were measured.
Index of sinus Valsalva (SVI) was calculated as the ratio between measured and predicted diameter (pSV) at the level of the sinus of Valsalva. Predicted diameter at the level of the sinus Valsalva (pSV) was calculated according to regression formula pSV(cm) = 1,92 + 0,74xBSA(m2), where BSA stands for body surface area [5]. Sinotubular junction index (STJI) was calculated as the ratio between measured and predicted diameter at the level of sinotubular junction. Predicted diameter at the level of sinotubular junction was calculated according to regression formula pSTJ(cm) = 1,69 + 0,62xBSA(m2) [5].
Indexes AscA/AA and STJ/AA were calculated as the ratio between AscAA or STJ diameters and AA diameter, respectively.
Preparation of arterial samples for analysis
Preparation of tissue for light microscopy and Histomorphometry
The tissue was prepared for morphological and morphometric analysis according to the procedure described in the previously published studies of our group [6,7,8]. Out of 30 serial sections per patient, three sections were chosen for the analysis with respect to following rules: oblique sections were excluded from the analysis, as well as sections with major technical flaws. In addition, minimal distance between chosen sections must be at least 100 μm.
Sections were stained with the application of selective techniques for elastic fibers: Weigert van Gieson technique with resorcin fuchsine, Verhoeff van Gieson method or Pincus’ staining with acid orcein.
Grading of morphological changes
Grading of morphological changes in the ascending aorta was established to test the hypothesis that aortic stenosis induces progressive histopathological changes and that subsequent grades follow the natural history of these alterations. All sections were graded according to the principle described by Schlatmann and Becker [9] for gradation of aortic wall changes during aging and Niwa et al. [10] for the gradation of congenital aortic stenosis.
Using both methods for morphometric analyses of elastic skeleton parameters, we found statistically significant differences among the grades (data not shown). However, we decided to proceed with Schlatmann and Becker gradation system since criteria for grading were more precise, hence, the reproducibility of the results was also higher with this system.
The grades were established according to the most severe changes at the magnification ×200 of the Olympus BX41 microscope.
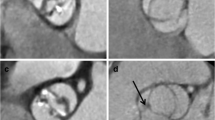
Grade 1 slides had fewer than five foci of elastic lamellae fragmentation in one microscopic field. Focus of elastic lamellae fragmentation comprises 2 to 4 neighboring elastic lamellae (Fig. 1a-b).
The ascending aorta in patients with severe aortic stenosis – a) and e) (Weigert van Gieson staining, original magnification ×100, bar = 500 μm); b) and f) (Weigert van Gieson staining, original magnification ×200, bar = 200 μm); c) (PTAH staining, original magnification ×200, bar = 200 μm); d) (PTAH staining, original magnification, ×400): a) – b) grade 1; three foci of elastic lamellae fragmentation in 1 microscopic field of the Olympus BX41 microscope, magnification ×200; focus of elastic lamellae fragmentation comprises 2 to 4 neighboring elastic lamellae; c) grade 2; eight foci of elastic lamellae fragmentation in 1 microscopic field of the Olympus BX41 microscope, magnification ×200; confluent or scattered foci throughout the media of the aorta; d) - f) the presence of foci with elastic fragmentation in 10 or more neighboring elastic lamellae. As opposite to control aortas, aortas of aortic stenosis patients have thin subendothelial connective tissue with numerous elastic fibers. These samples are atherosclerosis free or with low grade atherosclerosis (types I – III atherosclerotic lesions)
Grade 2 sections had 5 to 10 foci of elastic lamellae fragmentation in one microscopic field and foci were confluent or scattered throughout the media of the aorta (Fig. 1c-d).
Grade 3 sections were distinguished by the presence of foci with elastic fragmentation in 10 or more neighboring elastic lamellae, irrespective of the number of foci per microscopic field, with disorganization of smooth muscle cells layers (Fig. 1e-f).
Pathologist, who performed the analysis, was blinded for patients’ data. The slides were reexamined twice to obtain the final data as advised in previous similar studies [11].
Atherosclerosis was graded according to established classification systems [12].
Statistical analysis
Descriptive statistics included the mean values or the median with 25th – 75th percentile values, the standard deviation (SD), the standard error (SE) and a 95% confidence interval (95% C.I.).
The tests were performed with the SPSS version 10.0 for Windows. Following tests were used where appropriate: One-Sample Kolmogorov-Smirnov Test, ANOVA, Mann-Whitney test, Bonferroni Post Hoc Multiple Comparison test, Tukey Post Hoc, Pearson Correlation and Spearman’s Rho, ROC Curves and Regression Analysis. The value of p < 0.05 was considered statistically significant.
Data are presented as the means ± SD or the median with 25th – 75th percentile value.
Data distribution pattern – Echocardiographic parameters
Values of echocardiographic parameters conform normal distribution as confirmed with the One-Sample Kolmogorov-Smirnov Test, hence they were analyzed with parametric tests.
Results
Overall patients’ data
Overall patients’ data are systematized in Table 1.
The distribution of grades are presented in Table 2.
Echocardiographic parameters and grades
Values of echocardiographic parameters are given in Table 3. With the increase in grade and severity of histopathological defect, values of echocardiographic parameters increase (Fig. 2). Statistical significance was confirmed with ANOVA among echocardiographic parameters of different grades for STJ (F = 5.417; p = 0.006 (p < 0.05)) and AscA (F = 3.924; p = 0.023 (p < 0.05)) (Table 2 and Fig. 2). By using Bonferroni Post Hoc Analysis statistical significance among grades was confirmed for STJ, but not for AscA (GR1 vs GR2 p = 0.079; GR 1vs GR3 p = 0.093; GR2vs GR3 p = 1.000).
Values of echocardiographic parameters in different grades: a) values of diameters at the level of ventriculo-aortic junction (AA), sinus Valsalva (SV), sinotubular junction (STJ) and the largest diameter of visualized ascending aorta (AscA); b) values of sinus Valsalva index (SVI), sinotubular junction index (STJI), STJ/AA and AscA/AA
Additional testing with Spearman Correlation Coefficient test confirmed the ANOVA results. Statistically significant and positive correlations were established between grades and parameters STJ and AscA. Testing also revealed significant positive association of echocardiographic parameter STJI and the defined grades (Table 4).
Influence of aging
Patients age did not differ significantly among different grades as confirmed with ANOVA and post hoc Tukey HSD analysis (F = 0.398; p = 0.673 (p > 0.05)). Also, when distribution of grades was counted in a group <65 years and ≥65 years, Pearson Chi-square test revealed no statistically significant difference (Pearson Chi-square = 0.405; p = 0.817 (p > 0.05)).
Two-way ANOVA that tested influence of age and grade to different echocardiographic parameters revealed no statistically significant difference (Fig. 3).
Influence of age and grade to echocardiographic parameters – there is an increase in values of echocardiographic parameters with grade and a decrease with age. However differences are not statistically significant (blue - grade 1; green – grade 2; beige – grade 3): simultaneous effects of age and grade to a) diameters at the level of ventriculo-aortic junction (AA), b) diameters at the level of sinus Valsalva (SV), c) diameters at the level of sinotubular junction (STJ), d) the largest diameter of visualized ascending aorta (AscA), e) values of sinus Valsalva index (SVI), f) values of sinotubular junction index (STJI), g) STJ/AA and h) AscA/AA
Influence of gender
Distribution of grades did not differ among genders as confirmed with Pearson Chi-Square test (5.849; p = 0.054 (p > 0.05)). All echocardiographic parameters except indexes STJ/AA and AscA/AA were statistically significantly higher in males than in females, but simultaneous effects of grade and gender to echocardiographic parameters was not statistically significant as confirmed with Two-Way ANOVA (Fig. 4).
Influence of gender and grade to echocardiographic parameters: simultaneous effects of gender and grade to a) diameters at the level of ventriculo-aortic junction (AA), b) diameters at the level of sinus Valsalva (SV), c) diameters at the level of sinotubular junction (STJ), d) the largest diameter of visualized ascending aorta (AscA), e) values of sinus Valsalva index (SVI), f) values of sinotubular junction index (STJI), g) STJ/AA and h) AscA/AA
Multifactorial analysis
Echocardiographic parameters had no differences when tested for the presence or absence of arterial hypertension, diabetes mellitus and atherosclerosis (data not shown).
Application of Regression Analysis by using multiple predictors: age, gender, presence of arterial hypertension, diabetes mellitus, atherosclerosis with changes of grade identified statistically significant differences among echocardiographic parameters (Table 5).
Based on the regression analysis ROC Curves were constructed to test the sensitivity and specificity of different echocardiographic parameters for different grades (Fig. 5).
According to ROC Curves analysis following threshold values were detected to identified specific grades (Table 6).
Discussion
The debate about concomitant replacement of ascending aorta with aortic valve replacement spins around aortic diameter, etiology of valve disease, structural changes in the aortic wall and the influence of mechanical stress on the aortic wall. Our study focuses on TAV stenosis and structural derangements in the aortic wall that it causes.
According to the current guidelines, surgery should be considered in patients who have aortic root disease (whatever the severity of aortic regurgitation or stenosis) with maximal ascending aortic diameter ≥ 45 mm for patients with Marfan syndrome with risk factors, ≥50 mm for patients with bicuspid valve with risk factors, and ≥55 mm for other patients [13].
Aortic valve disease is associated with the ascending aortic dilatation because of the “hemodynamic burdens caused by forceful jets” [14, 15]. Due to mechanical stress, the size of the dilatation is related to the degree of turbulence induced by the stenotic valve and the severity of stenosis [16]. However, it seems that there is no independent association between the severity of aortic stenosis and the aortic diameter, indicating that factors other than the aortic stenosis itself (geometry of aortic orifice, flow distribution pattern and histopathological changes in the aortic wall) could affect the echocardiographic parameters of the aorta [17].
Gaudino et al. [16] published the results of follow-up study of patients submitted to AVR only and showed moderate dilatation of the ascending aorta with the expansion rate of 0.3 ± 0.2 mm/year after 10 years postoperatively [16]. Similarly, Yasuda et al. [18] reported a mean ascending aorta expansion rate of 0.08 mm/m2/year in a series of 14 patients followed for 9.7 years after surgery [18]. They speculated that correction of the aortic stenosis in these patients stabilized the hemodynamics and prevented further development of the aortic wall changes. Andrus et al. [19] reported results of a vast study that comprised 107 patients with an aortic diameter ≥ 3.5 cm. He found no evidence of further dilation in the first 3 years after isolated AVR, and concluded that in patients with aortic valve stenosis and with accompanying mild or moderate ascending aortic dilatation (3.5 cm to 4.9 cm) AVR alone may be reasonable [19].
Botzenhardt et al. [20] have even described a reduction of the aortic diameter in 10 patients with pre-operative aortic diameter ≥ 4 cm, 4.8 years after the isolated valve surgery [20].
As opposite to these studies, Matsuyama et al. [21] concluded that the clinical course of patients with a dilated ascending aorta is unpredictable and that aortic events may occur in patients with an aortic diameter of <5 cm. The author also found that patients with TAV stenosis and a slightly dilated aorta are at risk of late aortic events. Therefore, suggested preventive aortic surgery and AVR, even in patients with slightly dilated ascending aorta with a diameter of 4 cm to 5 cm, except in cases of high operative risk [21].
Ergin et al. [22] advocate more liberal ascending aorta replacement in conjunction with AVR since it significantly improves postoperative outcome in comparison to patients with AVR and already dilated aorta [22].
Only few studies investigated histopathological defects of aortic wall elastic skeleton in patients with the aortic valve dysfunction, utilizing limited number of elastic skeleton parameters. Roberts et al. [11] using a semi-quantitative method, found that there is no significant loss of elastic fibers in patients with stenosis of the TAV as compared to the control group [11]. Bauer et al. [4] showed that the thickness of elastic lamellae is decreased and the distance between elastic lamellae is increased significantly in patients with dilatation of the ascending aorta and with TAV stenosis [4]. Bechtel et al. [23] found that patients with TAV stenosis and the ascending aorta dilatation have more severe defects of the ascending aorta than patients with bicuspid valve and the same degree of dilatation [23].
Von Kodolitsch et al. [24] concluded that any patient at aortic valve replacement with an aortic diameter ≥ 43 mm and the presence of aortic wall fragility, aortic thinning or aortic regurgitation, will likely benefit from prophylactic aortic surgery. The combined presence of these parameters identifies a disease process of the entire aortic root rather than isolated valve disease [24].
Tsutsumi et al. [25] portrayed clinical entity of the patients prone to postsurgical aortic complications. They suggested that patients with aortic regurgitation combined with systemic hypertension, male sex, and thinned or fragile aortic walls in patients with ascending aortic dilatation (≥45 mm diameter) at the time of aortic valve replacement, should be considered for concomitant replacement of the ascending aorta [25].
Beller et al. [26] found that, in cases of aortic stenosis, restored aortic valve competence (by replacing the diseased valve) is associated with increased aortic root motion, theoretically heightening the threat of dissection posed to the aortic wall by mechanical stress. Mechanical principles command to include the higher magnitude of aortic root motion during follow-up of patients after AVR as an additional risk factor for dissection [26].
We have previously found significant thinning of the ascending aorta wall and all its tunics in patients with aortic stenosis [27]. Similar changes have already been described in a different model of exaggerated hemodynamic forces and its influence to the arterial wall [28]. Rabkin, Jue and Tsang [29] proved echocardiographically that after the adjustment for body surface area, wall thickness of the sinus Valsalva is a good indicator of the aortic wall stress associated with the aortic valve sclerosis even in those cases when luminal diameters of the aorta are not dilated [29].
We applied Schlatmann and Becker grading system to demonstrate three different histopathological grades. Furthermore, our supposition is that these three grades follow the natural progression and evolution of aortic stenosis and its hemodynamic impact to the aortic wall. In our previous study, we confirmed significant progress of elastic lamellae disruption through different grades as well as spatial distribution of these changes in the aortic wall as they affect the internal media first [27]. These observations are in keeping with previous similar studies [6,7,8, 30, 31].
Grade 3 with destructive changes in numerous elastic lamellae and disorganization of smooth muscles resembled irreversible changes in the aortic wall.
Morphological and morphometric characteristics of elastic skeleton are changing during aging. Even the “perfect” internal thoracic artery is prone to elastic skeleton changes induced by aging [6, 7]. Nakashima et al. [30] proved that the number of elastic lamellae fenestrations increase with aging, as does the number of interlamellar elastic fibers, their ramifications and the number of their fenestrations [30]. It was very important to prove that the observed grades are not merely effects of aging. Our study showed there is no difference between patient age distribution in different histological grades. Dividing patients in two age groups (˂65 and ≥65), there is no difference in the distribution of grades. Described changes persisted in both groups of patients, younger and older than 65 years, they are potentiated with aging, but they are not the effect of aging entirely.
Combined effect of gender and grade had no effect to echocardiographic parameters and the distribution of grades among genders did not differ significantly.
Girdauskas et al. [32], using cardiac magnetic resonance imaging, showed that systolic transvalvular flow jet is hitting the right-lateral segment of the tubular ascending aorta, in patients with aortic valve stenosis. This finding confermed that we sampled aorta from the right place [32]. By using multiple predictors in the setting of Regression analysis, statistically significant differences among grades were reached for AA, SV, STJ, AscA and SVI. With further ROC curves analysis, threshold values for different grades were recognized. Grade 2 is identified in patients with AscA > 3.3 cm, while grade 3 is identified in patients with values of AscA > 3.5 cm, STJ > 2.9 cm and STJI > 1.
Limitations of the study
Our study obviously lacks post festum echo analisys – to determine what happens with aortas in patients with different grades, following AVR. Nevertheless, we focused on proving the existence of different, progressive, histological changes in the aortic wall, and to correlate them with various echo parameters, in patients exclusively with severe stenosis of tricuspid aortic valve.
Conclusion
Our findings strongly support the view that aortas of patients with TAV stenosis are submitted to hemodynamic stress that subsequently leads to gradual elastic lamellae disruption that could be histologically identified and graded. The changes in the aortic wall correlated statistically significant with echocardiographic parameters. Grade 2 is identified in patients with AscA > 3.3 cm, while grade 3 is identified in patients with values of AscA > 3.5 cm, STJ > 2.9 cm and STJI > 1. Although current guidelines suggest simultaneous replacement of ascending aorta with AVR when aortic diameter is ≥55 mm, we propose more radical approach, with diameter > 3.5 cm as a cutoff, in patients with severe TAV stenosis, especially in patients with long life expectancy.
Abbreviations
- AA:
-
Ventriculo-aortic junction
- AscA:
-
Ascending aorta
- AVR:
-
Aortic valve replacement
- BSA:
-
Body surface area
- GR:
-
Grade
- pSTJ:
-
Predicted diameter at the level of sinotubular junction
- pSV:
-
Predicted diameter at the level of the sinus of Valsalva
- STJ:
-
Sinotubular junction
- STJI:
-
Sinotubular junction index
- SV:
-
Sinus Valsalva
- SVI:
-
Sinus Valsalva index
- TAV:
-
Tricuspid aortic valve
References
Taghizadeh H, Tafazzoli-Shadpour M, Shadmehr MB. Analysis of arterial wall remodeling in hypertension based on lamellar modeling. J Am Soc Hypertens. 2015;9(9):735–44.
Yapei Y, Xiaoyan R, Sha Z, Li P, Xiao M, Shuangfeng C, Lexin W, Lianqun C. Clinical significance of arterial stiffness and thickness biomarkers in type 2 diabetes mellitus: an up-to-date meta-analysis. Med Sci Monit. 2015;21:2467–75.
Kohn JC, Chen A, Cheng S, Kowal DR, King MR, Reinhart-King CA. Mechanical heterogeneities in the subendothelial matrix develop with age and decrease with exercise. J Biomech. 2016;49(9):1447–53.
Bauer M, Pasic M, Meyer R, Goetze N, Bauer U, Siniawski H, Hetzer R. Morphometric analysis of aortic media in patients with bicuspid and tricuspid aortic valve. Ann Thorac Surg. 2002;74:58–62.
Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Members of the Chamber Quantification Writing Group. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s guidelines and standards committee and the chamber quantification writing group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005, 18:1440–63.
Labudović BM, Borović S, Marinković-Erić J, Todorović V, Puškaš N, Kočica M, Radak Đ, Lačković V. A comprehensive morphometric analysis of the internal thoracic artery with emphasis on age, gender and left-to-right specific differences. Histol Histopathol. 2013a;28:1299–314.
Labudović BM, Borović S, Perić M, Vuković P, Marinković-Eric J, Todorović V, Radak Đ, Lačković V. The internal thoracic artery as a transitional type of artery: a morphological and morphometric study. Histol Histopathol. 2010;25:561–76.
Labudović Borović M., Borović S., Radak Đ., Marinković-Erić J., Maravić-Stojković V., Vučević D., Stojšić Z., Milićević Ž., Čolić M., 2013b. Morphometric model of abdominal aortic aneurysms and the significance of the structural changes in the Aortic Wall for rupture risk assessment, In: Fischhof D. and Hatig F., editors. Aortic aneurysms: risk factors, diagnosis, surgery and repair. Hauppauge, New York: Nova Science Publishers, Inc.; pp. 81-117.
Schlatmann TJM, Becker AE. Histological changes in the normal aging aorta: implications for dissecting aortic aneurysm. Am. J Cardiol. 1977;39:13–20.
Niwa K, Perloff JK, Bhuta SM, Laks H, Drinkwater DC, Child JS, Miner PD. Structural abnormalities of great arterial walls in congenital heart disease: light and electron microscopic analyses. Circulation. 2001;103:393–400.
Roberts WC, Vowels TJ, Ko JM, Filardo G, Hebeler RF Jr, Henry AC, Matter GJ, Hamman BL. Comparison of the structure of the aortic valve and ascending aorta in regurgitation and resection of the ascending aorta for aneurysm. Circulation. 2011;123:896–903.
American Heart Association Committee on Vascular Lesions of the Council of Atherosclerosis, Stary H.C. (chair). A definition of advanced types of atherosclerosis lesions and a histological classification of atherosclerosis. Circulation. 1995;92:1355–74.
Vahanian A, et al. Guidelines on the management of valvular heart disease (version 2012). Eur J Cardiothorac Surg. 2012;42:S1–S44.
Robiscek F. Editorial: bicuspid versus tricuspid aortic valves. J. Heart Valve Dis. 2003;12:52–3.
Glower DD. Indications for ascending aortic replacement. Size alone is not enough. J Am Coll Cardiol. 2011;58:585–6.
Gaudino M, Anselmi A, Morelli M, Pragliola C, Tsiopoulos V, Glieca F, Possati G. Aortic expansion rate in patients with dilated post-stenotic ascending aorta submitted only to aortic valve replacement. J Am Coll Cardiol. 2011;58:581–4.
Linhartová K, Beránek V, Šefrna F, Hanisová I, Sterbáková G, Pesková M. Aortic stenosis severity is not a risk factor for poststenotic dilatation of the ascending aorta. Circ J. 2007;71:84–8.
Yasuda H, Nakatani S, Stugaard M, Tsujita-Kuroda Y, Bando K, Kobayashi J, Yamagishi M, Kitakaze M, Kitamura S, Miyatake K. Failure to prevent progressive dilatation of ascending aorta by aortic valve replacement in patients with bicuspid aortic valve: comparisons with tricuspid valve. Circulation. 2003;108:II291–4.
Andrus BW, O’Rourke DJ, Dacey LJ, Palac RT. Stability of ascending aortic dilatation following aortic valve replacement. Circulation. 2003;108:II295–9.
Botzenhardt F, Hoffmann E, Kemkes BM, Gansera B. Determinants of ascending aortic dimensions after aortic valve replacement with a stented bioprosthesis. J. Heart Valve Dis. 2007;16:19–26.
Matsuyama K, Usui A, Akita T, Yoshikawa M, Murayama M, Yano T, Takenaka H, Katou W, Toyama M, Okada M, Sawaki M, Ueda Y. Natural history of a dilated ascending aorta after aortic valve replacement. Circ J. 2005;69:392–6.
Ergin MA, Spielvogel D, Apaydin A, Lansman SL, McCullough JN, Galla JD, Griepp RB. Surgical treatment of the dilated ascending aorta: when and how? Ann Thorac Surg. 1999;67:1834–9.
Bechtel JFM, Noack F, Sayk F, Erasmi AW, Bartels C, Sievers HH. Histopathological grading of ascending aortic aneurysm: comparison of patients with bicuspid versus tricuspid aortic valve. J Heart Valve Dis. 2003;12:54–61.
Von Kodolitsch Y, et al. Predictors of proximal aortic dissection at the time of aortic valve replacement. Circulation. 1999;100(suppl II):II-287–94.
Tsutsumi K, et al. Risk factor analysis for acute type a aortic dissection after aortic valve replacement. Gen Thorac Cardiovasc Surg. 2010;58:601–5.
Beller C, et al. Aortic root motion remodeling after aortic valve replacement – implications for late aortic dissection. Interact Cardiovasc Thorac Surg. 2008;7:407–11.
Borović S. Korelacija između ehokardiografskih parametara i histoloških promena u zidu ascendentne aorte kod bolesnika sa degenerativnom stenozom aortne valvule [correlation between echocardiographic parameters and histological changes in the ascending aorta wall in patients with degenerative aortic valve stenosis], Master of Sciences Thesis, University of Belgrade, Belgrade, 2009; pp. 47-55.
Masuda H, Zhuang YJ, Singh TM, Kawamura K, Murakami M, Zarins CK, Glagov S. Adaptive remodeling of internal elastic lamina and endothelial lining during flow-induced arterial enlargement. Arterioscler Thromb Vasc Biol. 1999;19:2298–307.
Rabkin SW, Jue J, Tsang MY. Aortic valve sclerosis is associated with an echocardiographically determined thinner aortic wall. J Heart Valve Dis. 2006;15:158–64.
Nakashima Y, Shiokawa Y, Sueishi K. Alterations of elastic architecture in human aortic dissecting aneurysm. Lab Investig. 1990;62:751–9.
Agozzino L, Ferraraccio F, Esposito S, Trocciola A, Parente A, Della Corte A, De Feo M, Cotrufo M. Medial degeneration does not involve uniformly the whole ascending aorta: morphological, biochemical and clinical correlations. Eur J Cardiothorac Surg. 2002;21:675–82.
Girdauskas et al. Functional aortic root parameters and expression of aortopathy in bicuspid versus tricuspid aortic valve stenosis, 2016. J Am Coll Cardiol. 19;67(15):1786-1796.
Acknowledgments
Not applicable.
Funding
The research activities were supported by grants No. 175005, 175,061, III45005, III41002 and III41022 from the Ministry of Education, Science and Technological Development of Republic of Serbia. The Ministry of Education, Science and Technological Development was not involved in study design, the collection, analysis and interpretation of data, the writing of the report and the decision to submit the article for publication.
Availability of data and materials
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Contributions
SB and MLB made study concept and design, SB and MLB collected the data, SB, MLB, IZ and JME performed analysis and interpretation, SB, MLB and VT drafted the manuscript, SB, MLB, PM, VT, JR, IZ and PD performed critical revision of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Ethics Committee of the Dedinje Cardiovascular Institute. Informed consent was obtained from all patients.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Borović, S.D., Labudović Borović, M.M., Zaletel, I.V. et al. Correlation of structural defects in the ascending aortic wall to ultrasound parameters: benefits for decision-making process in aortic valve surgery. J Cardiothorac Surg 13, 12 (2018). https://doi.org/10.1186/s13019-017-0671-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13019-017-0671-8