Abstract
Background
In knee osteoarthritis (KOA), treatments involving knee injections of bone marrow-derived mesenchymal stem cells (BM-MSC), adipose tissue-derived mesenchymal stem cells (AD-MSC), or umbilical cord-derived mesenchymal stem cells (UC-MSC) have shown promise in alleviating symptoms. However, which types of mesenchymal stem cells (MSCs) have the best therapeutic outcomes remain uncertain.
Method
We systematically searched PubMed, OVID, Web of Science, and the Cochrane Library until January 1, 2024. The study evaluated five endpoints: Visual Analog Score (VAS) for Pain, Range of Motion (ROM), Whole-Organ Magnetic Resonance Imaging Score (WORMS), Western Ontario McMaster Universities Osteoarthritis Index (WOMAC), and adverse events (ADs). Standard meta-analysis and network meta-analysis were performed using Stata 16.0.
Results
Fifteen studies involving 585 patients were included in the meta-analysis. Standard meta-analysis revealed significant improvements with MSCs in VAS score (P < 0.001), knee ROM (P < 0.001), and WOMAC (P < 0.016) compared to traditional therapy. In the network meta-analysis, autologous MSCs significantly improved VAS score [SMD = 2.94, 95% CI (1.90, 4.56)] and knee ROM [SMD = 0.26, 95% CI (0.08, 0.82)] compared to traditional therapy. Similarly, BM-MSC significantly improved VAS score [SMD = 0.31, 95% CI (0.11, 0.91)] and knee ROM [SMD = 0.26, 95% CI (0.08, 0.82)] compared to hyaluronic acid. However, compared with traditional therapy, autologous or allogeneic MSCs were associated with more adverse reactions [SMD = 0.11, 95% CI (0.02, 0.59)], [SMD = 0.13, 95% CI (0.002, 0.72)]. Based on the surface under the cumulative ranking results, autologous BM-MSC showed the most improvement in ROM and pain relief in KOA patients, UC-MSC (SUCRA 94.1%) were most effective for positive WORMS, and AD-MSC (SUCRA 70.6%) were most effective for WOMAC-positive patients.
Conclusion
MSCs transplantation effectively treats KOA patients, with autologous BM-MSC potentially offering more excellent benefits.
Similar content being viewed by others
Introduction
Osteoarthritis (OA) stands as a prominent cause of joint pain and disability among adults, with over 30 million symptomatic adults in the United States alone [1]. The estimated annual cost of OA and related disorders is $461 billion when considering direct and indirect expenses [2]. While OA can affect any joint, the knee is the most commonly affected, with 10% of men and 13% of women over 60 experiencing symptomatic knee osteoarthritis (KOA) [3]. This prevalence is expected to rise due to increasing life expectancy and the obesity epidemic [4, 5]. Presently, nonoperative treatment options include physical therapy, nonsteroidal anti-inflammatory drugs, and intraarticular injections of corticosteroids and hyaluronic acid (HA) [6, 7]. Cartilage degeneration remains irreversible despite these options, highlighting the need for novel and practical treatment approaches for KOA to address its complex pathology [8].
Recent extensive studies have suggested mesenchymal stem cells (MSCs) as a promising alternative for treating symptomatic KOA due to their multifaceted effects on the local environment [9,10,11]. MSCs have shown effectiveness in healing and regenerating cartilage defects, potentially enhancing cartilage regeneration and mitigating the degenerative process when injected intra-articularly in KOA cases [12]. MSCs possess diverse properties, including anti-inflammatory, anti-microbial, analgesic, regenerative, immunomodulatory, and immune-evasive capabilities [7]. These cells can be sourced from bone marrow, adipose tissue, umbilical cord, amniotic fluid, placenta, menstrual blood, dental pulp, and endometrium. Bone marrow, adipose tissue, and umbilical cord are the most accessible sources [13]. Nonetheless, debates persist among orthopedic and translational medicine researchers regarding the choice of MSC types and sources for KOA treatment.
Bone marrow-derived mesenchymal stem cells (BM-MSC) have been extensively studied and have shown potential to improve knee pain and function and restore cartilage morphology in some instances [12, 14]. Adipose tissue-derived mesenchymal stem cells (AD-MSC) are garnering attention due to their simplicity in extraction, low complication rates, and minimal donor site morbidity [15, 16]. Additionally, umbilical cord-derived mesenchymal stem cells (UC-MSC) exhibit promising clonogenic, proliferative, and migratory capabilities and enhanced secretion of chondrogenic factors [17].
Numerous meta-analyses have explored the efficacy of single MSCs in treating KOA and chondral defects, primarily focusing on pain and physical function [18,19,20]. While these studies support the use of MSCs in clinical practice, few systematic reviews have evaluated the relative efficacy and safety of different MSCs types and sources in KOA treatment or compared these strategies. Thus, we designed and conducted this network meta-analysis to comprehensively assess the clinical efficacy and safety of various MSC sources and types for treating KOA and identify the optimal strategy.
Methods
The meta-analysis followed the requirements of the Cochrane Handbook for Systematic Reviews of Interventions [21], registered in PROSPERO (CRD42022351142), and PRISMA reporting guidelines [22].
Data source and search strategy
Two independent reviewers independently searched the following four databases for comprehensive literature information: PubMed, OVID, Web of Science, and the Cochrane Library; searches were conducted from their inception until January 1, 2024, on all related papers. The following free words or phrases and their abbreviations were used to develop a search strategy. The literature search strategy consisted of MeSH terms and the free words “knee osteoarthritis” and “mesenchymal stem cells” (Supplementary materials. Table 1). In addition, reference sections in the searched articles were manually checked to ensure that no relevant studies were overlooked.
Study selection criteria
The meta-analysis included clinical studies investigating the outcomes of patients who received MSCs therapy in the knee joint to treat osteoarthritis of any degree. Each randomized controlled trials (RCTs) compared MSC transplantation directly with other established treatment modalities, highlighting the comparative effectiveness of these approaches. Studies examining patients receiving other cell therapies alone or in combination were excluded. Table 1 presents the study inclusion and exclusion criteria in detail.
Types of studies
We included only RCTs that tested the effectiveness and safety of MSCs in treating KOA.
Types of participants
This study included participants who were diagnosed with KOA. To ensure that all relevant studies were included, we included all patients with KOA regardless of their age, the cause, the type, the time, the site, or the degree of KOA, as long as they met the inclusion criteria outlined in Table 1.
Types of interventions
The included studies investigated a variety of interventions. Specifically, the treatment groups received MSCs therapy, which was sometimes combined with other conventional treatments such as HA or platelet-rich plasma injection. The control groups received placebo or other conventional treatments. There were no specific limitations on the dose, frequency, or method of administration of MSCs. The detailed interventions for each study are provided in Tables 2 and 3.
Types of outcome measures
The outcome measures we evaluated included Visual Analog Score (VAS) for Pain [23], Range of motion (ROM), Whole-Organ Magnetic Resonance Imaging Score (WORMS) [20, 24], Western Ontario McMaster Universities Osteoarthritis Index (WOMAC) [25], and adverse events (ADs).
Data extraction and management
Using a standardized form, data were independently extracted and checked by two independent reviewers (Liu and Li), and another reviewer (Yin) adjusted the differences of opinion. The form contains the following items: the first author’s name, journal, year of publication, country of study, language, sample size, sex, age, diagnosis method, Kellgren-Lawrence Grade, MSCs Harvest location, MSCs Type, MSCs Source, details of treatment and control intervention, treatment duration, intervention duration, follow-up, outcome measure, and summary of results according to the Cochrane Handbook for Systematic Reviews of Interventions (version 6.1.0) [26].
Bias assessment of the included studies
Two reviewers (Liu and Li) independently assessed the risk of bias utilizing the risk of bias tool developed by Cochrane [27]. The following criteria were used to evaluate each trial: random sequence generation (selection bias), allocation concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), selective outcome reporting (reporting bias), and other bias. The judging criteria were rated as low risk of bias, unclear of bias, or low risk of bias. When two reviewers could not reach an agreement, a third experienced reviewer (Yin) made the final decision.
Quality of evidence
Based on the risk of bias tool developed by Cochrane, two independent reviewers (Liu and Li) assessed the methodological quality of the included studies. When disagreements could not be resolved through discussion, a third experienced reviewer (Yin) added his perspective and made the final decision. Each study was assessed for selection bias, performance bias, detection bias, attrition bias, reporting bias, and other biases. Each domain was rated as high risk of bias, low risk of bias, or unclear risk of bias.
Data synthesis and statistical analysis
The methodological quality and risk of bias assessment for all included studies were conducted using RevMan (Review Manager, Version 5.4) software. Network meta-analyses (NMAs) and standard meta-analyses were performed using STATA 16.0 software (Stata Corporation, Texas) for dichotomous data such as ADs, odds ratios (OR), and their corresponding 95% confidence intervals (CI) were calculated. Continuous outcomes, including ROM, VAS, WOMAC, and WORMS, were presented as standard mean difference (SMD) with 95% CI. Heterogeneity among the trials was assessed using the chi-square test and I2 statistics. A fixed-effects model was utilized for data analysis when p > 0.1 and I2 < 50%; otherwise, a random-effects model was applied for p < 0.1 and I2 > 50%.
In network meta-analysis, estimates are derived from either indirect or mixed evidence. When direct evidence is lacking, the analysis estimates indirect evidence based on trials comparing interventions with a common comparator. Conversely, when direct evidence is available, a mixed treatment effect is estimated using the weighted average of both direct and indirect evidence [28]. Pairwise meta-analyses based on random effects models were employed to derive effects from direct evidence. Global inconsistency and node-split tests were conducted, and the consistency model was adopted if no inconsistency was detected (p-value of Z-test > 0.05)29.
Publication bias for each endpoint was assessed using funnel plots and Egger’s tests in Stata. Surface under the cumulative ranking (SUCRA), treatment rankings, and probabilities of the best treatment were determined using Bayesian frameworks based on random-effects models. The SUCRA score, ranging from 0 to 100, indicates the treatment’s relative efficacy, with higher scores representing better treatments. These rankings and probabilities aid in understanding the preferred order of treatments for the average patient, as determined by clinicians and policymakers. However, it is essential to note that treatment effects are most significant, as a favorable rank does not necessarily imply a substantial or clinically significant effect [30].
Results
Literature Selection
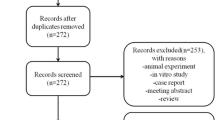
A total of 2397 studies were initially identified through a thorough search of 4 electronic databases and 18 additional studies referenced in relevant literature. Subsequently, 1983 studies were excluded after screening their titles and abstracts against predefined inclusion criteria. Additionally, 146 duplicated studies were removed. This process left 268 trials for further consideration. After reviewing the full texts of these trials, an additional 131 studies were excluded based on the exclusion criteria. Ultimately, 15 studies involving 585 patients met the criteria for inclusion in the meta-analysis (Fig. 1).
Characteristics of included trials
The characteristics of the included trials, along with intervention types, are summarized in Table 2. All included studies demonstrated no significant differences in baseline characteristics. These randomized controlled trials (RCTs) involved 585 patients with Kellgren-Lawrence Grade ranging from I to IV. Among them, 294 patients received intra-articular injections of MSCs, while 261 patients were treated with traditional drugs, primarily HA. The follow-up duration varied from 6 to 48 months, most falling within the 6 to 12 months range.
Of the fifteen studies, nine utilized autologous MSCs, while the remainder employed allogeneic MSCs. Six out of fifteen studies utilized MSCs derived from adipose tissue, eight from bone marrow, and one from umbilical cords. In all studies, cell transplantation only involved intra-articular injection in the treatment groups. The dosage of transplanted MSCs varied among the studies, and the frequency of transplantation ranged from one to four times, with most studies employing a single treatment session. Table 3 provides an overview of the general characteristics of the MSCs transplantation protocols used in the included studies. Clinical outcomes assessed included ROM, VAS score, WOMAC, WORMS, and ADs.
Methodological quality of included trials
To assess the risk of bias in the studies included, we used the standard Cochrane collaborative tool, and the risk of bias assessment for the included studies is shown in Fig. 2. Overall, the study included in this review was of acceptable methodological quality.
Standard meta-analysis
VAS score for pain
Ten studies, comprising 319 cases, reported VAS scores. Analysis revealed high statistical heterogeneity among the studies (p for heterogeneity < 0.0001, I2 = 82.5%). Subgroup analysis based on follow-up duration (6, 12, 16, and 48 months) was conducted, with one subgroup maintaining an I2 of over 50%, necessitating a random-effects model. The meta-analysis indicated a significant reduction in VAS scores with MSCs transplantation therapy compared to the control group (SMD = 2.21; 95% CI = [1.22, 3.21]; P < 0.001). (Fig. 3a)
ROM
Three studies involving 121 patients reported knee ROM. With an I2 of 0.0% and p for heterogeneity = 0.669, indicating low heterogeneity, a fixed-effect model was applied for meta-analysis. Results showed a significant improvement in knee ROM with MSCs transplantation therapy compared to the control group (SMD = -14.49; 95% CI = [-19.72, -9.27]; P < 0.001). (Fig. 3b)
WORMS
WORMS were reported in three studies comprising 100 patients. Low heterogeneity was observed among the included studies (I2 = 0.0%, p for heterogeneity = 0.397), warranting a fixed-effect model. However, no significant difference in WORMS was noted between the MSCs transplantation therapy group and the control group (SMD = 0.73; 95% CI = [-9.38, 10.84]; P = 0.888). (Fig. 3c)
WOMAC
Eleven studies, encompassing 301 cases, reported WOMAC total scores. Analysis revealed a high level of statistical heterogeneity among the studies (p for heterogeneity < 0.0001, I2 = 85.2%). Subgroup analysis based on follow-up duration (6, 12, and 48 months) was conducted, with two out of three subgroups showing an I2 more significant than 50%, leading to the utilization of a random-effects model. The results indicated a higher WOMAC total score in the MSCs transplantation therapy group compared to the control group (SMD = -12.29; 95% CI = [-22.26, -2.33]; P < 0.016). (Fig. 3d)
ADs
Eight studies involving 254 patients described adverse events during treatment and follow-up. None reported severe complications with permanent effects such as tumors, abnormal tissue proliferation, or immune reactions. The most commonly reported adverse reactions included minor discomfort, bruising, fever, and headache, which resolved spontaneously or with symptomatic treatment. Meta-analysis showed low heterogeneity among the included studies (I2 = 22.1%, P = 0.235), warranting a fixed-effects model for analysis. MSCs transplantation therapy was associated with a higher incidence of adverse events compared to the control group (OR = 0.49; 95% CI = [0.30, 0.80]; P = 0.005) (Fig. 3e). The remaining studies did not report any adverse events or side effects.
Network meta-analysis
Inconsistency analysis
We calculated the absolute difference between direct and indirect evidence using the relative odds ratio (ROR) with 95% confidence intervals. Consistency between direct and indirect evidence is indicated when ROR approximates one, or the 95% CI includes 0. No closed loop was formed in analyzing VAS scores, WORMS, WOMAC, and ADs outcome measures. Hence, no inconsistency analysis was performed. Additionally, no significant inconsistency was detected for ROM comparisons across different sources and types of MSCs, suggesting that the consistency model is more appropriate than the inconsistency model. Consequently, the consistency model was applied for VAS scores, ROM, WORMS, WOMAC, and ADs.
Comparison between different MSCs sources
Network plot
We generated five network plots for the five outcomes, each representing different sources of MSCs. A summary network plot of these comparisons is presented in Fig. 4a–e.
VAS score for pain
Ten pairwise comparisons were analyzed in this network. NMAs revealed a significant reduction in VAS scores with both autologous MSCs therapy [SMD = 2.33, 95% CI (1.25, 4.37)] and allogeneic MSCs therapy [SMD = 2.94, 95% CI (1.90, 4.56)] compared to traditional therapy. However, no significant differences were observed between autologous and allogeneic MSCs therapy (Fig. 5a). Autologous MSCs therapy demonstrated the lowest VAS scores (SUCRA 86.1%), followed by allogeneic MSCs therapy (SUCRA 63.8%). (Fig. 5f)
ROM
Three studies were assessed in this network, one of which was a 3-arm study. NMAs showed that autologous MSCs therapy [SMD = 0.26, 95% CI (0.08, 0.82)] improved knee ROM compared to traditional therapy. However, no significant differences were found between allogeneic MSCs therapy and traditional therapy, nor between autologous and allogeneic MSCs therapy (Fig. 5b). Autologous MSCs therapy had the highest probability of being the best option for improving knee ROM (SUCRA 81.7%), followed by allogeneic MSCs therapy (SUCRA 40.2%) and traditional therapy (SUCRA 28.1%). (Fig. 5g)
WORMS
Three articles assessing knee WORMS were included in this network. Similar to standard meta-analysis results, no significant differences in WORMS scores were observed in the network meta-analysis. According to the SUCRA rank, autologous MSCs therapy was theoretically the best strategy for positive WORMS scores (SUCRA 54.6%), followed by allogeneic MSCs therapy (SUCRA 53.3%), with traditional therapy having the lowest outcome (SUCRA 42.1%). (Figure 5c and h)
WOMAC
Eleven articles were included in the WOMAC network. Similar to the WORMS network results, no significant differences were found in WOMAC outcomes through a network meta-analysis. According to the SUCRA rank, traditional therapy was the most effective treatment strategy for positive WOMAC scores (SUCRA 94.1%), followed by autologous MSCs therapy (SUCRA 28.2%) and allogeneic MSCs therapy (SUCRA 27.7%). (Figure 5d and i)
ADs
Eight studies assessed adverse reactions in this network. Both autologous MSCs therapy [SMD = 0.11, 95% CI (0.02, 0.59)] and allogeneic MSCs therapy [SMD = 0.13, 95% CI (0.002, 0.72)] were associated with increased adverse events compared to traditional therapy. No significant difference was observed between autologous and allogeneic MSCs therapy. According to SUCRA values, traditional therapy was the best option to avoid adverse events (SUCRA 99.1%), followed by autologous MSCs therapy (SUCRA 28.9%) and allogeneic MSCs therapy (SUCRA 22.0%), which might be the least preferable strategy. (Figure 5e and j)
Comparison between different MSCs types
Network plot
We generated five network plots for each outcome, each representing different types of MSCs. Figure 6a–e presents a summary network plot of these comparisons.
Network meta-analysis of different MSCs types, Network plot of the subgroup: a VAS score; b knee ROM; c WORMS score; d WOMAC; e Adverse events. BM-MSC: bone marrow-derived mesenchymal stem cells; AD-MSC: adipose tissue-derived mesenchymal stem cells; UC-MSC: umbilical cord-derived mesenchymal stem cells, HA: Hyaluronic acid
VAS scores for pain
Five pairwise comparisons were analyzed in this network. NMAs indicated a significant decrease in VAS scores with AD-MSC therapy [SMD = 2.14, 95% CI (1.16, 3.94)] and BM-MSC therapy [SMD = 0.31, 95% CI (0.11, 0.91)] compared to UC-MSC therapy. According to the SUCRA rank, BM-MSC therapy was the most effective strategy (SUCRA 83.9%), followed by AD-MSC therapy (SUCRA 62.1%), HA therapy (SUCRA 52.0%), and UC-MSC therapy (SUCRA 1.9%) being the least preferable option. (Figure 7a and f)
ROM
Three studies were assessed in this network, including one 3-arm study. NMAs showed that BM-MSC therapy [SMD = 0.26, 95% CI (0.08, 0.82)] improved knee ROM compared to HA therapy. However, no significant differences were observed between AD-MSC therapy and HA therapy, nor between AD-MSC therapy and BM-MSC therapy (Fig. 7b). In terms of SUCRA ranking probability, BM-MSC therapy may be the best option to improve knee ROM (SUCRA 78.0%), followed by HA therapy (SUCRA 41.3%) and AD-MSC therapy (SUCRA 30.7%). (Fig. 7g)
WORMS
Three articles for WORMS scores were included in this network. No significant differences were found in WORMS scores through network meta-analysis. According to the SUCRA rank, UC-MSC therapy (SUCRA 94.1%) was the most effective treatment strategy for positive WORMS scores, followed by BM-MSC therapy (SUCRA 28.2%) and HA therapy (SUCRA 27.7%). (Figure 7c and h)
WOMAC
The WOMAC network meta-analysis included six articles. Similar to the WORMS network results, no significant differences were observed in WOMAC outcomes through network meta-analysis. Based on SUCRA rank, AD-MSC therapy (SUCRA 70.6%) may be the most effective option for WOMAC-positive patients, followed by UC-MSC therapy (SUCRA 68.3%) and BM-MSC therapy (SUCRA 43.9%). HA therapy (SUCRA 17.2%) may be the least effective treatment option. (Figure 7d and i)
ADs
Four studies assessed adverse reactions in this network. Compared with HA therapy, AD-MSC therapy [SMD = 0.03, 95% CI (0.00, 0.50)] and BM-MSC therapy [SMD = 0.06, 95% CI (0.01, 0.48)] resulted in increased adverse reactions. No significant differences were found between BM-MSC therapy and AD-MSC therapy. SUCRA values indicated that HA therapy (SUCRA 99.6%) was the most effective strategy, followed by AD-MSC therapy (SUCRA 31.3%) and BM-MSC therapy (SUCRA 19.1%). (Figure 7e and j)
Publication bias
Publication bias was assessed using the funnel plot and Egger’s test. Although the funnel plot showed some asymmetry, possibly due to small sample sizes or publication bias (Fig. 8), Egger’s test did not reveal significant evidence of publication bias (P = 0.061), suggesting minimal publication bias. (See Supplementary materials, Table 2)
Discussion
This trial assessed the safety and effectiveness of various sources and types of MSCs transplantation in patients with symptomatic KOA. Our study provides empirical evidence that MSCs transplantation effectively treats KOA patients. Specifically, our findings highlight that (1) autologous BM-MSC showed the most improvement in function and pain relief in KOA patients; (2) UC-MSC were most effective for positive WORMS, and AD-MSC were most effective for WOMAC-positive patients; (3) compared with traditional therapy, autologous or allogeneic MSCs were associated with more adverse reactions. To our knowledge, this is the first investigation to determine the optimal MSCs strategy for KOA and the initial comparison among BM-MSC, AD-MSC, and UC-MSC.
NMAs, by amalgamating direct and indirect comparisons, can augment study size, thereby enhancing statistical power [24]. According to NMAs findings, autologous MSC transplantation proved more effective than traditional therapy in terms of VAS scores and ROM. However, both autologous and allogeneic MSCs transplantations were associated with more reversible adverse reactions compared to traditional therapy, consistent with standard meta-analysis results. Considering clinical efficacy and safety probabilities, autologous MSCs transplants were more likely to be effective than allogeneic transplants. Moreover, BM-MSC showed the highest probability of being the most effective option in VAS scores and ROM. However, BM-MSC also elicited the highest frequency of adverse events, compared to HA with minimal complications, followed by AD-MSC. Among other MSC types, UC-MSC exhibited the highest probability of being the best treatment strategy for positive WORMS results. At the same time, AD-MSC ranked highest for WOMAC-positive patients. Except for BM-MSC and AD-MSC, which significantly reduced pain compared to UC-MSC, no significant differences were observed among the MSCs regarding ROM, WORMS, WOMAC, and adverse events.
The findings of previous meta-analyses align closely with those of our study concerning the effectiveness and safety of MSCs in treating KOA [18,19,20]. However, prior studies needed more clarity regarding the distinction between allogeneic and autologous MSCs and the impacts of various MSCs types. In contrast, our study offered the advantage of precisely defining the sources and types of MSCs. Additionally, we employed NMAs to rank subgroups derived from different cell sources that could not be directly compared. This approach allowed us to explore the optimal cell type through indirect comparisons, enhancing our understanding of the therapeutic efficacy of MSCs in KOA treatment.
Various MSCs sources can be harvested, including autologous bone marrow, adipose tissue, and allogeneic umbilical cord tissue. These cells exhibit differentiation, plasticity, immunomodulatory, immune evasive, and anti-inflammatory properties [23, 31, 32]. While MSCs have long been considered low immunogenic or immune-privileged, recent studies have indicated the production of antibodies and immune rejection against allogeneic MSCs, challenging this notion [33,34,35]. This suggests MSCs may not possess immune-privileged status as previously believed [35, 36]. Although the rejection of MSCs does not impact the efficacy of allogeneic MSCs therapy, safeguarding MSCs from immune responses and prolonging their persistence in vivo could enhance clinical outcomes and mitigate the development of antigen sensitivity [37, 38]. Our NMAs demonstrate that autologous MSCs outperform allogeneic MSCs regarding efficacy and safety. Thus, autologous MSCs may represent the most suitable cell source for treating KOA. However, this conclusion is drawn from indirect comparisons. Further well-designed and high-quality clinical randomized controlled trials are warranted to elucidate the impact of transplanted cell volume, frequency, duration, and KOA stage on treatment outcomes.
To achieve optimal functional outcomes in KOA treatment, selecting the appropriate MSCs source is crucial. Emerging evidence indicates that stem cell-based products such as BM-MSC, AD-MSC, and UC-MSC may ameliorate symptoms in osteoarthritis patients [7, 39]. Preclinical investigations evaluating clinical outcomes and cartilage repair post stem cell therapy in KOA have predominantly utilized BM-MSC, followed by AD-MSC and UC-MSC [25, 40]. Studies suggest that BM-MSC yield, particularly in the elderly, is relatively low compared to AD-MSC, despite the challenges associated with AD-MSC preparation compared to BM-MSC [41]. Notably, adipose tissue yields an MSC volume approximately 500 times greater than bone marrow [42, 43]. While some researchers assert that BM-MSC exhibits superior cartilage generation capabilities compared to AD-MSC, others propose that augmenting the stromal vascular fraction derived from AD-MSC with growth factors and cytokines can also enhance cartilage growth [44, 45]. Synovium-derived MSCs have been explored for assessing efficacy and functional outcomes in osteoarthritic knees, with several studies suggesting their superior chondrogenesis potential [46, 47]. Contrary to our findings, Lee et al. [48] observed that allogeneic human UC-MSC was more effective than autologous BM-MSC in cartilage regeneration in KOA, although clinical outcomes improved regardless of treatment type. Given the relatively recent exploration of UC-MSC in KOA treatment and the limited number of studies, further high-quality randomized controlled trials are warranted to validate their efficacy [49]. Regenerative and translational medicine holds promise in managing MSCs in KOA [50]. However, large RCTs are imperative to refine therapeutic protocols concerning MSCs type, isolation methods, and the quality and quantity of transplanted MSCs [19]. Addressing ethical concerns regarding tissue and cellular product manipulation and their functional outcomes is also essential [3, 13]. Given the complexities involved, an interdisciplinary approach is necessary to translate stem cell research into optimal clinical practice for KOA management.
This study has several limitations. Firstly, heterogeneity was observed across studies in most outcomes, possibly due to variations in treatment protocols, including the different traditional therapies utilized in the control groups of the included RCTs. This heterogeneity potentially impacts the validity of our findings. Secondly, the novelty of stem cell therapy for KOA and its limited availability in clinical practice restricted the number of patients and studies included in the analysis. Thirdly, studies were scarce assessing ROM and WORMS outcomes, particularly in AD-MSC and UC-MSC subgroups. Lastly, the included studies encompassed patients at different stages of KOA, contributing to the heterogeneity of the results. Therefore, further confirmation of our findings necessitates large multicentric trials with standardized dosage and frequency protocols and uniform outcome assessment measures, excluding adjuvant procedures.
Conclusion
Transplanting MSCs in KOA yields superior outcomes to traditional therapies, notably enhancing function and alleviating pain. Furthermore, no significant disparities were noted when comparing various stem cell types and sources. However, BM-MSC therapy was most effective in improving the VAS and the ROM, while other types of MSCs were more effective in improving functional outcomes, such as WORMS and WOMAC scores. HA is the most advisable choice for treatment-associated adverse events, followed by AD-MSC and BM-MSCs. Although these adverse events are generally mild, they could adversely impact treatment compliance and satisfaction.
Data availability
No datasets were generated or analysed during the current study.
References
Cisternas MG, Murphy L, Sacks JJ, Solomon DH, Pasta DJ, Helmick CG. Alternative methods for defining Osteoarthritis and the impact on estimating prevalence in a US Population-based survey. Arthritis Care Res. 2016;68(5):574–80. https://doi.org/10.1002/acr.22721.
Zhang Y, Jordan JM. Epidemiology of osteoarthritis. Clin Geriatr Med. 2010;26(3):355–69. https://doi.org/10.1016/j.cger.2010.03.001.
Doyle EC, Wragg NM, Wilson SL. Intraarticular injection of bone marrow-derived mesenchymal stem cells enhances regeneration in knee osteoarthritis. Knee surgery, sports traumatology, arthroscopy: official journal of the ESSKA 2020; 28 (12): 3827–42. https://doi.org/10.1007/s00167-020-05859-z.
Aratikatla A, Maffulli N, Gupta M, Potti IA, Potty AG, Gupta A. Wharton’s jelly and osteoarthritis of the knee. Br Med Bull. 2024;149(1):13–31. https://doi.org/10.1093/bmb/ldad030.
Sharma L. Osteoarthritis of the knee. N Engl J Med. 2021;384(1):51–9. https://doi.org/10.1056/NEJMcp1903768.
Mianehsaz E, Mirzaei HR, Mahjoubin-Tehran M, Rezaee A, Sahebnasagh R, Pourhanifeh MH, et al. Mesenchymal stem cell-derived exosomes: a new therapeutic approach to osteoarthritis? Stem Cell Res Ther. 2019;10(1):340. https://doi.org/10.1186/s13287-019-1445-0.
Song Y, Zhang J, Xu H, Lin Z, Chang H, Liu W, et al. Mesenchymal stem cells in knee osteoarthritis treatment: a systematic review and meta-analysis. J Orthop Translation. 2020;24:121–30. https://doi.org/10.1016/j.jot.2020.03.015.
Aletto C, Giordano L, Quaranta M, Zara A, Notarfrancesco D, Maffulli N. Short-term results of intra-articular injections of stromal vascular fraction for early knee osteoarthritis. J Orthop Surg Res. 2022;17(1):310. https://doi.org/10.1186/s13018-022-03196-0.
Pers YM, Ruiz M, Noël D, Jorgensen C. Mesenchymal stem cells for the management of inflammation in osteoarthritis: state of the art and perspectives. Osteoarthr Cartil. 2015;23(11):2027–35. https://doi.org/10.1016/j.joca.2015.07.004.
Schmitz C, Alt C, Pearce DA, Furia JP, Maffulli N, Alt EU. Methodological flaws in Meta-analyses of clinical studies on the management of knee osteoarthritis with stem cells: a systematic review. Cells. 2022;11(6). https://doi.org/10.3390/cells11060965.
Gupta A, Maffulli N, Rodriguez HC, Mistovich RJ, Delfino K, Cady C, et al. Cell-free stem cell-derived extract formulation for treatment of knee osteoarthritis: study protocol for a preliminary non-randomized, open-label, multi-center feasibility and safety study. J Orthop Surg Res. 2021;16(1):514. https://doi.org/10.1186/s13018-021-02672-3.
Bastos R, Mathias M, Andrade R, Amaral R, Schott V, Balduino A et al. Intra-articular injection of culture-expanded mesenchymal stem cells with or without addition of platelet-rich plasma is effective in decreasing pain and symptoms in knee osteoarthritis: a controlled, double-blind clinical trial. Knee surgery, sports traumatology, arthroscopy: official journal of the ESSKA 2020; 28 (6): 1989–1999. https://doi.org/10.1007/s00167-019-05732-8.
Zhu C, Wu W, Qu X. Mesenchymal stem cells in osteoarthritis therapy: a review. Am J Translational Res. 2021;13(2):448–61.
Lu L, Dai C, Zhang Z, Du H, Li S, Ye P, et al. Treatment of knee osteoarthritis with intra-articular injection of autologous adipose-derived mesenchymal progenitor cells: a prospective, randomized, double-blind, active-controlled, phase IIb clinical trial. Stem Cell Res Ther. 2019;10(1):143. https://doi.org/10.1186/s13287-019-1248-3.
Freitag J, Bates D, Wickham J, Shah K, Huguenin L, Tenen A, et al. Adipose-derived mesenchymal stem cell therapy in the treatment of knee osteoarthritis: a randomized controlled trial. Regen Med. 2019;14(3):213–30. https://doi.org/10.2217/rme-2018-0161.
Lee WS, Kim HJ, Kim KI, Kim GB, Jin W. Intra-articular injection of autologous adipose tissue-derived mesenchymal stem cells for the treatment of knee osteoarthritis: a phase IIb, Randomized, Placebo-Controlled Clinical Trial. Stem Cells Translational Med. 2019;8(6):504–11. https://doi.org/10.1002/sctm.18-0122.
Matas J, Orrego M, Amenabar D, Infante C, Tapia-Limonchi R, Cadiz MI et al. Umbilical cord-derived mesenchymal stromal cells (MSCs) for knee osteoarthritis: repeated MSC Dosing is Superior to a single MSC dose and to Hyaluronic Acid in a controlled Randomized Phase I/II Trial. Stem cells translational medicine 2019; 8 (3): 215–24. https://doi.org/10.1002/sctm.18-0053.
Kim SH, Ha CW, Park YB, Nam E, Lee JE, Lee HJ. Intra-articular injection of mesenchymal stem cells for clinical outcomes and cartilage repair in osteoarthritis of the knee: a meta-analysis of randomized controlled trials. Arch Orthop Trauma Surg. 2019;139(7):971–80. https://doi.org/10.1007/s00402-019-03140-8.
Maheshwer B, Polce EM, Paul K, Williams BT, Wolfson TS, Yanke A, et al. Regenerative potential of mesenchymal stem cells for the treatment of knee osteoarthritis and Chondral defects: a systematic review and Meta-analysis. Arthroscopy: J Arthroscopic Relat Surg : Official Publication Arthrosc Association North Am Int Arthrosc Association. 2021;37(1):362–78. https://doi.org/10.1016/j.arthro.2020.05.037.
Tan SHS, Kwan YT, Neo WJ, Chong JY, Kuek TYJ, See JZF, et al. Intra-articular injections of mesenchymal stem cells without adjuvant therapies for knee osteoarthritis: a systematic review and Meta-analysis. Am J Sports Med. 2021;49(11):3113–24. https://doi.org/10.1177/0363546520981704.
Cumpston M, Li T, Page MJ, Chandler J, Welch VA, Higgins JP et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. The Cochrane database of systematic reviews 2019; 10 Ed000142. https://doi.org/10.1002/14651858.ed000142.
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. https://doi.org/10.1371/journal.pmed.1000097.
Pintore A, Notarfrancesco D, Zara A, Oliviero A, Migliorini F, Oliva F, et al. Intra-articular injection of bone marrow aspirate concentrate (BMAC) or adipose-derived stem cells (ADSCs) for knee osteoarthritis: a prospective comparative clinical trial. J Orthop Surg Res. 2023;18(1):350. https://doi.org/10.1186/s13018-023-03841-2.
Singh H, Knapik DM, Polce EM, Eikani CK, Bjornstad AH, Gursoy S et al. Relative Efficacy of Intra-articular Injections in the Treatment of Knee Osteoarthritis: A Systematic Review and Network Meta-analysis. The American journal of sports medicine. 2021; 3635465211029659. https://doi.org/10.1177/03635465211029659.
Garza JR, Campbell RE, Tjoumakaris FP, Freedman KB, Miller LS, Santa Maria D, et al. Clinical efficacy of intra-articular mesenchymal stromal cells for the treatment of knee osteoarthritis: a double-blinded prospective randomized controlled clinical trial. Am J Sports Med. 2020;48(3):588–98. https://doi.org/10.1177/0363546519899923.
Higgins J, Thomas J, Chandler J, Cumpston M, Welch VA. Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Handbook for Systematic Reviews of Interventions; 2019.
Cumpston M, Li T, Page MJ, Chandler J, Welch VA, Higgins JP, et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev. 2019;10(10):Ed000142. https://doi.org/10.1002/14651858.ed000142.
Cipriani A, Higgins JP, Geddes JR, Salanti G. Conceptual and technical challenges in network meta-analysis. Ann Intern Med. 2013;159(2):130–7. https://doi.org/10.7326/0003-4819-159-2-201307160-00008.
Dias S, Welton NJ, Caldwell DM, Ades AE. Checking consistency in mixed treatment comparison meta-analysis. Stat Med. 2010;29(7–8):932–44. https://doi.org/10.1002/sim.3767.
Rücker G, Schwarzer G. Ranking treatments in frequentist network meta-analysis works without resampling methods. BMC Med Res Methodol. 2015;15(58). https://doi.org/10.1186/s12874-015-0060-8.
Andia I, Maffulli N. Mesenchymal stromal cell products for intra-articular knee injections for conservative management of osteoarthritis. Therapeutic Adv Musculoskelet Disease. 2021;13:1759720x21996953. https://doi.org/10.1177/1759720x21996953.
Gupta A, El-Amin SF 3rd, Levy HJ, Sze-Tu R, Ibim SE, Maffulli N. Umbilical cord-derived Wharton’s jelly for regenerative medicine applications. J Orthop Surg Res. 2020;15(1):49. https://doi.org/10.1186/s13018-020-1553-7.
Fu X, Liu G, Halim A, Ju Y, Luo Q, Song AG. Mesenchymal Stem Cell Migration Tissue Repair Cells. 2019;8(8). https://doi.org/10.3390/cells8080784.
Han Y, Li X, Zhang Y, Han Y, Chang F, Ding J. Mesenchymal stem cells for Regenerative Medicine. Cells. 2019;8(8). https://doi.org/10.3390/cells8080886.
Yin N, Wu C, Qiu J, Zhang Y, Bo L, Xu Y, et al. Protective properties of heme oxygenase-1 expressed in umbilical cord mesenchymal stem cells help restore the ovarian function of premature ovarian failure mice through activating the JNK/Bcl-2 signal pathway-regulated autophagy and upregulating the circulating of CD8(+)CD28(-) T cells. Stem Cell Res Ther. 2020;11(1):49. https://doi.org/10.1186/s13287-019-1537-x.
Ankrum JA, Ong JF, Karp JM. Mesenchymal stem cells: immune evasive, not immune privileged. Nat Biotechnol. 2014;32(3):252–60. https://doi.org/10.1038/nbt.2816.
Zanotti L, Sarukhan A, Dander E, Castor M, Cibella J, Soldani C, et al. Encapsulated mesenchymal stem cells for in vivo immunomodulation. Leukemia. 2013;27(2):500–3. https://doi.org/10.1038/leu.2012.202.
Atanasova E, Milosevic D, Bornschlegl S, Krucker KP, Jacob EK, Carmona Porquera EM, et al. Normal ex vivo mesenchymal stem cell function combined with abnormal immune profiles sets the stage for informative cell therapy trials in idiopathic pulmonary fibrosis patients. Stem Cell Res Ther. 2022;13(1):45. https://doi.org/10.1186/s13287-021-02692-0.
He L, He T, Xing J, Zhou Q, Fan L, Liu C, et al. Bone marrow mesenchymal stem cell-derived exosomes protect cartilage damage and relieve knee osteoarthritis pain in a rat model of osteoarthritis. Stem Cell Res Ther. 2020;11(1):276. https://doi.org/10.1186/s13287-020-01781-w.
Dilogo IH, Canintika AF, Hanitya AL, Pawitan JA, Liem IK, Pandelaki J. Umbilical cord-derived mesenchymal stem cells for treating osteoarthritis of the knee: a single-arm, open-label study. Eur J Orthop Surg Traumatology: Orthopedie Traumatologie. 2020;30(5):799–807. https://doi.org/10.1007/s00590-020-02630-5.
Wolfstadt JI, Cole BJ, Ogilvie-Harris DJ, Viswanathan S, Chahal J. Current concepts: the role of mesenchymal stem cells in the management of knee osteoarthritis. Sports Health. 2015;7(1):38–44. https://doi.org/10.1177/1941738114529727.
Hass R, Kasper C, Böhm S, Jacobs R. Different populations and sources of human mesenchymal stem cells (MSC): a comparison of adult and neonatal tissue-derived MSC. Cell Communication Signaling: CCS. 2011. https://doi.org/10.1186/1478-811x-9-12. 9 12.
Kim KI, Lee MC, Lee JH, Moon YW, Lee WS, Lee HJ, et al. Clinical efficacy and safety of the intra-articular injection of autologous adipose-derived mesenchymal stem cells for knee osteoarthritis: a phase III, Randomized, Double-Blind, placebo-controlled trial. Am J Sports Med. 2023;51(9):2243–53. https://doi.org/10.1177/03635465231179223.
Kim HJ, Im GI. Chondrogenic differentiation of adipose tissue-derived mesenchymal stem cells: greater doses of growth factor are necessary. J Orthop Research: Official Publication Orthop Res Soc. 2009;27(5):612–9. https://doi.org/10.1002/jor.20766.
Sadri B, Hassanzadeh M, Bagherifard A, Mohammadi J, Alikhani M, Moeinabadi-Bidgoli K, et al. Cartilage regeneration and inflammation modulation in knee osteoarthritis following injection of allogeneic adipose-derived mesenchymal stromal cells: a phase II, triple-blinded, placebo controlled, randomized trial. Stem Cell Res Ther. 2023;14(1):162. https://doi.org/10.1186/s13287-023-03359-8.
Angthong C, Kunkanjanawan H. Ankle synovium-derived mesenchymal stem cells for the treatment of osteochondral lesion of the talus: a novel cell harvesting technique and clinical applications. Eur Rev Med Pharmacol Sci. 2020;24(16):8273–80. https://doi.org/10.26355/eurrev_202008_22623.
Kim YS, Kim YI, Koh YG. Intra-articular injection of human synovium-derived mesenchymal stem cells in beagles with surgery-induced osteoarthritis. Knee. 2021;28:159–68. https://doi.org/10.1016/j.knee.2020.11.021.
Lee NH, Na SM, Ahn HW, Kang JK, Seon JK, Song EK. Arthroscopy: J arthroscopic Relat Surg : official publication Arthrosc Association North Am Int Arthrosc Association. 2021;37(8):2521–30. https://doi.org/10.1016/j.arthro.2021.02.022. Allogenic Human Umbilical Cord Blood-Derived Mesenchymal Stem Cells Are More Effective Than Bone Marrow Aspiration Concentrate for Cartilage Regeneration After High Tibial Osteotomy in Medial Unicompartmental Osteoarthritis of Knee.
Mautner K, Gottschalk M, Boden SD, Akard A, Bae WC, Black L, et al. Cell-based versus corticosteroid injections for knee pain in osteoarthritis: a randomized phase 3 trial. Nat Med. 2023;29(12):3120–6. https://doi.org/10.1038/s41591-023-02632-w.
Gupta PK, Maheshwari S, Cherian JJ, Goni V, Sharma AK, Tripathy SK, et al. Efficacy and safety of Stempeucel in Osteoarthritis of the knee: a phase 3 Randomized, Double-Blind, Multicenter, Placebo-controlled study. Am J Sports Med. 2023;51(9):2254–66. https://doi.org/10.1177/03635465231180323.
Acknowledgements
Not applicable.
Funding
This study was supported by the Sanming Project of Medicine in Shenzhen (No. SZZYSM202108013), the President Foundation of The Third Affiliated Hospital of Southern Medical University (No. YP202210).
Author information
Authors and Affiliations
Contributions
H.L., Y.L., and L.Y. led the conception and design of the study. H.L. and X.C. did the database search, screening, quality assessment, and data extraction. X.C. and J.Z. designed the experiments and workflow, and L.Y. did the statistical analysis and interpreted the data. X.C. wrote the paper. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Ethical Approval and Consent to participate are not applicable. The study protocol was registered at PROSPERO (CRD42022351142).
Consent for publication
All authors have stated their consent of publication.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Chen, X., Zheng, J., Yin, L. et al. Transplantation of three mesenchymal stem cells for knee osteoarthritis, which cell and type are more beneficial? a systematic review and network meta-analysis. J Orthop Surg Res 19, 366 (2024). https://doi.org/10.1186/s13018-024-04846-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13018-024-04846-1