Abstract
Osteoporotic fractures impose a substantial burden on patients with diabetes due to their unique characteristics in bone metabolism, limiting the efficacy of conventional fracture prediction tools. Artificial intelligence (AI) algorithms have shown great promise in predicting osteoporotic fractures. This review aims to evaluate the application of traditional fracture prediction tools (FRAX, QFracture, and Garvan FRC) in patients with diabetes and osteoporosis, review AI-based fracture prediction achievements, and assess the potential efficiency of AI algorithms in this population. This comprehensive literature search was conducted in Pubmed and Web of Science. We found that conventional prediction tools exhibit limited accuracy in predicting fractures in patients with diabetes and osteoporosis due to their distinct bone metabolism characteristics. Conversely, AI algorithms show remarkable potential in enhancing predictive precision and improving patient outcomes. However, the utilization of AI algorithms for predicting osteoporotic fractures in diabetic patients is still in its nascent phase, further research is required to validate their efficacy and assess the potential advantages of their application in clinical practice.
Similar content being viewed by others
Introduction
Osteoporotic fractures (OF) remains a prevalent clinical disorder that severely impacts patients' quality of life, leading to hospitalization, disability, and even mortality [1]. The diagnosis of osteoporosis is established when the bone mineral density (BMD) value falls below − 2.5 standard deviations (T-score − 2.5) [2]. Latest globalized data has indicated that the prevalence of osteoporosis and osteopenia were 19.7% and 40.4%, respectively [3]. OFs is the most common complication of osteoporosis, including hip fracture (HF), vertebral fracture (VF), wrist fractures (WF), and distal radius fracture (DRF). An estimated 9 million cases of OF were reported worldwide in 2000, including 1.6 million HF, 1.7 million WF, and 1.4 million VF [4]. Notably, the mortality rate after HF may be as high as 20% [5]. Given the substantial morbidity and serious consequences associated with OF, this condition imposes a significant economic burden on society that cannot be ignored. In 2000, osteoporosis in the UK was accountable for a financial burden of 1.8 billion pounds, and this figure is estimated to rise to 2.2 billion pounds by 2025 [6]. Similarly, China is projected to experience 5.99 million osteoporotic fractures annually, with an annual cost of 25.43 billion US dollars by 2050 [7]. Aging remains the primary etiological factor that underlies osteoporosis, while secondary causes include chronic kidney disease, diabetes, thyroid disease, treatment of glucocorticoids, proton pump inhibitors, antiepileptic drugs, and selective serotonin reuptake inhibitors [8]. With the global trend of population aging, OF will undoubtedly become more prevalent [9]. Thereby emphasizing the need for accurate risk assessment to minimize the substantial socioeconomic costs associated with this condition.
In addition to the rising incidence of osteoporosis, diabetes is also a growing public health concern. Interestingly, although patients with type 1 diabetes mellitus (T1DM) tend to have lower BMD, patients with type 2 diabetes mellitus (T2DM) often exhibit higher BMD [10]. Despite these differences in BMD, both T1DM and T2DM increase the risk of fracture [11]. FRAX, QFracture algorithm and the Garvan Fracture Risk Calculator (Garvan FRC) are the most common fracture risk prediction tools internationally. However, these tools fail to predict the fracture risk of patients with diabetes accurately [12]. Specifically, FRAX and Garvan FRC underestimate fracture risk in patients with T2DM by failing to incorporate T2DM as an independent predictor. On the other hand, although QFracture includes both T1DM and T2DM as independent predictors, its performance has not been validated in diabetic populations [12]. As such, there is a pressing need for more accurate fracture risk prediction tools for individuals with diabetes.
This comprehensive review aims to provide insights into the unique features of bone metabolism and fracture risk in patients with osteoporosis and diabetes mellitus. Specifically, we critically analyze the limitations of currently available osteoporosis prediction tools for diabetic populations and explore the potential of artificial intelligence (AI) in enhancing the accuracy of fracture prediction. Our findings highlight the urgent need for innovative approaches to personalized fracture risk assessment and management.
Correlation of diabetes and osteoporotic fracture
Distinct pathophysiological mechanisms of T1DM and T2DM underlie the heightened fracture risk observed in patients with diabetes. The risk for fractures in patients with T1DM is six-fold higher than in the general population, primarily due to low BMD, alterations in bone quality, microarchitecture, and impaired bone turnover state [13]. Non-osseous factors such as recurrent hypoglycemic episodes, peripheral neuropathy, autonomic neuropathy, retinopathy, and low body weight further increase the risk of falls in this population [14]. Although the decrease of BMD is not significant, T2DM patients still have a higher risk of fracture than non-diabetes patients [13, 15]. A meta-analysis comprising 54 clinical studies conducted in China has demonstrated that the prevalence of osteoporosis in patients with type 2 diabetes is significantly higher (37.8%) than the overall prevalence of osteoporosis (27.96%) [16]. In sharp contrast to the effect of secondary OF in T1DM, T2DM patients tend to present with higher BMD [17]. Alteration in bone microarchitecture that result in poor bone quality may account for the increased risk of fracture in T2DM patients. Studies have shown that trabecular bone score (TBS) in patients of T2DM and pre-diabetes stage is significantly lower than that in non-type 2 diabetes patients [18]. The changes of bone microstructure in T2DM with OF were characterized by higher endocortical bone surface, intracortical pore volume and greater relative porosity at the distal tibia and ultra-distal radius [19]. In addition to the risk factors above mentioned, chronic hyperglycemia, tissue-specific accumulation of advanced glycation end-products (AGE), changes in vitamin D homeostasis, diabetes microvascular disease, and insulin pharmacotherapy [20], which are common to the two types of diabetes, have adverse effects on the bone health of diabetes patients. In view of the large population base of diabetes patients, the unique bone metabolism characteristics, and higher prevalence of osteoporosis, OF should not be neglected as a complication of diabetes.
Prediction effect of traditional prediction tools on fracture in diabetes patients
At present, there are various risk assessment tools for OF. The most recommended risk assessment tool is the FRAX® fracture risk assessment tool [21], followed by the QFracture algorithm [22] and the Garvan Fracture Risk Calculator (Garvan FRC) [23].
FRAX (https://www.sheffield.ac.uk/FRAX/) is a computer-based algorithm that calculates the fracture risk in the next 10 years for people aged 40–90. Since it was developed in 2008, FRAX has been an open access tool provided convenience for clinicians all over the world. The algorithm incorporates independent variables included age, weight (kg), height (cm), previous fracture history, parent hip fracture history, smoking history, glucocorticoid use history, rheumatoid arthritis history, secondary osteoporosis history, alcohol intake history, and femoral neck BMD value. Obviously, race, diet, geographical factors that vary from regions to regions are not included. The reason that FRAX is recommended by most osteoporosis related guidelines or consensus all over the world is that FRAX has been calibrated to countries or regions where the epidemiology of fracture and death is known (currently 64 countries) [21]. FRAX regards T1DM but not T2DM as a cause of secondary osteoporosis, and the current FRAX algorithm does not acquire T2DM input, which may be the reason why FRAX has insufficient ability to predict fracture in patients with diabetes [24]. As for T1DM, scientists haven made efforts to use FRAX algorithm to predict fracture in patients with T1DM without BMD value in a clinical cohort of 346 patients with T1DM and 411 controls, and concluded that the FRAX without BMD exerted good prediction efficiency in detecting patients with T1DM at risk of major osteoporotic fracture (MOF) [25]. However, it has been recognized that FRAX underestimates the fracture risk of individuals with T2DM when applied to populations with equivalent BMD and T score [26]. In 2012, a clinical study including 3518 diabetes patients and 36,085 non diabetes patients showed that FRAX underestimated the risk of MOF and HF observed in diabetes patients after adjusting for competitive mortality [27]. Furthermore, a cohort study of 49,098 non-diabetic women and 8840 women with diabetes in 2016 showed that FRAX underestimated the risk of HF in diabetes patients, while in diabetes patients with diabetes for more than 10 years, FRAX underestimated the risk of MOF [28]. To improve the performance of FRAX for T2DM, four feasible methods have been proposed [29]: (1) including the rheumatoid arthritis (RA) input to FRAX; (2) making a TBS adjustment to FRAX; (3) reducing the femoral neck T-score input to FRAX by 0.5 SD; and (4) increasing the age input to FRAX by 10 years. However, there has not been a method that is optimal in all cases.
QFracture (https://qfracture.org/) was developed by Julia Hippisley Cox and Carol Coupland based on data from the United Kingdom to estimate1 to 10-year risk of MOF and HF in people aged 30–99 without BMD measurement. It is characterized by no need for imaging or laboratory examination data. The variables in this algorithm included body mass index (BMI), age, gender, ethnicity, smoking status, alcohol status, history of diabetes, parent’s history of osteoporosis/hip fracture, residence history of nursing or care home, history of wrist spine hip or shoulder fracture, history of falls, dementia, cancer, asthma or chronic obstructive pulmonary disease (COPD), heart attack, angina, stroke or transient ischemic attack (TIA), chronic liver disease, chronic kidney disease (stage 4 or 5), Parkinson's disease, rheumatoid arthritis or systemic lupus erythematosus (SLE), malabsorption such as Crohn's disease, ulcerative colitis, coeliac disease, steatorrhea or blind loop syndrome, endocrine problems such as thyrotoxicosis, hyperparathyroidism, Cushing's syndrome, epilepsy or treatment of anticonvulsants, antidepressants, treatment of steroid tablets regularly, and estrogen replacement therapy. Compared with FRAX, the advantage of QFracture algorithm applied in the prediction of osteoporotic fracture in diabetics is that it directly includes the presence or absence of diabetes as its calculation variable. However, there are currently no studies specifically evaluating the performance of QFacture prediction of OF in patients with diabetes. Despite the disadvantages including not taking BMD as an input variable, ignoring mortality as a competitive risk, and only calibrated for use in populations in UK, its clinical use is still recognized. The Fremantle Diabetes Study Phase I (FDS1) in 2019 [30] proposed a simple HF risk prediction tool which took QFacture as comparison to evaluate the prediction efficiency of MOF. QFracture demonstrated excellent discrimination, calibration, and accuracy. During 10 years of follow-up, 48 (3.94%) out of the 1219 members of the FDS1 cohort with T2DM had an incident HF, and the predicted risk by the QFracture hip fracture risk equation is 4.06% (49.5 cases).
Garvan FRC was developed based on the Dubbo Osteoporosis Epidemiology Study (DOES) cohort study in Australia in 2007 [31]. Garvan FRC incorporates four clinical risk factors including age, sex, number of previous fractures, and number of recent falls with BMD or weight (when BMD is not available) to estimate 5-year and 10-year risk of OF and HF [32]. Although diabetes is not incorporated in Garvan FRC’s risk prediction algorithm, history of recent falls may substitute for the increased risk of diabetes to a certain extent. A registry-based cohort study was performed in 2022 to estimate the performance of Garvan FRC in patients with diabetes [33]. Individuals aged 50–95 years consisting of 2618 women with and 14,064 without diabetes, and 636 men with and 2201 without diabetes were recruited and their 5-year fracture risk rate was calculated. Results showed that Garvan FRC provided similar fracture risk stratification in individuals with versus without diabetes, however, OF risk in women with diabetes was underestimated. Interestingly, after lowering the femoral neck T-score by 0.3 in women with diabetes for re-calculation, the effect of diabetes on OF and HF provided by Garvan FRC was largely attenuated.
As mentioned above, clinicians use of FRAX, QFracture, and Garvan FRC for estimation of fracture risk in an individual with diabetes should be aware of this limitation.
Performance of AI in predicting osteoporotic fracture
Since the above three tools have limitation in predicting osteoporotic fracture in patients with diabetes, there is an urgent need to develop a convenient and accurate tool to predict the risk of OF in patients with diabetes. In recent years, the rapid development of artificial intelligence has brought a feasible answer.
Artificial intelligence (AI) technology has been increasingly utilized in the field of osteoporosis, primarily through machine learning (ML) methodologies. ML is to apply specific traits to identify patterns that can be used to analyze a particular situation [34]. Algorithms of ML are typically classified into three typical types: supervised learning, unsupervised learning, and semi supervised learning. The typical supervised learning algorithms include linear regression, logistic regression, neural network, decision tree, random forest, support vector machine, least absolute shrinkage and section operator, ensemble learning, and deep learning, etc. Unsupervised learning algorithms include K-means clustering, principal component analysis, support vector domain description, and local outlier factor, etc. Semi supervised learning is an algorithm that combines supervised learning and unsupervised learning but rarely used in the medical field. Supervised learning is frequently used to estimate risk. The area under the receiver operating characteristic curve (AUROC) and precision-recall curve (PRC) are comparatively common patterns to demonstrate the performance of AI algorithms [35]. These powerful algorithms allow for the identification of complex relationships and patterns within large datasets, offering great potential in advancing our understanding and management of osteoporosis.
To assess the current predictive value of AI in estimating osteoporotic fracture risk, a systematic literature search was conducted across multiple electronic databases, including PubMed and Web of Science. Studies published till March 2023 were included. A restriction for English language has been applied. Searches relevant to the use of AI to predict osteoporotic fracture were made using the search terms (“Fracture” OR “Fragility Fracture” OR “Osteoporotic Fracture”) AND (“Artificial Intelligence” OR “Deep Learning” OR “Machine Learning”) AND “Osteoporosis”. Records identified from searches were screened by the Li Zeting and Zhao Wen.
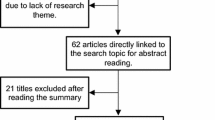
The study selection flow diagram is presented in Fig. 1. 272 literature results were identified by the search stragety.76 records were removed as duplicates. 5 records were removed as introduction of patents or techniques. 33 records were removed as conference articles. 31 records were removed as reviews or comments. 108 records were removed after review of titles, abstracts, and full-texts, among which 16 were irrelevant to the theme, and 92 identified as AI on osteoporosis in other fields, including 2 about applications of AI on omics, 44 about applications of AI on prediction of BMD and screening and diagnosis of osteoporosis, 24 about applications of AI on diagnosis of fracture, 17 about applications of AI on assessment of bone quality or bone microstructure, and 5 about applications of AI on iatrogenic intervention related to osteoporosis and its prognosis. A total of 19 of these records [36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54] concerned with the applications of AI on prediction of fracture risks were considered eligible for inclusion in the current literature review as presented in Table 1.
According to the presented data, most researches (18/19) of application of AI on prediction of fracture risks showed up after 2017, which is related to the vigorous and rapid development of AI algorithm in recent years. Researchers from various localities have gained universally acknowledged achievements in this domain, especially researchers from North America [36, 39, 44, 48, 49, 51], Europe [41,42,43, 45,46,47, 50, 52, 53], and East Asia [37, 38, 40]. As for the application of algorithm patterns, supervised learning [41,42,43,44,45,46,47,48,49,50,51, 53, 54] is the most common pattern of risk prediction algorithm, and there are also reports on the application of unsupervised [36, 52] learning methods to define high-risk groups of OFs. Most of the existing studies are based on the existing cohort researches. In the field of fracture risk prediction, the application of AI is mainly divided into three types, among which the most common type is to improve the efficiency of fracture risk prediction by to establishing a new method or by applying the existing AI algorithm to the field of fracture risk prediction [38,39,40, 42,43,44, 46,47,48,49, 53, 54]. The following is to improve the prediction efficiency of the original ML prediction model or traditional prediction method by incorporating innovative imaging data or clinical characteristics [36, 37, 39, 41, 45, 50, 51]. Last but not least, to define the high-risk group [36, 52] through ML algorithm, and then calculate its fracture prediction risk.
The prediction efficiency of AI fracture prediction model is the most concerned in clinic. As shown in Table 1, most ML model excellent prediction efficiency, with AUC range from 0.74 to 0.99. Kruse et al. [53] made the greatest efforts in the application of the ML algorithm, that 24 ML algorithms were applied in their research to predict 5-year hip fracture and 10-year hip fracture for a population of 4722 women and 717 men, including Classification Tree, Bootstrap aggregated trees, Bayesian Generalized Linear Model, Partial Least Squares, k-Nearest Neighbours (kNN), Boosted Logistic Regression, Boosted Generalized Additive Model, High Dimensional Discriminant Analysis, Random Forest (RF), Conditional Inference Tree, Logistic Model Trees, Stochastic Gradient Boostin, Quadratic Discriminant Analysis, Linear Discriminant Analysis, Bagged Flexible Discriminant Analysis (BFDA), Bagged Multivariate Adaptive Regression Splines, Nearest Shrunken Centroids, Support Vector Machines with Radial Weights, Neural Network (NN), Neural Network with Feature Extraction, eXtreme Gradient Boosting (XGB), Conditional Inference Random Forest and Adaptive Boosting. Predictive performance and calibrated probabilities were good as AUC > 0.9 in ML algorithms such as XGB, RF, and BFDA, and the best performance is the BFDA in the female group with an AUC value of 0.91. These results surpassed the most widely used modified FRAX tools performance achieved by Lundin et al. (AUC = 0.73 [0.64;0.81]) [55]and that in the Spanish FRIDEX cohort (AUC = 0.88 [0.82; 0.95]) [56].
Adding new parameters to the existing ML model may obtain potentially beneficial effects. Dong et al. [39] modified GoogLeNet, a neural network algorithm, to 4461 subjects and 15,524 spine radiographs for osteoporotic compression fractures by combing radiographs Genant semiquantitative system, then achieved an AUC-ROC of 0.99 and an area under the precision-recall curve (AUC-PR) of 0.82, respectively. Meanwhile, a sensitivity of 59.8%, a specificity of 99.9%, a positive predictive value (PPV) of 91.2%, an F1 score of 0.72, and an accuracy of 99.5% were yielded.
In addition to improve the prediction efficiency, to identify the characteristics of high fracture risk population in the early stage is also of great clinical significance. Whittier et al. [36] classified a multinational cohort (n = 5873), into three phenotypes by fuzzy c-means clustering, an unsupervised ML method, with high-resolution peripheral quantitative computed tomography (HR-pQCT) data. These phenotypes were identified by their anatomically different characteristics in bone microarchitecture, and associated with stratified risk of osteoporotic fracture. Furthermore, within each phenotype, unique bone imaging biomarkers were associated with within-phenotype fracture risk. This fracture prediction model that predicted fracture risk through unsupervised learning method combined with imaging examination rather than other cumbersome laboratory examination results or clinical characteristics facilitated clinical workflow.
A growing body of research has compared the efficacy of various ML methods in fracture risk prediction. In general, newer ML techniques, such as random forest (RF) and neural networks (NN), have demonstrated superior performance compared to traditional approaches like logistic regression (LR). Furthermore, it is expected that deep learning (DL) methods will yield even better results in this domain. For instance, Wu et al. [44] established a prediction model of male osteoporosis fracture (n = 5130) with genetic risk score, BMD, and other risk factors as predictors by using ML methods such as RF, NN, gradient boosting, and LR. Unsurprisingly, performance of LR was significantly worse than the more advanced techniques of of RF, NN, gradient boosting. However, in contrast to previous studies, recent research has shown that traditional AI methods may still hold promise for fracture risk prediction. For example, when de Vries et al. [43] developed Cox regression, random survival forests (RSF) and an artificial neural network (ANN)-DeepSurv model to predict the risk of a future MOF, Cox regression outperformed RSF (p = 0.043 and p = 0.023) and ANN-DeepSurv (p = 0.043) with a C-index of 0.625 (0.562–0.689), pulling back a game for traditional AI methods These results suggest that the adage "new is good" may not always hold true in the field of AI, and reinforce the importance of rigorous comparative studies to identify the most effective methods for specific clinical applications.
In general, the development of AI algorithm has led the prediction of osteoporotic fracture into a new aera. Compared to traditional predictive tools, AI algorithms have achieved superior efficiency in fracture risk assessment. However, among the various types of AI algorithms currently available, no single algorithm has demonstrated consistently outstanding predictive performance. Additionally, issues related to universality and practicality still need to be addressed, highlighting the need for continued improvement in this field. To achieve optimal results, it is necessary to develop specific AI algorithms tailored to the needs of distinct populations, such as diabetic patients, which would enable the creation of specialized predictive tools for these high-risk groups.
Efficacy of AI in predicting osteoporotic fracture in diabetes population
As previously discussed, conventional fracture risk prediction tools have shown limited efficacy when applied to osteoporosis patients with diabetes. Now what about the performance of AI algorithm? 20 literature results were identified in PubMed using the search terms ("Fracture"[All Fields] OR "Fragility Fracture"[All Fields] OR "Osteoporotic Fracture"[All Fields]) AND ("Artificial Intelligence"[All Fields] OR "Deep Learning"[All Fields] OR "Machine Learning"[All Fields]) AND ("Diabetes"[All Fields] OR "Hyperglycemia"[All Fields] OR "Abnormal glucose metabolism"[All Fields]), and 23 literature results were identified in Web of Science with search terms (((TS = (Artificial Intelligence OR Deep Learning OR Machine Learning)) AND TS = (Fracture OR Fragility Fracture OR Osteoporotic Fracture)) AND TS = (Diabetes OR Hyperglycemia OR Abnormal glucose metabolism)). However, only 3 records were accurately related to application of AI on fracture risk prediction in diabetes patients after review of titles and abstracts.
In 2022, Chen et al. [40] developed a hybrid model combining XGBoost with deep neural network (DNN) to predict the fracture risk of patients using data of 14,419 diabetes patients. Various machine learning methods were used simultaneously in this study, including LR, RF, kNN, Support Vector Machine (SVM), Decision Tree (DT), Extremely Randomized Trees (ERT), Gradient Boosting Decision Tree (GBDT), AdaBoost, CatBoost, XGBoost, and multilayer perceptron (MLP, a DNN derived algorism). Accuracy of the ML methods ranged from 67.76% to 86.08%, precision ranged from 70.41% to 87.69%. LR presented the worst performance (accuracy of 67.76% and precision of 70.41%), while XGboost (accuracy of 86.08% and precision of 87.69%) and MLP (accuracy of 82.78% and precision of 84.18%). Furthermore, the authors combined the best two methods to develop a new one and achieved the best performance (accuracy of 90.38%, precision of 90.52%, and AUC of approximately 0.90). Under the premise that the fracture prediction ability of traditional tools in diabetes patients is greatly reduced, it is encouraging that AI shows such excellent prediction efficiency. In the following year, two exciting studies emerged. Chu et al. [57] developed Probabilistic Classification Vector Machines (PCVM) algorithm to construct risk prediction models for fractures apart from the 6 algorithms LR, SVM, RF, DT, GBDT, XGBoost, which were conducted in the research above. What is delightful is that PCVM achieved the best f1 scores (0.97), surpassing LR (0.75), SVM (0.83), RF (0.84), DT (0.85), GBDT (0.87), XGBoost (0.88). Research of Chen et al. [40] determined 18 influencing factors of fracture risks of patients with diabetes while Chu et al. [57] determined 17 influencing factors, these influencing factors were easy to obtain and do not require precise inspection. To predict the risk of hip fractures in a more accurate way, Yosibash et al. [58] developed a ML algorithm with autonomous finite element analyses (AFE) based on CT scans for hip fracture risk assessment in type 2 diabetic mellitus (T2DM). The research results showed a sensitivity of 92% and specificity of 88% (cross-validation area under the curve [AUC] 0.92) among T2DM patients, indicating that AL algorithm has the potential to showcase more advantages in the accuracy of fracture risk assessment for diabetes patient combining with the advancement of imaging technique.
Current evidences suggest that Denosumab, Ibandronate, and Teriparatide are considered the most successful drugs for postmenopausal osteoporosis-related fragility fractures [59, 60]. Risedronate, alendronate, zoledronate, denosumab, or etidronate have also shown good efficacy in preventing fractures in corticosteroid-induced osteoporosis (CIO) [61]. The effectiveness of osteoporosis treatment is believed to be related to factors such as polymorphisms of the vitamin D receptor (VDR) [62], and biochemical markers of bone turnover (BTMs), such as the bone alkaline phosphatase (bALP), procollagen type I N propeptide (PINP), serum cross-linked C-telopeptides of type I collagen (bCTx), and urinary cross-linked N-telopeptides of type I collagen (NTx) [63, 64]. However, there is a lack of large-scale clinical studies on osteoporosis patients with diabetes, which is related to the lack of prediction methods for osteoporotic fractures in patients with osteoporosis and diabetes, which leads to the lack of awareness of this aspect. Although research in this field is still limited, it is noteworthy that various machine learning methods were employed in these researches, leveraging large dataset for training purposes. As such, these findings lend credibility to the potential utility of AI-driven approaches for improving risk prediction in diabetic patients. Nevertheless, further research is warranted to validate and extend these results, as well as to explore the broader applicability of AI in addressing clinical challenges related to osteoporosis management in high-risk populations.
Conclusion
In the domain of osteoporotic fracture prediction, there remains a substantial need for improved performance of traditional tools such as FRAX, QFracture, and Garvan FRC when applied to patients with diabetes. Under such conditions, AI algorithm holds a bright future in enhancing the accuracy of fracture risk prediction in osteoporosis patients with diabetes. Notably, advanced AI techniques are rapidly evolving, while conventional methods such as linear regression continue to demonstrate utility. However, the limitations of AI methods comprising the demand of large amount of training data, inconvenience for clinical application, and unpredictable universality of the model. To address these challenges, it is urgent to develop new machine learning models using large, real-world data sets from patients with diabetes and osteoporosis exhibiting regional or national characteristics. By leveraging these approaches, it may be possible to establish a novel, widely accepted osteoporotic fracture prediction tool that can better serve the needs of high-risk patient populations.
References
Consensus development conference: diagnosis, prophylaxis, and treatment of osteoporosis. Am J Med. 1993;94(6):646–50.
Kanis JA, McCloskey EV, Johansson H, Oden A, Melton LJ 3rd, Khaltaev N. A reference standard for the description of osteoporosis. Bone. 2008;42(3):467–75.
Xiao PL, Cui AY, Hsu CJ, Peng R, Jiang N, Xu XH, Ma YG, Liu D, Lu HD. Global, regional prevalence, and risk factors of osteoporosis according to the World Health Organization diagnostic criteria: a systematic review and meta-analysis. Osteoporos Int. 2022;6:66.
Johnell O, Kanis JA. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int. 2006;17(12):1726–33.
Gourlay ML, Brown SA. Clinical considerations in premenopausal osteoporosis. Arch Intern Med. 2004;164(6):603–14.
Association BO. The care of patients with fragility fracture (Blue Book); 2009.
Si L, Winzenberg TM, Jiang Q, Chen M, Palmer AJ. Projection of osteoporosis-related fractures and costs in China: 2010–2050. Osteoporos Int. 2015;26(7):1929–37.
Barnsley J, Buckland G, Chan PE, Ong A, Ramos AS, Baxter M, Laskou F, Dennison EM, Cooper C, Patel HP. Pathophysiology and treatment of osteoporosis: challenges for clinical practice in older people. Aging Clin Exp Res. 2021;33(4):759–73.
Migliorini F, Giorgino R, Hildebrand F, Spiezia F, Peretti GM, Alessandri-Bonetti M, Eschweiler J, Maffulli N. Fragility fractures: risk factors and management in the elderly. Medicina. 2021;57(10):66.
Leidig-Bruckner G, Grobholz S, Bruckner T, Scheidt-Nave C, Nawroth P, Schneider JG. Prevalence and determinants of osteoporosis in patients with type 1 and type 2 diabetes mellitus. BMC Endocr Disord. 2014;14:33.
Khosla S, Samakkarnthai P, Monroe DG, Farr JN. Update on the pathogenesis and treatment of skeletal fragility in type 2 diabetes mellitus. Nat Rev Endocrinol. 2021;17(11):685–97.
Agarwal A, Leslie WD. Fracture prediction tools in diabetes. Curr Opin Endocrinol Diabetes Obes. 2022;29(4):326–32.
Janghorbani M, Van Dam RM, Willett WC, Hu FB. Systematic review of type 1 and type 2 diabetes mellitus and risk of fracture. Am J Epidemiol. 2007;166(5):495–505.
Palui R, Pramanik S, Mondal S, Ray S. Critical review of bone health, fracture risk and management of bone fragility in diabetes mellitus. World J Diabetes. 2021;12(6):706–29.
Jia P, Bao L, Chen H, Yuan J, Liu W, Feng F, Li J, Tang H. Risk of low-energy fracture in type 2 diabetes patients: a meta-analysis of observational studies. Osteoporos Int. 2017;28(11):3113–21.
Chen P, Li Z, Hu Y. Prevalence of osteoporosis in China: a meta-analysis and systematic review. BMC Public Health. 2016;16(1):1039.
Ma L, Oei L, Jiang L, Estrada K, Chen H, Wang Z, Yu Q, Zillikens MC, Gao X, Rivadeneira F. Association between bone mineral density and type 2 diabetes mellitus: a meta-analysis of observational studies. Eur J Epidemiol. 2012;27(5):319–32.
Ho-Pham LT, Nguyen TV. Association between trabecular bone score and type 2 diabetes: a quantitative update of evidence. Osteoporos Int. 2019;30(10):2079–85.
Patsch JM, Burghardt AJ, Yap SP, Baum T, Schwartz AV, Joseph GB, Link TM. Increased cortical porosity in type 2 diabetic postmenopausal women with fragility fractures. J Bone Miner Res. 2013;28(2):313–24.
Kalaitzoglou E, Fowlkes JL, Popescu I, Thrailkill KM. Diabetes pharmacotherapy and effects on the musculoskeletal system. Diabetes Metab Res Rev. 2019;35(2): e3100.
Kanis JA, Johansson H, Harvey NC, McCloskey EV. A brief history of FRAX. Arch Osteoporos. 2018;13(1):118.
Hippisley-Cox J, Coupland C. Predicting risk of osteoporotic fracture in men and women in England and Wales: prospective derivation and validation of QFractureScores. BMJ. 2009;339: b4229.
Sandhu SK, Nguyen ND, Center JR, Pocock NA, Eisman JA, Nguyen TV. Prognosis of fracture: evaluation of predictive accuracy of the FRAX algorithm and Garvan nomogram. Osteoporos Int. 2010;21(5):863–71.
El Miedany Y. FRAX: re-adjust or re-think. Arch Osteoporos. 2020;15(1):150.
Champakanath A, Keshawarz A, Pyle L, Snell-Bergeon JK, Shah VN. Fracture risk assessment (FRAX) without BMD and risk of major osteoporotic fractures in adults with type 1 diabetes. Bone. 2021;143: 115614.
Schwartz AV, Vittinghoff E, Bauer DC, Hillier TA, Strotmeyer ES, Ensrud KE, Donaldson MG, Cauley JA, Harris TB, Koster A, et al. Association of BMD and FRAX score with risk of fracture in older adults with type 2 diabetes. JAMA. 2011;305(21):2184–92.
Giangregorio LM, Leslie WD, Lix LM, Johansson H, Oden A, McCloskey E, Kanis JA. FRAX underestimates fracture risk in patients with diabetes. J Bone Miner Res. 2012;27(2):301–8.
Majumdar SR, Leslie WD, Lix LM, Morin SN, Johansson H, Oden A, McCloskey EV, Kanis JA. Longer duration of diabetes strongly impacts fracture risk assessment: the Manitoba BMD Cohort. J Clin Endocrinol Metab. 2016;101(11):4489–96.
Leslie WD, Johansson H, McCloskey EV, Harvey NC, Kanis JA, Hans D. Comparison of methods for improving fracture risk assessment in diabetes: the Manitoba BMD registry. J Bone Mineral Res. 2018;33(11):1923–30.
Davis WA, Hamilton EJ, Bruce DG, Davis TME. Development and validation of a simple hip fracture risk prediction tool for type 2 diabetes: the Fremantle Diabetes Study Phase I. Diabet Care. 2019;42(1):102–9.
Nguyen ND, Frost SA, Center JR, Eisman JA, Nguyen TV. Development of a nomogram for individualizing hip fracture risk in men and women. Osteoporos Int. 2007;18(8):1109–17.
Nguyen ND, Frost SA, Center JR, Eisman JA, Nguyen TV. Development of prognostic nomograms for individualizing 5-year and 10-year fracture risks. Osteoporos Int. 2008;19(10):1431–44.
Agarwal A, Leslie WD, Nguyen TV, Morin SN, Lix LM, Eisman JA. Performance of the garvan fracture risk calculator in individuals with diabetes: a registry-based cohort study. Calcif Tissue Int. 2022;110(6):658–65.
Kaul V, Enslin S, Gross SA. History of artificial intelligence in medicine. Gastrointest Endosc. 2020;92(4):807–12.
Smets J, Shevroja E, Hügle T, Leslie WD, Hans D. Machine learning solutions for osteoporosis-a review. J Bone Miner Res. 2021;36(5):833–51.
Whittier DE, Samelson EJ, Hannan MT, Burt LA, Hanley DA, Biver E, Szulc P, Sornay-Rendu E, Merle B, Chapurlat R, et al. Bone microarchitecture phenotypes identified in older adults are associated with different levels of osteoporotic fracture risk. J Bone Miner Res. 2022;37(3):428–39.
Shimizu H, Enda K, Shimizu T, Ishida Y, Ishizu H, Ise K, Tanaka S, Iwasaki N. Machine learning algorithms: prediction and feature selection for clinical refracture after surgically treated fragility fracture. J Clin Med. 2022;11(7):66.
Kong SH, Lee JW, Bae BU, Sung JK, Jung KH, Kim JH, Shin CS. Development of a spine X-ray-based fracture prediction model using a deep learning algorithm. Endocrinol Metab. 2022;6:66.
Dong Q, Luo G, Lane NE, Lui LY, Marshall LM, Kado DM, Cawthon P, Perry J, Johnston SK, Haynor D, et al. Deep learning classification of spinal osteoporotic compression fractures on radiographs using an adaptation of the genant semiquantitative criteria. Acad Radiol. 2022;6:66.
Chen Y, Yang T, Gao X, Xu A. Hybrid deep learning model for risk prediction of fracture in patients with diabetes and osteoporosis. Front Med. 2022;16(3):496–506.
Ulivieri FM, Rinaudo L, Piodi LP, Messina C, Sconfienza LM, Sardanelli F, Guglielmi G, Grossi E. Bone strain index as a predictor of further vertebral fracture in osteoporotic women: an artificial intelligence-based analysis. PLoS ONE. 2021;16(2): e0245967.
Nissinen T, Suoranta S, Saavalainen T, Sund R, Hurskainen O, Rikkonen T, Kröger H, Lähivaara T, Väänänen SP. Detecting pathological features and predicting fracture risk from dual-energy X-ray absorptiometry images using deep learning. Bone Rep. 2021;14: 101070.
de Vries BCS, Hegeman JH, Nijmeijer W, Geerdink J, Seifert C, Groothuis-Oudshoorn CGM. Comparing three machine learning approaches to design a risk assessment tool for future fractures: predicting a subsequent major osteoporotic fracture in fracture patients with osteopenia and osteoporosis. Osteoporos Int. 2021;32(3):437–49.
Wu Q, Nasoz F, Jung J, Bhattarai B, Han MV. Machine learning approaches for fracture risk assessment: a comparative analysis of genomic and phenotypic data in 5130 older men. Calcif Tissue Int. 2020;107(4):353–61.
Villamor E, Monserrat C, Del Rio L, Romero-Martin JA, Ruperez MJ. Prediction of osteoporotic hip fracture in postmenopausal women through patient-specific FE analyses and machine learning. Comput Methods Programs Biomed. 2020;193:66.
Galassi A, Martín-Guerrero JD, Villamor E, Monserrat C, Rupérez MJ. Risk assessment of hip fracture based on machine learning. Appl Bionics Biomech. 2020;2020:8880786.
Engels A, Reber KC, Lindlbauer I, Rapp K, Buechele G, Klenk J, Meid A, Becker C, Koenig H-H. Osteoporotic hip fracture prediction from risk factors available in administrative claims data—a machine learning approach. PLoS One. 2020;15(5):66.
Almog YA, Rai A, Zhang P, Moulaison A, Powell R, Mishra A, Weinberg K, Hamilton C, Oates M, McCloskey E, et al. Deep learning with electronic health records for short-term fracture risk identification: crystal bone algorithm development and validation. J Med Internet Res. 2020;22(10): e22550.
Su Y, Kwok TCY, Cummings SR, Yip BHK, Cawthon PM. Can classification and regression tree analysis help identify clinically meaningful risk groups for hip fracture prediction in older American men (The MrOS Cohort Study)? JBMR Plus. 2019;3(10): e10207.
Muehlematter UJ, Mannil M, Becker AS, Vokinger KN, Finkenstaedt T, Osterhoff G, Fischer MA, Guggenberger R. Vertebral body insufficiency fractures: detection of vertebrae at risk on standard CT images using texture analysis and machine learning. Eur Radiol. 2019;29(5):2207–17.
Ferizi U, Besser H, Hysi P, Jacobs J, Rajapakse CS, Chen C, Saha PK, Honig S, Chang G. Artificial intelligence applied to osteoporosis: a performance comparison of machine learning algorithms in predicting fragility fractures from MRI data. J Magn Resonan Imaging. 2019;49(4):1029–38.
Kruse C, Eiken P, Vestergaard P. Clinical fracture risk evaluated by hierarchical agglomerative clustering. Osteoporos Int. 2017;28(3):819–32.
Kruse C, Eiken P, Vestergaard P. Machine learning principles can improve hip fracture prediction. Calcif Tissue Int. 2017;100(4):348–60.
Schuler B, Fritscher KD, Kuhn V, Eckstein F, Link TM, Schubert R. Assessment of the individual fracture risk of the proximal femur by using statistical appearance models. Med Phys. 2010;37(6):2560–71.
Lundin H, Sääf M, Strender LE, Nyren S, Johansson SE, Salminen H. Gait speed and one-leg standing time each add to the predictive ability of FRAX. Osteoporos Int. 2017;28(1):179–87.
Azagra R, Roca G, Encabo G, Aguyé A, Zwart M, Güell S, Puchol N, Gene E, Casado E, Sancho P, et al. FRAX® tool, the WHO algorithm to predict osteoporotic fractures: the first analysis of its discriminative and predictive ability in the Spanish FRIDEX cohort. BMC Musculoskelet Disord. 2012;13:204.
Chu S, Jiang A, Chen L, Zhang X, Shen X, Zhou W, Ye S, Chen C, Zhang S, Zhang L, et al. Machine learning algorithms for predicting the risk of fracture in patients with diabetes in China. Heliyon. 2023;9(7): e18186.
Yosibash Z, Trabelsi N, Buchnik I, Myers KW, Salai M, Eshed I, Barash Y, Klang E, Tripto-Shkolnik L. Hip fracture risk assessment in elderly and diabetic patients: combining autonomous finite element analysis and machine learning. J Bone Miner Res. 2023;38(6):876–86.
Migliorini F, Colarossi G, Baroncini A, Eschweiler J, Tingart M, Maffulli N. Pharmacological management of postmenopausal osteoporosis: a Level I evidence based—expert opinion. Expert Rev Clin Pharmacol. 2021;14(1):105–19.
Migliorini F, Maffulli N, Colarossi G, Eschweiler J, Tingart M, Betsch M. Effect of drugs on bone mineral density in postmenopausal osteoporosis: a Bayesian network meta-analysis. J Orthop Surg Res. 2021;16(1):533.
Migliorini F, Colarossi G, Eschweiler J, Oliva F, Driessen A, Maffulli N. Antiresorptive treatments for corticosteroid-induced osteoporosis: a Bayesian network meta-analysis. Br Med Bull. 2022;143(1):46–56.
Conti V, Russomanno G, Corbi G, Toro G, Simeon V, Filippelli W, Ferrara N, Grimaldi M, D’Argenio V, Maffulli N, et al. A polymorphism at the translation start site of the vitamin D receptor gene is associated with the response to anti-osteoporotic therapy in postmenopausal women from southern Italy. Int J Mol Sci. 2015;16(3):5452–66.
Migliorini F, Maffulli N, Spiezia F, Tingart M, Maria PG, Riccardo G. Biomarkers as therapy monitoring for postmenopausal osteoporosis: a systematic review. J Orthop Surg Res. 2021;16(1):318.
Migliorini F, Maffulli N, Spiezia F, Peretti GM, Tingart M, Giorgino R. Potential of biomarkers during pharmacological therapy setting for postmenopausal osteoporosis: a systematic review. J Orthop Surg Res. 2021;16(1):351.
Funding
Sanming Project of Medicine in Shenzen Municipality, China (SZSM202011007).
Author information
Authors and Affiliations
Contributions
ZTL and WZ completed literature search and review writing, FPL and XHL conceived the research and revised the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Li, Z., Zhao, W., Lin, X. et al. AI algorithms for accurate prediction of osteoporotic fractures in patients with diabetes: an up-to-date review. J Orthop Surg Res 18, 956 (2023). https://doi.org/10.1186/s13018-023-04446-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13018-023-04446-5