Abstract
Background
Long non-coding RNAs (LncRNAs) are recognized as a pivotal element in the processes of fracture healing and the osteogenic differentiation of stem cells. This study investigated the molecular mechanism and regulatory significance of lncRNA MAGI2-AS3 (MAGI2-AS3) in fracture healing.
Methods
Serum levels of MAGI2-AS3 in patients with normal and delayed fracture healing were verified by RT-qPCR assays. The predictive efficacy of MAGI2-AS3 for delayed fracture healing was analyzed by ROC curve. Osteogenic markers were quantified by RT-qPCR assays. MC3T3-E1 cell viability was detected using CCK-8 assay, and flow cytometry was utilized to measure cell apoptosis. The dual-luciferase reporter gene assay was used to determine the targeted binding between MAGI2-AS3 and miR-223-3p.
Results
Serum MAGI2-AS3 expression was decreased in patients with delayed fracture healing compared with patients with normal healing. Elevated MAGI2-AS3 resulted in an upregulation of the proliferative capacity of MC3T3-E1 cells and a decrease in mortality, along with increased levels of both osteogenic markers. However, after transfection silencing MAGI2-AS3, the trend was reversed. Additionally, miR-223-3p was the downstream target of MAGI2-AS3 and was controlled by MAGI2-AS3. miR-223-3p mimic reversed the promoting effects of MAGI2-AS3 overexpression on osteogenic marker levels and cell growth, and induced cell apoptosis.
Conclusion
The upregulation of MAGI2-AS3 may expedite the healing of fracture patients by targeting miR-223-3p, offering a novel biomarker for diagnosing patients with delayed healing.
Similar content being viewed by others
Introduction
Fractures are a frequently encountered condition in clinical settings, with tibia fractures in adults being particularly prevalent [1]. The process of fracture repair is intricate and protracted, and delayed healing imposing substantial burdens on patients [2]. It involves not only physical and emotional distress but also entails considerable financial expenditure. Given the vulnerability of soft tissues and poor blood flow in the lower leg, tibial fractures carry a considerable risk of delayed or non-union [3]. Meanwhile, factors such as inadequate nutrition, infection, and blood circulation disruption serve as pivotal indicators of delayed fracture healing [4]. Calcitonin was found to potentially accelerate fracture healing in studies of means of fracture healing and that intermittent pneumatic compression care also aids in soft tissue healing [5, 6]. Moreover, low-intensity pulsed ultrasound was considered as an effective and less invasive treatment for patients with delayed healing [7]. Notably, the functional activity of osteoblasts has been implicated in fracture healing, and their proliferation and differentiation of osteoblasts are essential for the formation of new bone [8]. Furthermore, as osteoblasts become more metabolically active, there is a rise in the levels of osteogenic markers, which serve as indirect indicators to monitor the progress of fracture healing [9]. By understanding how to regulate osteoblast function and monitor osteogenic marker changes, we can gain a better understanding of the fracture healing process. Hence, the pursuit of convincing biomarkers to explore the mechanism of fracture healing holds significant practical value for the healing and monitoring of individuals suffering from fractures.
LncRNAs have been established as participants in the regulation of the cell cycle, chromatin modification and various life activities in vivo, as supported by extensive epigenetic and molecular studies [10]. In the recent years, lncRNAs have become a significant focus in bone metabolism research. For instance, Lei et al. discovered that lncRNAs contribute to bone destruction in rheumatoid arthritis by mediating cell autophagy [11]. Xue et al. realized the efficacy of lncRNA SNHG14/miR-493-5p/Mef2c regulatory network in alleviating osteoporosis [12]. Interestingly, MAGI2-AS3 targeting miR-374b-5p ameliorated the injury and inflammatory response of nucleus pulposus cells in intervertebral disc degeneration [13]. MAGI2-AS3 is a lncRNA transcribed from the antisense strand of MAGI2, located on chromosome 7q21.11, and was found to be abnormally expressed in human tumors and neurological disorders [14,15,16]. However, the specific relationship between MAGI2-AS3 and fracture healing has not been widely acknowledged.
On this basis, the present study reflected the diagnostic potential of MAGI2-AS3 in delayed fracture healing by quantitatively measuring its levels in patients with normal and delayed fracture healing. Additionally, the molecular mechanism of MAGI2-AS3 in fracture healing was revealed by in vitro cellular assays, aiming to seek novel therapeutic strategies for fracture healing.
Materials and methods
Inclusion of patients
The subjects of the study were 119 patients, all over 18 years old, with unilateral tibial fractures. These patients underwent surgical treatment from November 2022 to September 2023 at Yidu Central Hospital of Weifang. Any patients with previous history of fracture, osteoporosis, or fractures at other sites were excluded from the study. The experimental protocol was reviewed and approved by the Ethics Committee of Yidu Central Hospital of Weifang, and all the participats provided their informed consent.
Grouping of patients
Patients were monitored for 4 months post-operation to observe fracture healing. They were categorized into two groups based on prognosis: the normal healing group (n = 63) and the delayed healing group (n = 56). All patients in the normal healing group displayed bone scabs formation at the tibial fracture site, and their activities returned to normal without pain [17]. In contrast, the delayed healing group exhibited a noticeable gap between the fracture ends on X-ray images, with no continuous callus and dense sclerotic bone.
Collection of serum samples
Blood samples were collected from the patients and centrifuged (2000 rpm, 15 min) after standing for 10 min. The upper layer of serum was subsequently collected and cryopreserved.
Cultivation of cells
MC3T3-E1 cells were purchased from the RIKEN Cell Bank (Tsukuba, Japan), and cultured in DMEM medium, which contains 10% FBS and 1% penicillin-streptomycin. The cells were seeded and placed in an incubator set to 37 °C, with an environment of 95% air and 5% CO2.
Transfection of cells
Transfection vectors pcDNA3.1-MAGI2-AS3, si-NC, si-MAGI2-AS3, mimic NC, and miR-223-3p mimic were synthesized by RiboBio (Guangzhou, China). Each of these vectors was mixed with the transfection reagent Lipofectamine 2000 (Invitrogen, Carlsbad, USA), and then added to MC3T3-E1 cells in the logarithmic phase for transfection assay. The medium was replaced every 6 h, and the cells were harvested 24 h after transfection.
RT-qPCR reaction
Total RNA was extracted from the serum and cells for testing using the TRIzol reagent. After confirming the RNA concentration, reverse transcription was executed using the HiFiScript cDNA Synthesis Kit (Cwbiotech, China). Once the cDNA was obtained, quantitative PCR analysis was conducted on an MX3000p Real-time PCR instrument via SYBR premix Ex Taq II Kit (Takara Bio, Japan). With β-actin and U6 as reference genes, the MAGI2-AS3, miR-223-3p, ALP mRNA, OC mRNA and Runx2 mRNA expression were calculated using the 2−ΔΔCt method.
CCK-8 assay
The transfected MC3T3-E1 cells were inoculated into 96-well plates at a concentration of 2 × 103 cells, and CCK-8 solution was added after incubation for 0, 24, 48, and 72 h. Incubation was terminated after 4 h at 37 °C, and the cells were then shaken in a shaker (Thermo Scientific, USA) for 5 min. Absorbance values were determined at 450 nm using a microplate reader (Thermo Scientific, USA).
Apoptosis assay
MC3T3-E1 cells treated with transfection were gathered and rinsed in PBS. Annexin V-FITC and PI reagents (Annexin V-FITC/PI Kit; Biotech, China) were added to the cells and incubated for 10 min in the dark. Following this, apoptotic cell counts were assessed via flow cytometry.
Dual-luciferase reporter assay
Fragments of MAGI2-AS3 containing miR-223-3p binding sites were inserted into the pmiRGLO vector to create either wild-type (WT) or mutant-type (MUT) MAGI2-AS3 fragments. These vectors were co-transfected with either a mimic NC or a miR-223-3p mimic into MC3T3-E1 cells. The luciferase activity was assessed 48 h post-transfection using a dual-luciferase reporter assay system (Promega, Wisconsin, USA).
Statistical analysis
SPSS 21.0 software was utilized to process the experimental data. Intergroup comparisons of clinical indicators between the two groups of patients were performed by chi-square test. The risk factors of delayed fracture healing were evaluated by Binary logistic regression. Levels of MAGI2-AS3 and miR-223-3p were compared between groups through t-test and ANOVA. The predictive value of MAGI2-AS3 expression in patients with delayed fractures was evaluated by ROC curve. The target and binding sites of MAGI2-AS3 were predicted by ENCORI and lncRNASNPV3 online databases. P < 0.05 was believed a statistically significant difference.
Results
Serum MAGI2-AS3 was lowly expressed in patients with delayed healing
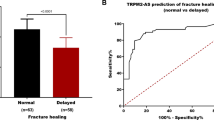
The RT-qPCR results of indicated that serum MAGI2-AS3 was downregulated in patients with delayed fracture healing compared to normal fracture healing (Fig. 1A, P < 0.001). Furthermore, the ROC curve elucidated that the sensitivity and specificity of MAGI2-AS3 in predicting delayed healing were 85.71% and 82.54%, with an AUC of 0.8934 (95% CI = 0.8343–0.9525), suggesting that abnormal MAGI2-AS3 levels may be associated with delayed fracture healing (Fig. 1B).
Analysis of MAGI2-AS3 expression and diagnostic value in included patients with tibial fractures. A. Serum MAGI2-AS3 levels were decreased in delayed healing patients compared to normal healing patients. B. AGI2-AS3 had high diagnostic ability in predicting delayed fracture healing in patients. *** P < 0.001, compared with normal healing
General information of the subjects and the clinical potential of MAGI2-AS3
By analyzing and comparing the clinical data of patients with normal and delayed fracture healing, it is observed in Table 1 that there were no significant differences in age, BMI, gender, fracture side, severity of fracture, osteosynthesis method, smoking and drinking habits between the two groups (P > 0.05). Moreover, MAGI2-AS3 may be a factor influencing delayed fracture healing, as revealed by Binary logistic regression used for assessing risk indicators of delayed healing (Table 2, P < 0.001).
Regulation of osteoblast activity by abnormal expression of MAGI2-AS3
In the study exploring the molecular mechanism of MAGI2-AS3 in fracture healing, the present study transfected pcDNA3.1-MAGI2-AS3 or si-MAGI2-AS3 into osteoblast MC3T3-E1 to upregulate or downregulate the content of MAGI2-AS3 (Fig. 2A). Subsequently, the examination of osteogenic marker levels revealed that increasing MAGI2-AS3 enhanced the ALP (Fig. 2B), OC (Fig. 2C), and Runx2 (Fig. 2D) levels. Conversely, dysregulation of MAGI2-AS3 suppressed these levels. MC3T3-E1 cell activity assay also revealed that prominently expressed MAGI2-AS3 significantly improved cell proliferation and reduced cell apoptosis, while silencing MAGI2-AS3 inhibited cell growth and induced cell apoptosis in Fig. 2E and F. These findings imply that overexpression of MAGI2-AS3 may promote fracture healing.
Regulation of osteoblast reproduction by up or down-regulation of MAGI2-AS3 expression. A. Transfection of pcDNA3.1-MAGI2-AS3 resulted in elevated levels of MAGI2-AS3 in cells, whereas the MAGI2-AS3 expression was suppressed by si-MAGI2-AS3 transfected. B-D. Effect of transfection with abnormal levels of MAGI2-AS3 on osteogenic marker levels. E-F. Regulation of osteoblast proliferation and apoptosis by aberrant expression of MAGI2-AS3. * P < 0.05, ** P < 0.01, *** P < 0.001, compared with control
MiR-223-3p is the direct target of MAGI2-AS3
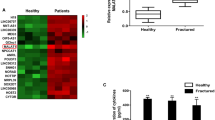
A search of the online databases ENCORI and lncRNASNPV3 revealed that miR-7153-5p, miR-146a-5p, miR-146b-5p, miR-2467-3p, miR-223-3p, and miR-625-5p are downstream targets of MAGI2-AS3 (Fig. 3A). RT-qPCR assays then validated that serum miR-223-3p was enriched in patients with delayed healing, as determined by the levels of the above miRNAs (Fig. 3B, P < 0.001). Further confirmation of the targeting relationship between MAGI2-AS3 and miR-223-3p came from bioinformatics software prediction. This predicted the presence of binding sites between MAGI2-AS3 and miR-223-3p. Additionally, luciferase activity was remarkably decreased after co-transfection of miR-223-3p mimic with MAGI2-AS3-WT (Fig. 3C, P < 0.01). After transfection of pcDNA3.1-MAGI2-AS3 or si-MAGI2-AS3 in MC3T3-E1 cells, the expression of miR-223-3p decreased or increased (Fig. 3D). These results imply that MAGI2-AS3 directly sponges and negatively regulates miR-223-3p expression in fracture healing.
MAGI2-AS3 directly sponges miR-223-3p. A. The downstream target genes of MAGI2-AS3 predicted by the bioinformatics website are shown in the Venn diagram. B. Expression of different miRNAs in the included fracture patients. C. The targeted combination of MAGI2-AS3 and miR-223-3p was analyzed by bioinformatics and dual-luciferase reporter gene detection. D. Regulation of miR-223-3p expression by transfection of abnormal levels of MAGI2-AS3. nsP < 0.05, ** P < 0.01, *** P < 0.001, compared with control
Upregulation of mir-223-3p attenuated the influence of elevated MAGI2-AS3 on osteoblasts
We further investigated the fracture healing mechanism by co-transfecting of pcDNA3.1-MAGI2-AS3 and miR-223-3p mimic in MC3T3-E1 cells. In Fig. 4A, MAGI2-AS3 elevation suppressed miR-223-3p expression, but miR-223-3p levels were restored after its upregulation. Concurrently, transfection with pcDNA3.1-MAGI2-AS3 upregulated the osteogenic markers ALP, OC, and Runx2 levels, whereas miR-223-3p mimic diminished their expression (Fig. 4B and D). In addition, MAGI2-AS3 overexpression promoted cell growth and reduced cell apoptosis. However, the combination of pcDNA3.1-MAGI2-AS3 and miR-223-3p mimic reversed MAGI2-AS3’s beneficial effects on cell activity and stimulated cell apoptosis (Fig. 4E and F). Therefore, the outstanding expression of MAGI2-AS3 may mediate the activity of osteoblasts by adsorbing miR-223-3p, thereby accelerating fracture healing.
Mediation of miR-223-3p mimic on osteoblasts. A. Upregulation of miR-223-3p expression in osteoblasts after transfection with pcDNA3.1-MAGI2-AS3 + miR-223-3p mimic. B-D. High levels of MAGI2-AS3 improved the levels of osteogenic markers, while co-transfection with overexpression of miR-223-3p reversed this promoting effect. E-F. pcDNA3.1-MAGI2-AS3 accelerated osteoblast growth and suppressed apoptosis, while miR-223-3p mimic had the opposite effect. * P < 0.05, ** P < 0.01, *** P < 0.001, compared with control; #P < 0.05, ##P < 0.01, ###P < 0.001, compared with pcDNA3.1-MAGI2-AS3
Discussion
Fracture healing is a complex biological process that involvese aspects like inflammatory response, neovascularization, and the generation of cartilage and bone tissue. Factors such as a patient’s nutritional status, circulatory status, metabolic level, and age also impact the speed and quality of fracture healing [18]. For effective healing, it’s essential to ensure the stability of the broken bone and maintenance of sufficient soft tissue coverage to provide an adequate blood supply, which helps to activate endogenous stem cells to repair damaged tissue [19]. Given the distinctive positioning of the tibia and the characteristics of the associated tissues, patients may experience delayed healing. Therefore, delving into the mechanism of lncRNAs in fracture healing holds practical value.
MAGI2-AS3 is a novel lncRNA that has been observed to have dysregulated expression in human diseases and biological processes by multiple reports [16, 20]. MAGI2-AS3 appears to related to the regulation of expression of the MAGI2 gene, and the protein encoded by MAGI2 participate in processes such as cell signaling and cell adhesion [21]. Silencing MAGI2-AS3 mediated HSPA8 protein level to delay cell senescence, which may assist cells in anti-aging regulation [22]. Additionally, it was observed that MAGI2-AS3 is poorly expressed in breast cancer [23], hepatic cancer [24], and esophageal cancer [25]. In the study on the mechanism of MAGI2-AS3, it inhibited inflammatory responses by downregulating the levels of miR-374b-5p and alleviated myeloid cell damage and outer matrix degradation by promoting IL-10 levels [13]. MAGI2-AS3 was determined to be downregulated in leukemic stem cells and inhibited cell renewal by upregulating LRIG1, thereby improving the deterioration of acute myeloid leukemia [26]. More critically, MAGI2-AS3 was revealed to be downregulated in patients with intervertebral disc degeneration, whereas elevated levels of MAGI2-AS3 suppressed Fas ligand expression and mediated the nucleus pulposus cells [27]. And upregulation of MAGI2-AS3 affected intervertebral disc therapy by repressing the levels of FasL gene and protein [28]. Consequently, we hypothesized that MAGI2-AS3 may be engaged in the mechanism of fracture healing. To test this hypothesis, 119 patients with tibial fractures were enrolled in this study, and among the total number of participants, there were 63 patients with normal healing fractures and 56 patients with delayed healing. The results proposed that serum MAGI2-AS3 expression was reduced in the delayed healing group, and low level of MAGI2-AS3 had a high diagnostic significance for distinguishing patients with delayed fracture healing, suggesting its possible involvement in the pathological process of fracture healing.
As healing proceeds, the number of osteoblasts increased progressively, and the differentiation and activity of the cells contribute to the acceleration of new bone formation [29]. Biomarkers of osteoblast activity and function, like ALP, OC, and Runx2, increase during fracture healing, reflecting osteoblast activity and new bone formation [30]. Our study elucidated that enhancing MAGI2-AS3 expression significantly increased the levels of osteogenic markers ALP, OC, and Runx2, whereas inhibiting MAGI2-AS3 reduced their levels. Furthermore, overexpression of MAGI2-AS3 enhanced the growth capacity of the osteoblast MC3T3-E1 and repaired apoptosis, while silencing MAGI2-AS3 affected cell viability and induced apoptosis. These findings confirmed that MAGI2-AS3 contributes to fracture healing when it is increased, and reduced MAGI2-AS3 level may lead to delayed fracture healing.
In addition to lncRNAs, miRNAs have also demonstrated their involvement in musculoskeletal diseases. For instance, research conducted by Lorenzo and colleagues highlighted the promising role of miRNAs in the clinical management of tendon injuries [31]. miR-223-3p is located on the X chromosome, which is identified as a pivotal regulator in bone diseases [32]. In a study by Long et al. the miR-223-3p/FOXO3 axis promoted osteogenic differentiation of bone marrow mesenchymal stem cells by elevating autophagy [33]. Dong et al. proposed that miR-223-3p impacts chondrocyte activity and inflammatory response and may be a target of sinomenine in treating osteoarthritis [34]. Furthermore, miR-223-3p was enriched for expression in osteoporosis patients skeletal muscle injured mice [35, 36]. In this research work, the bioinformatics website verified that miR-223-3p is a downstream target of MAGI2-AS3 and that there are targeted binding sequences between them. miR-223-3p was prominently expressed in patients with delayed fracture healing and was inversely regulated by MAGI2-AS3. Correspondingly, miR-223-3p levels were significantly elevated in fracture patients compared to healthy individuals and were involved in fracture healing therapy by mediating FGFR2 [37]. Additionally, transfecting cells with miR-223-3p mimic lessened the enhancingeffect of upregulated MAGI2-AS3 on osteogenic markers, and revived the increase in osteoblast viability. Furthermore, the function of small interfering RNAs (siRNAs) cannot be ignored. siRNAs was found to be involved in the therapeutic process of tendon repair, rheumatoid arthritis and osteoporosis by mediating gene regulation [38,39,40]. The specific pathological process and mechanism are also the content of our subsequent mining.
Conclusion
Briefly, the study examined the regulatory capacity of MAGI2-AS3 in fracture healing. MAGI2-AS3 expression was decreased in patients with delayed healing, and it regulated osteoblast activity of osteoblasts through sponge miR-223-3p. An increase in MAGI2-AS3 levels enhanced osteoblast proliferation, diminished cell apoptosis, and facilitated fracture healing in patients, while miR-223-3p mimic reversed the effect of MAGI2-AS3 overexpression. These discoveries offer novel insights for enhancing fracture healing and the accurate prediction of delayed healing.
Data availability
Corresponding authors may provide data and materials.
References
Wang D, Liu Y, Lv W, et al. Repetitive brief ischemia accelerates tibial shaft fracture healing: a 5-years prospective preliminary clinical trial (PCT). BMC Musculoskelet Disord. 2021;22(1):631.
Yang X, Shao J, Wu XM, et al. Troxerutin stimulates osteoblast differentiation of mesenchymal stem cell and facilitates Bone Fracture Healing. Front Pharmacol. 2021;12:723145.
Lee JY, Lee HJ, Yang SH et al. Treatment of soft tissue defects after minimally invasive plate osteosynthesis in fractures of the distal tibia: clinical results after reverse sural artery flap. Medicina (Kaunas, Lithuania). 2023;59(10).
Tang Z, Li W, Xie H, Jiang S, Pu Y, Xiong H. Taohong Siwu-Containing Serum Enhances Angiogenesis in Rat Aortic Endothelial Cells by Regulating the VHL/HIF-1α/VEGF Signaling Pathway. Evidence-based complementary and alternative medicine: eCAM. 2021;2021:6610116.
Migliorini F, Cocconi F, Vecchio G, Schäefer L, Koettnitz J, Maffulli N. Pharmacological agents for bone fracture healing: talking points from recent clinical trials. Expert Opin Investig Drugs. 2023;32(9):855–65.
Khanna A, Gougoulias N, Maffulli N. Intermittent pneumatic compression in fracture and soft-tissue injuries healing. Br Med Bull. 2008;88(1):147–56.
Martinez de Albornoz P, Khanna A, Longo UG, Forriol F, Maffulli N. The evidence of low-intensity pulsed ultrasound for in vitro, animal and human fracture healing. Br Med Bull. 2011;100:39–57.
Zhang H, Wang R, Wang G, et al. Single-cell RNA sequencing reveals B cells are important regulators in Fracture Healing. Front Endocrinol. 2021;12:666140.
Tunthasen R, Pripatnanont P, Meesane J. In Vitro Biocompatibility of a Novel Semi-rigid Shell Barrier System: as a new application for guided bone regeneration. Polymers. 2022;14(12).
Peng Y, Wang Z, Li M, Wang T, Su Y. Characterization and analysis of multi-organ full-length transcriptomes in Sphaeropteris brunoniana and Alsophila latebrosa highlight secondary metabolism and chloroplast RNA editing pattern of tree ferns. BMC Plant Biol. 2024;24(1):73.
Lei HT, Wang JH, Yang HJ, et al. LncRNA-mediated cell autophagy: an emerging field in bone destruction in rheumatoid arthritis. Volume 168. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie; 2023. p. 115716.
Xue J, Liu L, Liu H, Li Z. LncRNA SNHG14 activates autophagy via regulating miR-493-5p/Mef2c axis to alleviate osteoporosis progression. Commun Biology. 2023;6(1):1120.
Yu J, Li C. Role of lncRNA MAGI2-AS3 in lipopolysaccharide-induced nucleus pulposus cells injury by regulating miR-374b-5p/interleukin-10 axis. Immun Inflamm Dis. 2023;11(4):e772.
Yang G, Li T, Liu J, et al. lncRNA MAGI2-AS3 suppresses castration-resistant prostate cancer proliferation and migration via the miR-106a-5p/RAB31 axis. Genomics. 2023;115(2):110599.
Gong J, Ma L, Peng C, Liu J. LncRNA MAGI2-AS3 acts as a tumor suppressor that attenuates non-small cell lung cancer progression by targeting the miR-629-5p/TXNIP axis. Annals Translational Med. 2021;9(24):1793.
Taheri M, Askari A, Hussen BM, Ghafouri-Fard S, Rashnoo F. Role of MAGI2-AS3 in malignant and non-malignant disorders. Pathol Res Pract. 2023;246:154530.
Yao C, Sun J, Wu J, et al. Clinical outcomes of Ti-Ni shape-memory patella concentrator combined with cannulated compression screws in the treatment of C2 and C3 patella fracture: a retrospective study of 54 cases. BMC Musculoskelet Disord. 2020;21(1):506.
Ali AA, Mukhtar MM, Shaheen S, Mohamed AO. Assessment of plasma BMP-2, BMP-7, BMP-10, vitamin D, and TGF β1 in simple fractures among Sudanese patients. PLoS ONE. 2021;16(2):e0247472.
Yao W, Cai Q, Wang J, et al. Biological reconstruction in the treatment of extremity sarcoma in femur, tibia, and humerus. Medicine. 2020;99(27):e20715.
Xue C, Li G, Lu J, Luo J, Jia J. Novel insights for lncRNA MAGI2-AS3 in solid tumors. Volume 137. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie; 2021. p. 111429.
Zhong A, Ding N, Zhou Y, et al. Identification of Hub Genes Associated with the Pathogenesis of Intracranial Aneurysm via Integrated Bioinformatics Analysis. Int J Gen Med. 2021;14:4039–50.
Zhang Y, Qiao X, Liu L, et al. Long noncoding RNA MAGI2-AS3 regulates the H(2)O(2) level and cell senescence via HSPA8. Redox Biol. 2022;54:102383.
Zhang Z, Yi Y, Wang Z et al. LncRNA MAGI2-AS3-Encoded polypeptide restrains the Proliferation and Migration of breast Cancer cells. Mol Biotechnol. 2023.
Liu F, Deng W, Wan Z, et al. lncRNA MAGI2-AS3 overexpression had antitumor effect on hepatic cancer via miRNA-23a-3p/PTEN axis. Food Sci Nutr. 2021;9(5):2517–30.
Cheng W, Shi X, Lin M, Yao Q, Ma J, Li J. LncRNA MAGI2-AS3 overexpression sensitizes esophageal Cancer cells to Irradiation through Down-Regulation of HOXB7 via EZH2. Front cell Dev Biology. 2020;8:552822.
Chen L, Fan X, Zhu J, Chen X, Liu Y, Zhou H. LncRNA MAGI2-AS3 inhibits the self-renewal of leukaemic stem cells by promoting TET2-dependent DNA demethylation of the LRIG1 promoter in acute myeloid leukaemia. RNA Biol. 2020;17(6):784–93.
Cui S, Liu Z, Tang B, Wang Z, Li B. LncRNA MAGI2-AS3 is down-regulated in intervertebral disc degeneration and participates in the regulation of FasL expression in nucleus pulposus cells. BMC Musculoskelet Disord. 2020;21(1):149.
Leite Pereira C, Grad S, Gonçalves RM. Biomarkers for intervertebral disc and associated back pain: from diagnosis to disease prognosis and personalized treatment. JOR Spine. 2023;6(4):e1280.
Yeh PS, Chen JT, Cherng YG, Yang ST, Tai YT, Chen RM. Methylpiperidinopyrazole attenuates Estrogen-Induced mitochondrial energy production and subsequent osteoblast maturation via an estrogen receptor alpha-dependent mechanism. Molecules. 2020;25(12).
Wei H, Chen Y, Nian H et al. Abnormal bone metabolism may be a primary causative factor of keel bone fractures in laying hens. Animals: Open Access J MDPI. 2021;11(11).
Giordano L, Porta GD, Peretti GM, Maffulli N. Therapeutic potential of microRNA in tendon injuries. Br Med Bull. 2020;133(1):79–94.
Ko SY, Tsai SF, Hsu CT et al. Gender differences in microRNA expressions as related to long-term graft function in kidney transplant patients. Int J Mol Sci. 2022;23(21).
Long C, Cen S, Zhong Z, Zhou C, Zhong G. FOXO3 is targeted by mir-223-3p and promotes osteogenic differentiation of bone marrow mesenchymal stem cells by enhancing autophagy. Hum Cell. 2021;34(1):14–27.
Dong HC, Li PN, Chen CJ, et al. Sinomenine attenuates cartilage degeneration by regulating miR-223-3p/NLRP3 Inflammasome Signaling. Inflammation. 2019;42(4):1265–75.
Mäkitie RE, Hackl M, Niinimäki R, Kakko S, Grillari J, Mäkitie O. Altered MicroRNA Profile in osteoporosis caused by impaired WNT signaling. J Clin Endocrinol Metab. 2018;103(5):1985–96.
Cheng N, Liu C, Li Y, et al. MicroRNA-223-3p promotes skeletal muscle regeneration by regulating inflammation in mice. J Biol Chem. 2020;295(30):10212–23.
Wang B, Wu W, Xu K, Wu H. MicroRNA-223-3p is involved in fracture healing by regulating fibroblast growth factor receptor 2. Bioengineered. 2021;12(2):12040–8.
Gargano G, Oliviero A, Oliva F, Maffulli N. Small interfering RNAs in tendon homeostasis. Br Med Bull. 2021;138(1):58–67.
Gargano G, Oliva F, Oliviero A, Maffulli N. Small interfering RNAs in the management of human rheumatoid arthritis. Br Med Bull. 2022;142(1):34–43.
Gargano G, Asparago G, Spiezia F, Oliva F, Maffulli N. Small interfering RNAs in the management of human osteoporosis. Br Med Bull. 2023;148(1):58–69.
Acknowledgements
Not applicable.
Funding
This study was funded by Clinical study of latissimus dorsi muscle flap for repairing limb wounds (No. WFWSJK-2020-307).
Author information
Authors and Affiliations
Contributions
S.T. W and M.W. W designed the research study. Z.Q. D, B.B. H, S.L. S, X.S L, D.Z. L and D.J. W performed the research. Z.Q. D, B.B. H, S.T. W, M.W. W, S.L. S, X.S L, D.Z. L and D.J. W analyzed the data. S.T. W and M.W. W wrote the manuscript. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The experimental protocol was reviewed and approved by the Ethics Committee of Yidu Central Hospital of Weifang, and all the participating patients signed informed consent.
Consent for publication
Not applicable.
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Dong, Z., Hu, B., Wang, S. et al. LncRNA MAGI2-AS3 promotes fracture healing through downregulation of miR-223-3p. J Orthop Surg Res 19, 370 (2024). https://doi.org/10.1186/s13018-024-04850-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13018-024-04850-5