Abstract
Objective
Recent studies have shown that the mouth–gut–disc axis may play a key role in the process of disc structural failures (including intervertebral disc degeneration (IDD) and endplate change) in the cervical spine and neck pain. However, the potential mechanisms underlying the mouth–gut–disc axis remain elusive. Therefore, we explored whether periodontal disease is associated with disc structural failures in patients with cervical degeneration disorders and clinical outcomes.
Methods
Adults (aged > 18 years) who met open surgery criteria for cervical spine were enrolled in this prospective cohort study. Participants were allocated into two groups based on periodontal examinations before surgery: no/mild periodontitis group and moderate/severe periodontitis group. Data were evaluated using an independent t test and Pearson’s correlation analysis.
Results
A total of 108 patients were enrolled, including 68 patients in the no/mild periodontitis group and 40 patients in the moderate/severe periodontitis group. The number of common causes of missing teeth (P = 0.005), plaque index (PLI) (P = 0.003), bleeding index (BI) (P = 0.000), and probing depth (PD) (P = 0.000) significantly differed between the two groups. The incidence rate of endplate change (P = 0.005) was higher in the moderate/severe periodontitis group than in the no/mild periodontitis group. A moderate negative association was found between the neck disability index (NDI) score and periodontal parameters (PLI: r = − 0.337, P = 0.013; BI: r = − 0.426, P = 0.001; PD: r = − 0.346, r = − 0.010).
Conclusions
This is the first study to provide evidence that severe periodontitis is associated with a higher occurrence rate of disc structural failures and poor clinical outcomes in patients with cervical degenerative disorders.
Similar content being viewed by others
Introduction
Chronic neck pain is a significant public health problem, leading to disability and productivity loss worldwide [1, 2], especially in patients with cervical degenerative disorders. Disc structural failures such as intervertebral disc degeneration (IDD) and endplate change are regarded as the most common contributors to chronic neck pain [3,4,5]. Radiographic changes have been reported as the main criteria for evaluating disc structural failures, including scoring systems for IDD and endplate change developed by Pfirrmann et al. [5] and Modic et al. [6], respectively.
Inflammation is thought to be one of the main triggers for developing disc structural failure. Other potential factors for triggering disc structural failure include natural history, aging, smoking, mechanical and genetic factors, obesity, and inadequate metabolite transport. Growing evidence supports that infection of the intervertebral disc by low-virulence organisms may be crucial to the inflammatory response [7]. Our previous review reported a significant relationship between low-virulence organisms in IDD and endplate changes [7]. A further review demonstrated that the mouth–gut–disc axis (such as microbiome oral dysbiosis, gastrointestinal system, and intervertebral disc (IVD)) was potentially correlated with disc structural failure and spinal pain [8]. However, this hypothesis remains unclear.
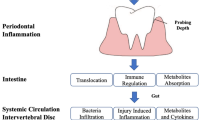
Diverse microbial communities inhabit the oral cavity, which contains about 50 species with a subset of 1,000 species [9, 10]. The dynamic and polymicrobial oral microbiome is considered one of the direct precursors to the development of periodontal health deterioration, such as periodontitis [11]. Periodontitis is thought to be driven by a feedforward loop between the microbiota and host factor, which favors the emergence and persistence of dysbiosis [12, 13]. Intriguingly, alterations in the microbiome composition in the periodontium are associated with regulating inflammation in many other diseases, like cardiovascular diseases, rheumatoid arthritis, diabetes, cancer, and chronic respiratory diseases [14,15,16]. Alterations in the oral microbiota promote systemic inflammation, which is thought to be the reason for the development of a metabolic syndrome in patients with periodontal diseases [17, 18]. Furthermore, previous studies have reported the detection of low-virulence organisms in degenerated cervical IVD tissue, which might play an important role in the disc structural failure process for stimulating inflammation [17, 18]. Three potential mechanisms by which the gut microbiota can induce disc structural failures are (1) bacteria infiltration across the gut epithelial barrier, (2) activation of the inflammation, and (3) modification of metabolites and cytokines absorption [8]. Taken together, these factors show the potential cascade link between the mouth–gut–disc axis and the development of disc structural failures. Oral dysbiosis may cause periodontal disease and hasten the occurrence of systematic inflammation, resulting in disc structural failures. However, the potential mechanisms underlying the mouth–gut–disc axis (Fig. 1) remain elusive. Therefore, investigating the relationship between periodontal disease and the occurrence of IDD and endplate changes in the cervical spine is of great significance.
Potential mechanisms for the mouth–gut–disc axis. Oral microorganisms are the main cause of the occurrence of periodontitis. In the meanwhile, alterations in the microbiome composition in the periodontium are causing the modulation of gut microbiota translocation and composition, increasing intestinal permeability and inflammation and modifying metabolite absorption. Microorganisms, related inflammatory cytokines, and metabolites are the potential essential mechanisms for leading intervertebral disc degeneration and endplate changes
The present prospective study investigated the relationships among periodontal disease, IDD, endplate changes, and clinical outcomes in patients with cervical degenerative disorders.
Materials and methods
Study design
This prospective cohort study was approved by the Medical Research Ethics Committee of our institution. All participants agree for their demographic, clinical, and radiological data to be used in this study.
Participant selection
Participants aged 18 years or old adults who met the criteria to undergo open cervical spine surgery were enrolled between July 2019 and December 2021. A periodic oral evaluation was performed before surgery at our institution.
Participants with a history of spinal deformity, trauma, infection, and tumor in the cervical spine; prior cervical spine surgery; and diagnosed with systemic metabolic, severe organic, and systematic diseases were excluded from this study.
Demographic data
Patients’ age, gender, height, weight, body mass index (BMI), duration of neck pain, positive signs, and final diagnosis were obtained during the admission period by surgeons.
Periodontal examination
Periodontal parameters were recorded during the basic periodontal examination by stomatologists (DX and YZ), including the history of periodontal treatment, periodontal maintenance, history of mouthwash, the number of loose teeth, the number of remaining teeth, common causes of missing teeth, plaque index (PLI), gingival recession (GR), bleeding index (BI), and probing depth (PD, measured from the gingival margin to the bottom of the pocket in six sites, including buccal, mesiobuccal, distobuccal, lingual, distolingual, and mesiolingual, except for the third molars) [19, 20]. All participants were classified into mild, moderate, and severe periodontitis following the Center for Disease Control and Prevention in partnership with the American Academy of Periodontology (CDC-AAP) criteria (Table 1) [21]. A combination of none and mild periodontitis and moderate and severe periodontitis was referred to as the no/mild group and the moderate/severe periodontitis group in this study.
Evaluation of clinical outcomes
Three questionnaires were evaluated before the surgery once written consent was obtained from the participants. The questionnaires included the Visual Analogue Scale (VAS) for evaluating neck pain and/or radicular pain, the neck disability index (NDI) for assessing function and disability, and the Japanese Orthopedic Association score (JOA) for the assessment of cervical myelopathy.
Evaluation of radiological outcomes
Participants’ magnetic resonance images, consisting of sagittal T1-weight fast spin-echo (FSE), sagittal T2-weighted FSE, and axial T2-weighted FSE, were obtained using 3.0 T Trio Tim scanner (Siemens, Germany). The middle slice of T2-weighted FSE images was selected for the measurement. To reduce the potential bias, the same Apple MacBook laptop and DICOM Viewer software (Philips, Netherlands) were used for the measurement.
The Pfirrmann grade was used to assess the degeneration of IVD [5, 22]. IDD was defined as Pfirrmann grade ≥ III [5, 22]. Based on IDD classification, all the participants were allocated into the non-IDD group (-, Pfirrmann grade < III) and the IDD group (+ , Pfirrmann grade ≥ III). For the endplate changes, participants were divided into the non-endplate change group (type 0) and the endplate change group (type I, II, and III) using Modic classification [23, 24].
Cervical lordosis, range of motion (ROM), and the anterior, middle, and posterior disc height of each cervical IVD level were measured using cervical spine X-rays (such as lateral, flexion, and extension images).
Statistical Analysis
Continuous and dichotomous data were expressed as mean ± standard deviation (SD) and number with percentage, respectively. One-way analysis of variance (ANOVA), independent samples t test, and Chi-square test were used to analyze differences in features and clinical scores between no/mild and moderate/severe periodontitis groups. The normality of continuous variables was assessed. Pearson’s correlation analysis was performed to evaluate the relationship between periodontal parameters and radiological and clinical outcomes. The correlation was ranked as very strong, strong, moderate, and weak based on the following values: > 0.7, 0.5–0.7, 0.3–0.5, and < 0.3, respectively. Intra-class correlation coefficient (ICC) and their 95% confidence intervals (95% CI) were used to evaluate intra- and inter-rater reliability [25]. SPSS v24.0 (SPSS Inc., USA) was used for statistical analysis. A P value of < 0.05 was considered statistically significant.
Results
Participants characteristics
A total of 108 consecutive patients with cervical degenerative disorders (56 males and 52 females) who underwent an anterior cervical discectomy and fusion surgery from July 2019 to December 2021 at our institution were enrolled. Of these, 68 subjects were allocated into the no/mild periodontitis group and the remaining 40 subjects were allocated into the moderate/severe periodontitis group. The mean of age, BMI, and duration of pain was 42.52 years (range, 35–63 years), 23.85 kg/m2, and 54.88 weeks (range, 360–20 weeks), respectively. Patients were divided based on the Pfirrmann grading system, with 38 patients in the IDD group and 70 in the non-IDD group. The mean preoperative scores of VAS neck pain, JOA, and NDI were 4.87 ± 1.08, 12.30 ± 1.77, and 25.43 ± 6.97, respectively.
Comparison of demographic, periodontal, clinical, and radiological data between the two periodontitis groups
No significant differences were found between no/mild and moderate/severe periodontitis groups regarding age, gender, and BMI (Table 2). The number of common causes of missing teeth (P = 0.005), PLI (P = 0.003), BI (P < 0.001), and PD (P < 0.001) significantly differed between the two groups (Table 2). No significant difference was observed between the two groups in terms of the number of periodontal treatment histories, periodontal maintenance, mouthwash histories, and remaining teeth and GR.
The preoperative NDI was significantly lower in the no/mild periodontitis group than in the moderate/severe periodontitis group (P = 0.004). No significant differences were found between the two groups regarding VAS neck pain, JOA, and the duration of pain (Table 3).
The incidence rate of cervical endplate changes was higher in the moderate/severe periodontitis group than in the no/mild periodontitis group (P = 0.005). No significant differences were found between the two groups in terms of the incidence rates of IDD, cervical lordosis, ROM, and disc height of each cervical IVD level (Table 3).
Relationship between periodontal and radiological data
No significant correlation was found between periodontal data (including PLI, GR, BI, and PD) and radiological data (including cervical lordosis, ROM, and disc height of each cervical IVD level) (Table 4).
Relationship between periodontal data and clinical outcomes
A moderate negative association was found between the NDI score and periodontal parameters (PLI: r = − 0.337, P = 0.013; BI: r = − 0.426, P = 0.001; PD: r = − 0.346, r = − 0.010), but no significant differences were observed in terms VAS neck pain and JOA scores. The GR and number of remaining teeth were unrelated to preoperative clinical outcomes, such as VAS neck pain, JOA, and NDI scores (Table 4).
Inter-rater reliability
The inter-rater reliability for periodontal data (including GR: 0.811 (0.802, 0.855) and PD: 0.824 (0.811, 0.868)) and radiological data (including ROM: 0.832 (0.812, 0.867), disc height: 0.822 (0.801, 0.878), and cervical lordosis: 0.835 (0.821, 0.877)) ranged from good to excellent.
Discussion
This study sought to determine the relationships between the severity of periodontitis and disc structural failures in adults with cervical degenerative disorders. There are two major findings. First, individuals with severe periodontitis had a higher incidence rate of endplate changes. Second, some specific parameters in periodontitis were negatively associated with cervical disability scores. These findings provide a better understanding of the potential mechanisms underlying the mouth–gut–disc axis in the cervical spine.
Association between microbial colonization and IDD in the cervical spine
The human microbiome plays a key role in regulating health and diseases. 16S ribosomal RNA sequencing as the most widely used polymerase chain reaction-dependent technique can be used to analyze the microbial diversity and characterize microbiota from IDD patients. A previous review strongly supports the changes in the microbial composition and metabolites, which might emerge as an important player in regulating and managing spinal pain in patients with IDD [7, 8]. Dysbiosis is defined as the imbalance in the gut microbial community, which interrupts the diversity of different microorganisms by regulating the number of bacterial communities. Some studies provided direct evidence supporting the existence of microbial colonization in patients with IDD of the cervical spine and/or neck pain [26,27,28,29]. Therefore, it is interesting to investigate the associations between low-virulence organisms and characteristics of IVDs and endplates in the cervical spine.
Association between microbial colonization and periodontitis
The human oral microbiome—as one of the largest microbial communities with approximately 600 bacterial species—plays a key role in balancing the equilibrium between symbiotic or pathogenic factors and the defense mechanisms of the immune system [9, 30]. Most of the organisms have been detected in the periodontal pocket in patients with periodontitis [9, 31]. A recent meta-analysis reported an association between alterations in the composition of the oral microbiome and the development of periodontitis [13]. Oral dysbiosis induces inflammatory mediators to activate the systematic inflammation status. Intriguingly, the composition of the oral microbiome has been implicated in the potential link between gut inflammation disorders and musculoskeletal system degeneration, such as the disc [32,33,34,35,36,37]. The cascade relationships among periodontitis, the occurrence of IDD, endplate changes, and clinical outcomes in adults with lumbar degenerative disorders have been previously reported [38].
Mouth–gut–disc axis
Although the cause of IDD is multifactorial, infection with low-virulence organisms has been considered one of the main triggers of inflammation in the degenerative process [39, 40]. Direct infectious and indirect mechano-immunological pathways theoretically accelerate tissue damage in IVDs. Notably, current studies supporting the associations between low-virulence organisms and IDD and endplate changes mainly focus on the lumbar spine [7], with a few reports on the cervical spine. Some observational studies reported Cutibacterium acnes as the primarily involved pathogen in disc structural failures in the cervical spine [26,27,28,29]. Recent evidence from an animal model of cervical IVD infection by Cutibacterium acnes showed that low-virulence organism infection of cervical IVDs can lead to degenerative changes [41]. However, the source of low-virulence organisms in the cervical spine remains unclear.
Recently, one study provided evidence for the existence of differences in the microbiome composition in healthy, degenerative, and herniated IVD [42]. In addition, overlapping in the bacterial species among IVDs, gut, and skin provides evidence for the presence of the potential gut–disc axis microbiome [42]. Alterations in the gut microbiome potentially affect IVD via direct infectious or indirect inflammatory status. The potential mouth–gut–disc axis warrants further attention. Direct delivery of organisms, immune system modifications, and metabolite formation have been listed as the three main mechanisms for the establishment of the mouth–gut–disc axis [8].
Although oral or gut microbiota is thought to be the essential trigger of inflammation in the degenerative process, the source of the low-virulence organism for the pathogenesis of disc structural failures in the cervical spine is yet to be discovered. Our study provided indirect evidence supporting the concept of the mouth–disc axis in the cervical spine. We found a higher incidence rate of endplate changes in individuals with moderate and/or severe periodontitis. A previous study reported a significant difference in the subgingival microbiome between mild and severe periodontitis groups. The microbial composition was positively correlated with the expression of systemic inflammatory markers [17]. Taken together, oral microbiome dysbiosis in severe periodontitis may induce systemic inflammation to regulate the IDD and endplate changes, which supports the hypothesis of the current study.
Association between periodontitis data and clinical outcomes
In theory, alterations in oral microbiome composition aggravate the severity of PLI, BI, and PD in periodontitis, which may induce local and systemic inflammatory responses for regulating bone development and the involution process. Moreover, the inflammatory state may trigger a change in the internal and external microenvironment of IVD. Furthermore, direct invasion of organisms into the IVDs and dysregulation of local and systemic inflammatory activity can stimulate the expression of inflammatory cytokines and activate immune cells, leading to IDD and endplate changes. Inflammatory cytokines and migration of immune cells can also induce the appearance of nociceptive nerve fibers in IVDs and dorsal root ganglia. Due to these cascade responses, the expression of pain-related channels was elevated for amplifying and transmitting pain signals to the dorsal root ganglia and brain as related spinal pain [43].
Limitations
Nonetheless, this study has several limitations. First, although this study reports indirect evidence, direct evidence of oral microbial pathogens is missing. Second, although our and previous studies supported the group classification, the group allocation has potential bias. Third, the number of patients with smoking status and diagnosis with diabetes among the study groups were not significant differences. However, the potential bias from the risk factors in the developing disc structural failures still exists. Fourth, the pathological and laboratory tests of tissue samples were not conducted. Future prospective randomized controlled studies with a larger sample size should be performed to investigate the direct relationships between oral microbiome composition and disc structural failures and clinical outcomes.
Conclusion
In summary, the present study identified the relationship between severe periodontitis and a high incidence rate of endplate changes and poor clinical outcomes in patients with cervical degenerative disorders. The discovery of these associations provides new insights into a novel mechanism through which the alterations in the composition of the oral microbiome potentially promote disc structural failures and pain in the cervical spine.
Availability of data and materials
The data that support the findings of this study are available from the corresponding author, Xiaolong Chen, upon reasonable request.
References
Fejer R, Kyvik KO, Hartvigsen J. The prevalence of neck pain in the world population: a systematic critical review of the literature. Eur Spine J. 2006;15:834–48. https://doi.org/10.1007/s00586-004-0864-4.
Peng B, DePalma MJ. Cervical disc degeneration and neck pain. J Pain Res. 2018;11:2853–7. https://doi.org/10.2147/JPR.S180018.
da Costa RC, De Decker S, Lewis MJ, Volk H, Injury CSC, C,. Diagnostic imaging in intervertebral disc disease. Front Vet Sci. 2020;7: 588338. https://doi.org/10.3389/fvets.2020.588338.
Haughton V. Imaging intervertebral disc degeneration. J Bone Joint Surg Am. 2006;88(Suppl 2):15–20. https://doi.org/10.2106/JBJS.F.00010.
Pfirrmann CW, Metzdorf A, Zanetti M, Hodler J, Boos N. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine Phila Pa 1976. 2001;26:1873–8. https://doi.org/10.1097/00007632-200109010-00011.
Fields AJ, Ballatori A, Liebenberg EC, Lotz JC. Contribution of the endplates to disc degeneration. Curr Mol Biol Rep. 2018;4:151–60. https://doi.org/10.1007/s40610-018-0105-y.
Chen X, Lu S, Chopra N, Cui P, Zhang S, Narulla R, Diwan AD. The association between low virulence organisms in different locations and intervertebral disc structural failure: a meta-analysis and systematic review. Jor Spine. 2023. https://doi.org/10.1002/jsp2.1244.
Li W, Lai K, Chopra N, Zheng Z, Das A, Diwan AD. Gut-disc axis: A cause of intervertebral disc degeneration and low back pain? Eur Spine J. 2022;31:917–25. https://doi.org/10.1007/s00586-022-07152-8.
Aas JA, Paster BJ, Stokes LN, Olsen I, Dewhirst FE. Defining the normal bacterial flora of the oral cavity. J Clin Microbiol. 2005;43:5721–32. https://doi.org/10.1128/JCM.43.11.5721-5732.2005.
Dewhirst FE, Chen T, Izard J, Paster BJ, Tanner AC, Yu WH, Lakshmanan A, Wade WG. The human oral microbiome. J Bacteriol. 2010;192:5002–17. https://doi.org/10.1128/JB.00542-10.
Abusleme L, Hoare A, Hong BY, Diaz PI. Microbial signatures of health, gingivitis, and periodontitis. Periodontol. 2021;86:57–78. https://doi.org/10.1111/prd.12362.
Lamont RJ, Koo H, Hajishengallis G. The oral microbiota: dynamic communities and host interactions. Nat Rev Microbiol. 2018;16:745–59. https://doi.org/10.1038/s41579-018-0089-x.
Guerra F, Mazur M, Ndokaj A, Corridore D, La Torre G, Polimeni A, Ottolenghi L. Periodontitis and the microbiome: a systematic review and meta-analysis. Minerva Stomatol. 2018;67:250–8. https://doi.org/10.23736/S0026-4970.18.04198-5.
Cardoso EM, Reis C, Manzanares-Cespedes MC. Chronic periodontitis, inflammatory cytokines, and interrelationship with other chronic diseases. Postgrad Med. 2018;130:98–104. https://doi.org/10.1080/00325481.2018.1396876.
Cecoro G, Annunziata M, Iuorio MT, Nastri L, Guida L. Periodontitis, low-grade inflammation and systemic health: a scoping review. Medicina Kaunas. 2020;56:272. https://doi.org/10.3390/medicina56060272.
Orlandi M, Munoz Aguilera E, Marletta D, Petrie A, Suvan J, D’Aiuto F. Impact of the treatment of periodontitis on systemic health and quality of life: A systematic review. J Clin Periodontol. 2022;49(Suppl 24):314–27. https://doi.org/10.1111/jcpe.13554.
Plachokova AS, Andreu-Sanchez S, Noz MP, Fu J, Riksen NP. Oral microbiome in relation to periodontitis severity and systemic inflammation. Int J Mol Sci. 2021;22:5876. https://doi.org/10.3390/ijms22115876.
Morita T, Yamazaki Y, Mita A, Takada K, Seto M, Nishinoue N, Sasaki Y, Motohashi M, Maeno M. A cohort study on the association between periodontal disease and the development of metabolic syndrome. J Periodontol. 2010;81:512–9. https://doi.org/10.1902/jop.2010.090594.
Ainamo J, Barmes D, Beagrie G, Cutress T, Martin J, Sardo-Infirri J. Development of the world health organization (WHO) community periodontal index of treatment needs (CPITN). Int Dent J. 1982;32:281–91.
Cutress TW, Ainamo J, Sardo-Infirri J. The community periodontal index of treatment needs (CPITN) procedure for population groups and individuals. Int Dent J. 1987;37:222–33.
Eke PI, Page RC, Wei L, Thornton-Evans G, Genco RJ. Update of the case definitions for population-based surveillance of periodontitis. J Periodontol. 2012;83:1449–54. https://doi.org/10.1902/jop.2012.110664.
Sharma A, Lancaster S, Bagade S, Hildebolt C. Early pattern of degenerative changes in individual components of intervertebral discs in stressed and nonstressed segments of lumbar spine: an in vivo magnetic resonance imaging study. Spine Phila Pa 1976. 2014;39:1084–90. https://doi.org/10.1097/BRS.0000000000000265.
Modic MT, Steinberg PM, Ross JS, Masaryk TJ, Carter JR. Degenerative disk disease: assessment of changes in vertebral body marrow with MR imaging. Radiology. 1988;166:193–9. https://doi.org/10.1148/radiology.166.1.3336678.
Modic MT, Masaryk TJ, Ross JS, Carter JR. Imaging of degenerative disk disease. Radiology. 1988;168:177–86. https://doi.org/10.1148/radiology.168.1.3289089.
Koo TK, Li MY. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med. 2016;15:155–63. https://doi.org/10.1016/j.jcm.2016.02.012.
Georgy MM, Vaida F, Stern M, Murphy K. Association between Type 1 modic changes and propionibacterium acnes infection in the cervical spine: an observational study. AJNR Am J Neuroradiol. 2018;39:1764–7. https://doi.org/10.3174/ajnr.A5741.
Chen Y, Wang X, Zhang X, Ren H, Huang B, Chen J, Liu J, Shan Z, Zhu Z, Zhao F. Low virulence bacterial infections in cervical intervertebral discs: a prospective case series. Eur Spine J. 2018;27:2496–505. https://doi.org/10.1007/s00586-018-5582-4.
Bivona LJ, Camacho JE, Usmani F, Nash A, Bruckner JJ, Hughes M, Bhandutia AK, Koh EY, Banagan KE, Gelb DE, Ludwig SC. The prevalence of bacterial infection in patients undergoing elective ACDF for degenerative cervical spine conditions: a prospective cohort study with contaminant control. Global Spine J. 2021;11:13–20. https://doi.org/10.1177/2192568219888179.
Rao PJ, Maharaj M, Tang K, Lambie N, Scherman D, Phan K, Mobbs RJ. Degenerate-disc infection study with contaminant control (DISC): application of a proposed histological scoring system. J Clin Neurosci. 2019;66:87–91. https://doi.org/10.1016/j.jocn.2019.05.016.
van der Meulen TA, Harmsen H, Bootsma H, Spijkervet F, Kroese F, Vissink A. The microbiome-systemic diseases connection. Oral Dis. 2016;22:719–34. https://doi.org/10.1111/odi.12472.
Wade WG. Has the use of molecular methods for the characterization of the human oral microbiome changed our understanding of the role of bacteria in the pathogenesis of periodontal disease? J Clin Periodontol. 2011;38(Suppl 11):7–16. https://doi.org/10.1111/j.1600-051X.2010.01679.x.
Boer CG, Radjabzadeh D, Medina-Gomez C, Garmaeva S, Schiphof D, Arp P, Koet T, Kurilshikov A, Fu J, Ikram MA, Bierma-Zeinstra S, Uitterlinden AG, Kraaij R, Zhernakova A, van Meurs JBJ. Intestinal microbiome composition and its relation to joint pain and inflammation. Nat Commun. 2019;10:4881. https://doi.org/10.1038/s41467-019-12873-4.
Biver E, Berenbaum F, Valdes AM, Araujo de Carvalho I, Bindels LB, Brandi ML, Calder PC, Castronovo V, Cavalier E, Cherubini A, Cooper C, Dennison E, Franceschi C, Fuggle N, Laslop A, Miossec P, Thomas T, Tuzun S, Veronese N, Vlaskovska M, Reginster JY, Rizzoli R. Gut microbiota and osteoarthritis management: an expert consensus of the European society for clinical and economic aspects of osteoporosis, osteoarthritis and musculoskeletal diseases (ESCEO). Ageing Res Rev. 2019;55: 100946. https://doi.org/10.1016/j.arr.2019.100946.
Zhang X, Zhang D, Jia H, Feng Q, Wang D, Liang D, Wu X, Li J, Tang L, Li Y, Lan Z, Chen B, Li Y, Zhong H, Xie H, Jie Z, Chen W, Tang S, Xu X, Wang X, Cai X, Liu S, Xia Y, Li J, Qiao X, Al-Aama JY, Chen H, Wang L, Wu QJ, Zhang F, Zheng W, Li Y, Zhang M, Luo G, Xue W, Xiao L, Li J, Chen W, Xu X, Yin Y, Yang H, Wang J, Kristiansen K, Liu L, Li T, Huang Q, Li Y, Wang J. The oral and gut microbiomes are perturbed in rheumatoid arthritis and partly normalized after treatment. Nat Med. 2015;21:895–905. https://doi.org/10.1038/nm.3914.
Asquith M, Sternes PR, Costello ME, Karstens L, Diamond S, Martin TM, Li Z, Marshall MS, Spector TD, le Cao KA, Rosenbaum JT, Brown MA. HLA alleles associated with risk of ankylosing spondylitis and rheumatoid arthritis influence the gut microbiome. Arthritis Rheumatol. 2019;71:1642–50. https://doi.org/10.1002/art.40917.
Tajik N, Frech M, Schulz O, Schalter F, Lucas S, Azizov V, Durholz K, Steffen F, Omata Y, Rings A, Bertog M, Rizzo A, Iljazovic A, Basic M, Kleyer A, Culemann S, Kronke G, Luo Y, Uberla K, Gaipl US, Frey B, Strowig T, Sarter K, Bischoff SC, Wirtz S, Canete JD, Ciccia F, Schett G, Zaiss MM. Targeting zonulin and intestinal epithelial barrier function to prevent onset of arthritis. Nat Commun. 2020;11:1995. https://doi.org/10.1038/s41467-020-15831-7.
Vijay A, Valdes AM. Role of the gut microbiome in chronic diseases: a narrative review. Eur J Clin Nutr. 2022;76:489–501. https://doi.org/10.1038/s41430-021-00991-6.
Chen X, Xue D, Zhao Y, Cui P, Wang P, Wang Y, Lu SB. Association between periodontitis and disc structural failure in older adults with lumbar degenerative disorders: A prospective cohort study. BMC Surg. 2023;23:57. https://doi.org/10.1186/s12893-023-01950-7.
Sadowska A, Touli E, Hitzl W, Greutert H, Ferguson SJ, Wuertz-Kozak K, Hausmann ON. Inflammaging in cervical and lumbar degenerated intervertebral discs: analysis of proinflammatory cytokine and TRP channel expression. Eur Spine J. 2018;27:564–77. https://doi.org/10.1007/s00586-017-5360-8.
Ganko R, Rao PJ, Phan K, Mobbs RJ. Can bacterial infection by low virulent organisms be a plausible cause for symptomatic disc degeneration? A systematic review. Spine Phila Pa 1976. 2015;40:E587-592. https://doi.org/10.1097/BRS.0000000000000832.
Li J, Chen Y, Wu H, Shan Z, Bei D, Gan K, Liu J, Zhang X, Chen B, Chen J, Zhao FD. Different responses of cervical intervertebral disc caused by low and high virulence bacterial infection: a comparative study in rats. Bioengineered. 2022;13:12446–61. https://doi.org/10.1080/21655979.2022.2075305.
Rajasekaran S, Soundararajan DCR, Tangavel C, Muthurajan R, Sri Vijay Anand KS, Matchado MS, Nayagam SM, Shetty AP, Kanna RM, Dharmalingam K. Human intervertebral discs harbour a unique microbiome and dysbiosis determines health and disease. Eur Spine J. 2020;29:1621–40. https://doi.org/10.1007/s00586-020-06446-z.
Lyu FJ, Cui H, Pan H, Mc Cheung K, Cao X, Iatridis JC, Zheng Z. Painful intervertebral disc degeneration and inflammation: from laboratory evidence to clinical interventions. Bone Res. 2021;9:7. https://doi.org/10.1038/s41413-020-00125-x.
Acknowledgements
None
Funding
This work was supported by Beijing Hospitals Authority 'Ascent Plan' (DFL20190802) and Beijing Authority Clinical Medicine Development of special funding support (XMLX202116).
Author information
Authors and Affiliations
Contributions
XLC and SBL conceived and designed the experiments; XLC, DX, and PC performed the experiments; XLC and PC analyzed and interpreted the data; XLC, YZ, and SBL contributed analysis tools or data; XLC wrote the paper. All the authors approved the final version of the manuscript. All authors contributed to the article and approved the submitted version.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Human Research Ethics Committee of Xuanwu Hospital Capital Medical University (KS2022151-1). The patients/participants provided their written informed consent to participate in this study.
Competing interests
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Chen, X., Xue, D., Cui, P. et al. Association between periodontitis and disc structural failures in patients with cervical degenerative disorders. J Orthop Surg Res 18, 884 (2023). https://doi.org/10.1186/s13018-023-04381-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13018-023-04381-5