Abstract
Background
In the literature, scarce data investigate the link between 25-hydroxyvitamin D (25[OH]D) and blood lipids in the osteoporosis (OP) population. 25(OH)D, as a calcium-regulating hormone, can inhibit the rise of parathyroid hormone, increase bone mineralization to prevent bone loss, enhance muscle strength, improve balance, and prevent falls in the elderly. This retrospective cross-sectional study aimed to investigate the association between serum 25(OH)D levels and lipid profiles in patients with osteoporosis, with the objective of providing insight for appropriate vitamin D supplementation in clinical settings to potentially reduce the incidence of cardiovascular disease, which is known to be a major health concern for individuals with osteoporosis.
Methods
This is a retrospective cross-sectional study from the Affiliated Kunshan Hospital of Jiangsu University, including 2063 OP patients who received biochemical blood analysis of lipids during hospitalization from January 2015 to March 2022. The associations between serum lipids and 25(OH)D levels were examined by multiple linear regression. The dependent variables in the analysis were the concentrations of serum lipoprotein, total cholesterol (TC), triglycerides (TGs), apolipoprotein-A, lipoprotein A, high-density lipoprotein cholesterol and low-density lipoprotein cholesterol (LDL-C). The independent variable was the concentration of blood serum 25(OH)D. At the same time, age, body mass index, sex, time and year of serum analysis, primary diagnosis, hypertension, diabetes, statins usage, beta-C-terminal telopeptide of type I collagen, procollagen type I N-terminal propeptide were covariates. Blood samples were collected in the early morning after the overnight fasting and were analyzed using an automated electrochemiluminescence immunoassay on the LABOSPECT 008AS platform (Hitachi Hi-Tech Co., Ltd., Tokyo, Japan). The generalized additive model was further applied for nonlinear associations. The inception result for smoothing the curve was evaluated by two-piecewise linear regression exemplary.
Results
Our results proved that in the OP patients, the serum 25(OH)D levels were inversely connected with blood TGs concentration, whereas they were positively associated with the HDL, apolipoprotein-A, and lipoprotein A levels. In the meantime, this research also found a nonlinear relationship and threshold effect between serum 25(OH)D and TC, LDL-C. Furthermore, there were positive correlations between the blood serum 25(OH)D levels and the levels of TC and LDL-C when 25(OH)D concentrations ranged from 0 to 10.04 ng/mL. However, this relationship was not present when 25(OH)D levels were higher than 10.04 ng/mL.
Conclusions
Our results demonstrated an independent relationship between blood lipids and vitamin D levels in osteoporosis patients. While we cannot establish a causal relationship between the two, our findings suggest that vitamin D may have beneficial effects on both bone health and blood lipid levels, providing a reference for improved protection against cardiovascular disease in this population. Further research, particularly interventional studies, is needed to confirm these associations and investigate their underlying mechanisms.
Similar content being viewed by others
Introduction
Osteoporosis (OP) represents a skeletal sickness described by weakened bone mass and bone microarchitecture that quickly leads to bone fractures [1]. OP diagnosis is based on bone mineral density (BMD) [1]. Data show that in China in 2019, the estimated age‐standardized occurrence of OP at the backbone or hip in males and females 50 years old and above was 6.46% and 29.13%, respectively [2]. Therefore, 10.9 million Chinese males and 49.3 million females were projected to have OP. Certain OP risk factors were further recognized, such as the way of living, food regimes, comorbid diseases and medications, and genetic predispositions [2, 3]. Recent data linked OP patients' metabolism and blood serum lipid profile with the disease [4,5,6]. It has been shown that vitamin D led to an increase in BMD in OP individuals [7], whereas the lipid profile was reported as a risk factor for OP [8, 9].
Vitamin D is a critical steroid-like vitamin for human health and is produced in humans by irradiation with ultraviolet radiation B (UVB) light [10]. In the liver, it metabolizes to calcifediol [25(OH)D] and then to 1.25-dihydroxy vitamin D [1.25(OH)D] in the kidneys. 1.25(OH)D is a transcription factor. It regulates the activity of more than 1000 different genes by binding to vitamin D receptors (VDRs) [11]. Recent data appointed the blood serum concentration of calcifediol as a medical indicator for assessing vitamin D metabolism and absorption in the body [12, 13]. Vitamin D affects the total mineralization of the bones, the rate of bone resorption and the incidence of bone breaks. Epidemiological investigations display the link between the shortage of it with low bone thickness, higher bone resorption and higher breakage occurrence. Therefore, additional uptake of this vitamin results in elevated BMD, a reduction in bone resorption and a drop in fracture occurrence [14]. On the other side, a meta-analysis of 41 RCTs evaluated that the benefits of vitamin D for lipid metabolism are well-known [15, 16].
Data show that medical conditions with abrogated lipid metabolism, also known as dyslipidemias, are the lead cause of a wide range of cardiological complications such as atherosclerotic cardiovascular diseases (ASCVD), among which the most common is coronary heart disease (CHD) [17]. OP and CHD share typical age-associated onset and fundamental pathogenetic mechanisms such as bone and vascular mineralization [18]. Furthermore, data show a link between the concentration of blood serum cholesterols [total cholesterol (TC), triglycerides (TGs), high-density (HDL-C), and low-density lipoprotein cholesterol (LDL-C)] and BMD [4, 5], thus proving that vitamin D is intricately related to the cholesterol metabolism biosynthesis pathway. This interplay is multifaceted.
Moreover, vitamin D shortage has been related to the augmented occurrence of cardiovascular diseases (CVD) [19, 20]. Some data further show that statin therapy, used in the management of hypercholesterolemia, does not affect the plasma levels of this vitamin [21]. Moreover, data confirm that vitamin D and cholesterol dysregulation are age-associated [22, 23]. However, there is little knowledge about the relationship between the two in OP patients.
Here, this research proves the independent association between the serum cholesterol levels (TC, HDL-C, TG, and LDL-C) in males and females, age ≥ 50 years, with 25(OH)D blood serum concentration in Chinese patients with OP.
Materials and methods
Study design and patients' data
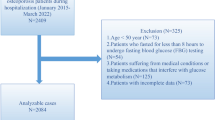
This study has performed a retrospective investigation, which included patients' data collected between January 2015 and March 2022. The patients' medical data were retrieved from the Affiliated Kunshan Hospital of Jiangsu University, Suzhou, China. 2409 OP patients were included in the study. All of them received medical blood checks during hospitalization. OP diagnosis was made on the following inclusion criteria: (1) occurrence of bone instability and breaks in the lack of other metabolic bone illnesses, with physiological BMD (T-score), and (2) OP confirmed based on a T-score of − 2.5 or less, even in the lack of a predominant bone rupture [7]. The exclusion criteria were: (1) patients with secondary OP (n = 76), or (2) patients with a history of hepatitis, liver cirrhosis, or cancer (n = 144); (3) medical history of kidney disease (n = 32), and (4) statins usage (n = 21); (5) age < 50 years (n = 73). After applying the inclusion criteria, 2063 patients were acquired for the study. Figure 1 represents patients' data and medical history. The study was approved by the Ethics Committee at the Affiliated Kunshan Hospital of Jiangsu University, Suzhou, China (approval No. 2020-03-046-K01) and was compliant with the Declaration of Helsinki. The patients' identity was hidden for an unbiased investigation. All patients signed a written consent form.
Dependent variables
The blood analyses were done on early morning fasting blood, and the lipids' quantitation was performed with an automated electro-chemiluminescence immunoassay on the LABOSPECT 008AS platform (Hitachi High-Tech Co., Tokyo, Japan). In our study, the dependent variables were the serum concentrations of TC, LDL-C, TG, apolipoprotein-A (APO-A), HDL-C, and lipoprotein A (LPA).
Exposure variables
Our study's exposure variable was 25(OH)D in its superabundant serum form, 25(OH)D. An automatic electro-chemiluminescence immune assay measured its concentrations through the Roche Cobas 8000/e602 analyzer (Roche Diagnostics, Mannheim, Germany). The blood collection moment was included in the analysis, together with the seasons.
Covariates
Covariates were age, sex, BMI, season and year of blood collection, primary diagnosis (OP without fractures/OPF), hypertension, diabetes, statins usage, beta-C-terminal telopeptide of type I collagen(β-CTX), and procollagen type I N-terminal propeptide (P1NP). All clinical variables were quantified within 3 days of hospital admittance.
Statistics
All the results are presented as mean ± standard deviation (SD), as a median (Q1 Q3) and as frequency (%) for constant and categorical variables, respectively. Pearson's chi-square or Fisher's exact tests were applied for univariate data quantitating absolute values. The t test and the Mann–Whitney U test were applied for continuous variables with standard and non-normally distributed continuous data, respectively. The univariate logistic regression data quantitation was utilized for assessing the link between the serum 25(OH)D levels and blood lipids.
The generalized estimating equations [24] studied the independent relations between the blood lipids' concentrations and the serum 25(OH)D by controlling the influence of covariances. Data were quantified through unadjusted (basic), negligibly accustomed (Model I) or fully adjusted (Model II) models. The variance inflation factor (VIF) data evaluation allowed adjustment of covariances following criteria: (1) the covariate was included in the crude model or detached from the full one, while the similar odds ratio (OR) was altered by at least 10%; (2) when the covariate from criterion 1 had P value of < 0.1 in the univariate model [22]. So, in terms of fully adjusted models, Model II was developed based on Criteria 1.
The generalized additive model (GAM) identified nonlinear relationships among our data. The two-piecewise linear regression model sets the threshold for line smoothing. The recursive method was used to spontaneously evaluate the turning point in the case of an apparent ratio in the smoothing curve [23]. Furthermore, this research performed subgroup analyses and estimated their robustness and potential variations, stratifying different covariates. Finally, the modifications and interactions of the subgroups were analyzed using the likelihood ratio test (LRT).
To enhance the generalizability of the findings for future studies, a sensitivity analysis was performed to examine the impact of BMI classification. Specifically, BMI was classified according to both the WHO international classification [25] and the classification used in the Chinese adult population [26]. The sensitivity analysis was conducted with the aim of determining whether the results were affected by the selected BMI classification system and to ensure that the findings could be applied to diverse populations beyond China.
All statistical analyses were performed using the Empower Stats (www.empowerstats.com, X&Y Solutions, Inc., Boston, MA, USA). The R software version 3.6.3 was also applied (http://www.r-project.org). P-values less than 0.05 were accepted as statistically significant.
Results
Features of study contributors
A population-descriptive analysis was performed to characterize the OP population included in the study. A total of 2063 patients, 83.86% (n = 1730), average age = 68.5 years, were females who passed the inclusion criteria and enrolled in this retrospective investigation. The medical data are included in Table 1. The blood serum 25(OH)D levels were measured, and the mean (SD) and medium (Q1–Q3) concentrations were 21.13 (8.83) ng/mL and 20.00 (15.00–25.83) ng/mL, respectively. The patients diagnosed with OPF accounted for 32.574% (n = 672). The mean and median concentrations of serum TC were 4.564 (0.997) ng/mL and 4.525 (3.890–5.160) ng/mL, respectively. These values for TG were 1.361 (0.876) ng/mL and 1.130 (0.820–1.647) ng/mL, individually, whereas for HDL concentrations were 1.467 (0.331) ng/mL and 1.430 (1.230–1.660) ng/mL, correspondingly. LDL-C mean (SD) and median (Q1-Q3) concentrations were 2.713 (0.787) ng/mL and 2.660 (2.160–3.210) ng/mL and of APO-A were 1.368 (0.274) ng/mL and 1.340 (1.180–1.530) ng/mL. For the blood serum concentrations these values were 186.168 (166.013) ng/mL and 131.000 (73.250–243.000) ng/mL, respectively, whereas for HCY were 12.968 (6.373) ng/mL and 11.360 (9.270–15.040) ng/mL. The concentrations of serum Ca, β-CTx and P1NP were correspondingly measured. This research classified the patients into five groups based on the estimated BMI (kg/m2). The groups were the following (1) ≤ 18.5 (underweight); (2) > 18.5 and ≤ 23.9 (normal); (3) > 23.9 and ≤ 28 (overweight); (4) > 28 and ≤ 35 (obesity); (5) > 35 (severe obesity). The BMI for the study participants was normal at 60.834% (n = 1255). The variables like season and year of blood sampling were measured too. The seasons of blood sampling were spring, summer, autumn and winter, whereas the years of blood collection were distinguished from 2015 to 2022. 31.168% (n = 643) of the study participants had hypertension, while 14.106% (n = 291) had diabetes.
Univariate analysis of blood lipids
The results of the univariate logistic regression analysis are displayed in Table 2. This research has revealed that the blood serum concentrations of 25OH(D) were negatively related to the concentrations of TG (β, -0.005; 95% CI [confidence interval], − 0.010, − 0.000; P = 0.04553), and positively associated with HDL (β, 0.005; 95% CI [confidence interval], 0.003, 0.006; P < 0.00001) and APO-A (β, 0.004; 95% CI [confidence interval], 0.003, 0.006; P < 0.00001).
In the univariate data quantitation of covariates like blood lipids and serum Ca2+ concentrations, the results showed a significant positive association with the concentrations of TC (β, 1.006; 95% CI, 0.715, 1.296; P < 0.00001), TG (β, 0.367; 95% CI, 0.108, 0.626; P value = 0.00548), HDL (β, 0.218; 95% CI, 0.121, 0.316; P = 0.00001), LDL-C (β, 0.663; 95% CI, 0.433, 0.893; P < 0.00001), APO-A (β, 0.357; 95% CI, 0.278, 0.436; P < 0.00001). Furthermore, this research detected a significant negative association with the levels of blood serum HCY (β, − 3.271; 95% CI, − 5.150, − 1.391; P = 0.00066). Females appeared with 54%, 24% and 31%greater TC, TG and LDL-C levels compared to males (P < 0.00001) and 13% higher LDL-C levels compared to men (P < 0.00001). OPF patients exhibited lower blood lipid levels compared with OP patients without fractures.
Independent relation between the blood serum 25(OH)D concentrations and blood lipid profiles of patients
Multiple regression equations were used to analyze serum 25(OH)D and blood lipids to exclude the effect of confounding factors on the correlation between the two factors. Table 3 summarizes the independent link between serum 25(OH)D level and blood lipids using multivariate linear regression analysis. This research employed a two-level adjustment based on the covariance analysis applying the following models: the crude unadjusted, the one accustomed for sex, patients' years at the time of blood collection, time and season of sampling, the serum concentration of calcium, patients' BMI, primary diagnosis and Model II in sync to Model I including comorbidities like hypertension, diabetes, β-CTX and P1NP. The results demonstrated a major adverse relationship between TG and serum 25(OH)D levels in both the crude (β, − 0.005; 95% CI, − 0.01 to 0; P = 0.04553) and Model II (β, − 0.006; 95% CI, − 0.011 to − 0.001; P = 0.02954). These results showed that a 10 ng/mL increase in the serum concentration of 25(OH)D led to a 5% (β, − 0.05; 95% CI, − 0.1 to − 0.01, P = 0.05107) decrease in the TG, Model I or a 6% (β, − 0.06; 95% CI, − 0.11 to − 0.01, P = 0.02954) decrease in the TG in Model II. Furthermore, this research estimated that this increase in the concentration of serum 25(OH)D was linked with a 4% (β, 0.04; 95% CI, 0.03–0.06, P < 0.00001) increase in the HDL in Model I or a 4% (β, 0.04; 95% CI, 0.02–0.06, P < 0.00001) increase in the HDL in Model II.
Threshold analysis and spline smoothing plot
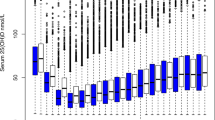
To demonstrate the nonlinear relationship between serum 25(OH)D and blood Lipids, this research utilized threshold effect analysis to accomplish this task. Table 4 displays the results from the threshold effect analysis, which examined the link between the serum concentration of 25(OH)D and the blood lipid profiles of OP patients in the fully adjusted Model II. The P value (< 0.05) for LRT indicated a nonlinear relation between 25(OH)D, TC and LDL-C. The two-piecewise linear regression model allowed us to set the turning point (K) of 10.04 ng/mL for the serum 25(OH)D concentration to obtain an accustomed flattened curve. Specifically, an expressive, positive association between the blood serum 25(OH)D levels and TC among the studied individuals was detected when 25(OH)D concentration ranged from 0 to 10.04 ng/mL (β, 0.107; 95% CI, 0.022–0.192; P = 0.014). This research did not detect any association between 25(OH)D and TC when 25(OH)D concentration was > 10.04 ng/mL (β, 0.001; 95% CI, − 0.005 to 0.007; P value = 0.819). Similarly, a highly positive relationship was proven between LDL-C and 25(OH)D concentrations, when 25(OH)D ranged from 0 to 10.04 ng/mL (β, 0.098; 95% CI, 0.03–0.165; P = 0.005) and no obvious association was detected between LDL-C and 25(OH)D when the serum levels of 25(OH)D were > 10.04 ng/mL (β, − 0.003; 95% CI, − 0.008 to 0.001; P = 0.179). Figure 2 shows the relationship between them.
Adjusted smoothed curves corresponding to the relationship between 25(OH)D levels and TC (A), LDL-C (B). A generalized additive model revealed a threshold nonlinear relationship between 25(OH)D and TC, LDL-C in OP patients. The upper and lower curves represent the range of the 95% confidence interval, and the middle curve represents the correlation between 25(OH)D and TC, LDL-C. Models were adjusted for Sex; Age of blood collection; Year of blood collection; Calcium; BMI; Season; Main diagnosis; Hypertension; Diabetes; β-CTX. The middle curve exhibited an inflection point (K) at 10.04 ng/mL. 25(OH)D 25-hydroxy vitamin D, TC total cholesterol, LDL-C low-density lipoprotein, BMI body mass index, β-CTX beta-C-terminal telopeptide of type I collagen
Subgroup analysis
This research performed subgroup analyses in the fully adjusted Model II to confirm robust findings. The potential confounders were the patients' age, gender, BMI, primary diagnosis, the season of blood collection, P1NP, Ca, and comorbidities like hypertension, diabetes, year of blood collection, and β-CTX concentrations. All analyses were adjusted for the above eleven covariates except the subgroup variable. Additional file 1: Table S1 shows that all layers were stable.
Sensitivity analysis
Stratified analyses of both BMI and lipid profiles were conducted using both the WHO [25] and Chinese adult standards [26] for BMI classification. The results of these analyses are presented in Additional file 1: Table S2, showing that significant differences in several lipid biomarkers were observed between different BMI subgroups of the osteoporosis population.
These findings highlight the importance of considering the BMI classification when studying the relationship between osteoporosis and lipid metabolism. Specifically, we found significant differences in lipid biomarkers between the different BMI subgroups established using both the WHO and Chinese adult standards, indicating that the choice of BMI classification may impact the results.
In the TC group, significant differences were observed between osteoporosis patients and those of normal weight range according to the WHO standard, while there were no significant differences between the categories when using the Chinese adult standard. In the HDL group, significant differences were observed between normal-weight and overweight osteoporosis patients using under both the WHO and Chinese adult standards. In the LDL group, a significant difference was observed in the overweight range under both the WHO and Chinese adult standards while in the APO-A group, there were significant differences within the overweight range when using both the WHO and Chinese adult standards. In the LPa group, significant differences were observed between normal, overweight, and obese individuals with osteoporosis when using the WHO standard, while significant differences were only in normal-weight people with osteoporosis when weight was defined according to the Chinese adult standard. In the triglyceride group, a significant difference was found in the overweight range of the osteoporosis population under both the WHO and Chinese adult standards.
Overall, these findings suggest that the BMI classification may be an important factor to consider when studying the relationship between osteoporosis and lipid metabolism. The choice of BMI classification may impact the results, and further research is needed to confirm these findings and explore the potential mechanisms underlying the observed associations.
Discussion
Although previous cross-sectional studies have investigated the association between osteoporosis and lipid profiles, with some studies finding that higher total cholesterol and triglyceride levels were associated with an increased risk of osteoporosis, the findings of our study add to the existing literature by demonstrating an independent association between vitamin D levels and blood lipid profiles in osteoporosis patients [27]. Our study is the first epidemic Chinese investigation that studied the independent association between the blood serum levels of 25(OH)D and the blood lipid profiles of OP individuals. This research has revealed that in the studied OP patients, the blood serum levels of 25(OH)D were inversely linked with the attention of TG. In contrast, 25(OH)D levels were positively linked with the HDL concentrations. Furthermore, the detected association was nonlinear with the cuff-off value of 25(OH)D concentration of 10.04 ng/mL.
Vitamin D plays a vital role in human physiology, though its exact mechanisms of action are yet unknown. Some authors doubt its improving-the-health effect. On the other side, others have data highlighting its positive impact on the immune system, hormonal regulation and cellular proliferation. Moreover, evidence confirms the link between vitamin D and lipid metabolism without solid details, in which different lipid parameters are affected by changes in the blood serum [28, 29]. Vitamin D insufficiency has been proposed to be linked with CVD [30, 31] without knowing the exact mechanisms through which this is realized. Some results show that it exerted a regulatory effect on cardiomyocytes and vascular smooth muscle cells [32]. Other authors showed that the decreased blood concentrations of 25(OH)D stimulated the renin-angiotensin system and increased blood pressure [33]. In addition, vitamin D has anti-inflammatory activities, thus controlling atherogenesis [32, 34, 35].
The abrogated lipid profile expressed with increased LDL-C and TG concentrations and decreased HDL-C levels has been reported as a CVD risk factor, mainly for atherosclerotic CVD [36, 37]. Moreover, data show that it is a risk factor for an ischemic cerebrovascular stroke [38]. Another risk factor for the pathologies mentioned above is insulin resistance, which is linked with metabolic syndrome [39]. The reason for this is the fact that insulin resistance leads to elevated concentrations of plasma LDL-C and TG and a reduced amount of HDL-C [40]. The results from our study upgrade the above-reported other authors' results with the received data for OP individuals. This research found a positive association between the blood serum concentrations of HDL-C and 25(OH)D, APO-A, and LPA levels in all participants after regulating significant confounders, including age and BMI. In addition, this research established an inverse link between 25(OH)D concentrations and TG levels in all participants. Interestingly, this research found a nonlinear relationship between serum levels of vitamin D, TC and LDL-C. Particularly, when the concentration of 25(OH)D was less than 10.04 ug/mL, they were positively correlated, while it was above 10.04 ug/mL, there was no correlation. These data are unique as they are not reported in previous studies.
Interestingly, our study revealed linear associations between the levels of vitamin D, TC, and LDL-C. When levels of 25(OH)D were less than 10.04 ng/mL, these variables were positively correlated, whereas no such correlations were evident when these concentrations rose above 10.04 ng/mL. These results are also unique, as no one has published them before. Jungert et al. [41] established that 25(OH)D concentrations were negatively related to TC and LDL-C in old German females. The exact correlation was estimated for middle-aged men from Finland [42]. J-m Wang et al. also found that vitamin D serum levels were adversely linked with TC and LDL-C in diabetic individuals [43]. These findings are different from our results. The causes may be the inconsistencies in this study population as it did not include OP patients from China. Although the age group was similar to our cohort of patients, the baseline levels of vitamin D were less in the Chinese population, thus leading to different results.
This research found an inverse link between TG and vitamin D in people with OP, similar to previous studies. Jungert et al. [41] found that 25(OH)D levels were negatively linked with TG among older German women. Karhapää et al. [42] revealed that 25(OH)D serum levels are negatively linked with TG in middle-aged Finnish males. Other authors' results presented that vitamin D concentrations above 10 nmol/l were linked with a decrease in TG (0.52%) among Danish grownups [44]. The same results were found in middle-aged Chinese individuals [45]. Mohammad Ali Arif et al. reported that vitamin D deficiency led to a severe drop in blood serum concentrations of LDL-C, TG and TC. These individuals displayed the highest LDL-C, TG and TC levels, whereas those with mild deficiency had lower levels of the lipids above [46].
Our results showed a positive link between 25(OH)D and HDL concentration in OP individuals. This is in unison with the results of Wang et al. [47], proving that low HDL levels were linked with low vitamin D amounts in Saudi males after correcting senility, BMI, smoking, and physical movement. Other authors also proved that the serum level of 25(OH)D concentration was positively linked with HDL-C without considering factors of obesity in middle-aged men and women [48, 49]. All the above-discussed data prove the link between vitamin D and the blood serum lipid profile in different populations. This suggests that this vitamin has a favorable result on the blood lipid profile. These data, though, need further confirmation.
Preceding reports suggested that the increased calcium absorption in the intestine decreased the synthesis and secretion of hepatic TG [50]. Vitamin D inhibits these processes by activating calcium uptake in the intestines. Data show that the elevated concentrations of intestinal calcium decreased the uptake of fatty acids in the intestines. Moreover, it was proven that the serum levels of LDL-C reduced fat absorption, predominantly saturated fats [51]. Furthermore, calcium promotes the change of cholesterol into bile acids, thus reducing the concentrations of blood serum cholesterol [52]. The elevated concentrations of the parathyroid hormone (PTH) led to an increase in TG, while 25(OH)D suppressed the levels of blood serum PTH [53, 54]. These data prove that vitamin D could affect TG concentrations by modifying the PTH levels. The solid indication that vitamin D shortage was linked with impaired b-cell function and insulin resistance has been demonstrated, thus further showing that this affected lipoprotein metabolism and reduced TG and HDL-C levels [55,56,57]. Furthermore, vitamin D was proven to be involved in bile acid synthesis in the liver [58]. This proves the potential direct link between it and patients' lipid profile and metabolism.
There are data that low vitamin D levels increase cholesterol. Some authors linked the increased calcium absorption as a risk factor that decreased TG synthesis and secretion in the liver [51]. This proves that insufficient vitamin D concentrations may control these processes. Other authors suggest that insoluble calcium–fatty acid complexes are shaped and constrain fatty acids' intestinal absorption—the last results in cholesterol reduction [59]. A link between the parathyroid hormone, TG and vitamin D has been proposed, in which significant amounts of PTH were linked with enhanced TG and low vitamin D concentrations.
Moreover, data show that increased vitamin D concentrations decreased serum PTH concentrations [47]. Also, there is a strong indication that the lack of vitamin D influences the function of beta-cells, thus causing insulin resistance, lipoprotein metabolism disruption, and ultimately elevated TG and reduced HDL cholesterol concentrations [55]. Hereafter, various types of machinery are most likely to act concurrently and link vitamin D shortage with abrogated lipid profiles.
Specific data link the elevated TG concentrations with overweight. Overweight people lack vitamin D because of the high quantity of subcutaneous fat [60]. Dyslipidemia is also connected with high blood sugar levels, leading to decreased vitamin D concentrations [61]. Physical activity recovers HDL cholesterol, and it facilitates vitamin D concentrations. This research proposes that a vigorous routine including consistent bodily movement may not only support refining dyslipidemia but can also prevent vitamin D deficiency.
This research is the first epidemic Chinese investigation that studied the independent association between the blood serum levels of 25(OH)D and the blood lipid profiles of OP individuals. Our study might have some direct implications for clinical practice. First, supplementation to a serum 25(OH)D concentration of 10.04 ng/mL may benefit TC and LDL-C concentrations in vitamin D-deficient OP patients, although no such benefit is expected for higher 25(OH)D concentrations. Secondly, vitamin D supplementation not only improved bone and muscular health in OP patients [62], but also negatively regulated TG levels and positively regulated HDL, apolipoprotein-A, and lipoprotein A levels, thereby reducing the risk of cardiovascular and cerebrovascular diseases. Third, it helped manage the blood lipid levels in OP patients, especially those with cardiovascular and cerebrovascular diseases. Finally, this research found helpful information for formulating relevant medical guidelines from an evidence-based perspective.
The study has some limitations. First, this study was a retrospective cross-sectional study, so the associations between blood lipids and 25(OH)D do not represent a causal relationship. Furthermore, meaningful endpoint events, such as the occurrence of CVD, were not used as dependent variables in these analyses. Future prospective cohort studies should be conducted based on our study. Next, some significant parameters, including the parathyroid hormone (PTH) levels, cardiac functions, and dietary habits, were not examined. Future studies should thus incorporate these parameters. Third, this research used a single-center design with comparatively minor people numbers; thus, the results could not be generalized to other ethnic groups. Therefore, this study highlights the need for additional research encompassing extensive analyses that include additional biochemical indicators, multi-center RCTs, and people of different ethnicities to better ensure the reliability of these study results.
Conclusions
Our results demonstrated that the blood serum concentrations of 25(OH)D were negatively correlated with TG levels and positively correlated with HDL, APO-A, and LPA levels in OP patients. In particular, a nonlinear relationship between 25(OH)D levels and concentrations of TC and LDL-C was detected, with positive associations between serum 25(OH)D levels and TC and LDL-C when 25(OH)D concentrations ranged from 0 to 10.04 ng/mL. However, this relationship was not present when 25(OH)D levels were higher than 10.04 ng/mL. Therefore, in the context of the clinical diagnosis and treatment of OP patients, these findings suggest that vitamin D not only has beneficial effects on bone health, but also on blood lipid levels, potentially providing some benefit as a means of preventing CVD.
Availability of data and materials
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Ensrud KE, Crandall CJ. Osteoporosis. Ann Intern Med. 2017;167:Itc17-itc32.
Hendrickx G, Boudin E, Van Hul W. A look behind the scenes: the risk and pathogenesis of primary osteoporosis. Nat Rev Rheumatol. 2015;11:462–74.
Lems WF. Fracture risk estimation may facilitate the treatment gap in osteoporosis. Ann Rheum Dis. 2015;74:1943–5.
Makovey J, Chen JS, Hayward C, Williams FM, Sambrook PN. Association between serum cholesterol and bone mineral density. Bone. 2009;44:208–13.
Ackert-Bicknell CL. HDL cholesterol and bone mineral density: is there a genetic link? Bone. 2012;50:525–33.
Bagger YZ, Rasmussen HB, Alexandersen P, Werge T, Christiansen C, Tankó LB. Links between cardiovascular disease and osteoporosis in postmenopausal women: serum lipids or atherosclerosis per se? Osteoporos Int. 2007;18:505–12.
Camacho PM, Petak SM, Binkley N, Diab DL, Eldeiry LS, Farooki A, Harris ST, Hurley DL, Kelly J, Lewiecki EM, et al. American Association of Clinical Endocrinologists/American College of Endocrinology clinical practice guidelines for the diagnosis and treatment of postmenopausal osteoporosis-2020 update. Endocr Pract. 2020;26:1–46.
Kim MR, Jeong SJ. Relationship between vitamin D level and lipid profile in non-obese children. Metabolites. 2019;9:125.
Leung RYH, Li GHY, Cheung BMY, Tan KCB, Kung AWC, Cheung CL. Serum metabolomic profiling and its association with 25-hydroxyvitamin D. Clin Nutr. 2020;39:1179–87.
Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–81.
Hossein-nezhad A, Holick MF. Vitamin D for health: a global perspective. Mayo Clin Proc. 2013;88:720–55.
Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, Durazo-Arvizu RA, Gallagher JC, Gallo RL, Jones G, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96:53–8.
Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911–30.
Lips P, van Schoor NM. The effect of vitamin D on bone and osteoporosis. Best Pract Res Clin Endocrinol Metab. 2011;25:585–91.
Dibaba DT. Effect of vitamin D supplementation on serum lipid profiles: a systematic review and meta-analysis. Nutr Rev. 2019;77:890–902.
Challoumas D. Vitamin D supplementation and lipid profile: what does the best available evidence show? Atherosclerosis. 2014;235:130–9.
Hajar R. Risk factors for coronary artery disease: historical perspectives. Heart Views. 2017;18:109–14.
Anagnostis P, Karagiannis A, Kakafika AI, Tziomalos K, Athyros VG, Mikhailidis DP. Atherosclerosis and osteoporosis: age-dependent degenerative processes or related entities? Osteoporos Int. 2009;20:197–207.
Skaaby T. The relationship of vitamin D status to risk of cardiovascular disease and mortality. Dan Med J. 2015;62:1–17.
Mozos I, Marginean O. Links between vitamin D deficiency and cardiovascular diseases. Biomed Res Int. 2015;2015: 109275.
Iqbal K, Islam N, Azam I, Mehboobali N, Iqbal MP. Lack of association of statin use with vitamin D levels in a hospital based population of type 2 diabetes mellitus patients. Pak J Med Sci. 2018;34:204–8.
Mc Auley MT, Wilkinson DJ, Jones JJ, Kirkwood TB. A whole-body mathematical model of cholesterol metabolism and its age-associated dysregulation. BMC Syst Biol. 2012;6:130.
Veldurthy V, Wei R, Oz L, Dhawan P, Jeon YH, Christakos S. Vitamin D, calcium homeostasis and aging. Bone Res. 2016;4:16041.
Pazianas M, van der Geest S, Miller P. Bisphosphonates and bone quality. Bonekey Rep. 2014;3:529.
Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser 2000;894:i-xii, 1–253.
Yang G, Kong L, Zhao W, Wan X, Zhai Y, Chen LC, Koplan JP. Emergence of chronic non-communicable diseases in China. Lancet. 2008;372:1697–705.
Kan B, Zhao Q, Wang L, Xue S, Cai H, Yang S. Association between lipid biomarkers and osteoporosis: a cross-sectional study. BMC Musculoskelet Disord. 2021;22:759.
Christakos S, Ajibade DV, Dhawan P, Fechner AJ, Mady LJ. Vitamin D: metabolism. Endocrinol Metab Clin N Am. 2010;39:243–53 (table of contents).
Hewison M, Burke F, Evans KN, Lammas DA, Sansom DM, Liu P, Modlin RL, Adams JS. Extra-renal 25-hydroxyvitamin D3–1alpha-hydroxylase in human health and disease. J Steroid Biochem Mol Biol. 2007;103:316–21.
Nsengiyumva V, Fernando ME, Moxon JV, Krishna SM, Pinchbeck J, Omer SM, Morris DR, Jones RE, Moran CS, Seto SW, Golledge J. The association of circulating 25-hydroxyvitamin D concentration with peripheral arterial disease: a meta-analysis of observational studies. Atherosclerosis. 2015;243:645–51.
Heidari B, Nargesi AA, Hafezi-Nejad N, Sheikhbahaei S, Pajouhi A, Nakhjavani M, Esteghamati A. Assessment of serum 25-hydroxy vitamin D improves coronary heart disease risk stratification in patients with type 2 diabetes. Am Heart J. 2015;170:573-579.e575.
Motiwala SR, Wang TJ. Vitamin D and cardiovascular risk. Curr Hypertens Rep. 2012;14:209–18.
Ajabshir S, Asif A, Nayer A. The effects of vitamin D on the renin-angiotensin system. J Nephropathol. 2014;3:41–3.
Al Mheid I, Patel RS, Tangpricha V, Quyyumi AA. Vitamin D and cardiovascular disease: is the evidence solid? Eur Heart J. 2013;34:3691–8.
Jouni ZE, Winzerling JJ, McNamara DJ. 1,25-Dihydroxyvitamin D3-induced HL-60 macrophages: regulation of cholesterol and LDL metabolism. Atherosclerosis. 1995;117:125–38.
Đokić B, Đonović N, Tadić B, Nikolić D. Factors and estimation of risk for cardiovascular diseases among patients in primary health care in central SERBIA. Cent Eur J Public Health. 2015;23:195–9.
Mazidi M, Rezaie P, Chaudhri O, Karimi E, Nematy M. The effect of Ramadan fasting on cardiometabolic risk factors and anthropometrics parameters: a systematic review. Pak J Med Sci. 2015;31:1250–5.
Nicholls S, Lundman P. The emerging role of lipoproteins in atherogenesis: beyond LDL cholesterol. Semin Vasc Med. 2004;4:187–95.
Wild SH, Byrne CD, Tzoulaki I, Lee AJ, Rumley A, Lowe GD, Fowkes FG. Metabolic syndrome, haemostatic and inflammatory markers, cerebrovascular and peripheral arterial disease: the Edinburgh Artery Study. Atherosclerosis. 2009;203:604–9.
Rodriguez-Colon SM, Mo J, Duan Y, Liu J, Caulfield JE, Jin X, Liao D. Metabolic syndrome clusters and the risk of incident stroke: the atherosclerosis risk in communities (ARIC) study. Stroke. 2009;40:200–5.
Jungert A, Roth HJ, Neuhäuser-Berthold M. Associations of serum 25-hydroxycholecalciferol and parathyroid hormone with serum lipids differ by sex and vitamin D status. Public Health Nutr. 2015;18:1684–91.
Karhapää P, Pihlajamäki J, Pörsti I, Kastarinen M, Mustonen J, Niemelä O, Kuusisto J. Diverse associations of 25-hydroxyvitamin D and 1,25-dihydroxy-vitamin D with dyslipidaemias. J Intern Med. 2010;268:604–10.
Wang JM, Ye SD, Li SM, Hu W. Correlations of 25(OH)D level with blood lipid, inflammatory factors and vascular endothelial function in diabetic patients. Eur Rev Med Pharmacol Sci. 2018;22:731–5.
Skaaby T, Husemoen LL, Pisinger C, Jørgensen T, Thuesen BH, Fenger M, Linneberg A. Vitamin D status and changes in cardiovascular risk factors: a prospective study of a general population. Cardiology. 2012;123:62–70.
Guan C, Fu S, Zhen D, Li X, Niu J, Cheng J, Zhao N, Liu J, Yin H, Tang X. Correlation of serum vitamin D with lipid profiles in middle-aged and elderly Chinese individuals. Asia Pac J Clin Nutr. 2020;29:839–45.
Arif MA, Niazi R, Arif SA. Association of dyslipidaemia in patients with varying degrees of Vitamin D deficiency in the Asian population. J Pak Med Assoc. 2017;67:1843–7.
Wang Y, Si S, Liu J, Wang Z, Jia H, Feng K, Sun L, Song SJ. The associations of serum lipids with vitamin D status. PLoS ONE. 2016;11: e0165157.
Auwerx J, Bouillon R, Kesteloot H. Relation between 25-hydroxyvitamin D3, apolipoprotein A-I, and high density lipoprotein cholesterol. Arterioscler Thromb. 1992;12:671–4.
Carbone LD, Rosenberg EW, Tolley EA, Holick MF, Hughes TA, Watsky MA, Barrow KD, Chen TC, Wilkin NK, Bhattacharya SK, et al. 25-Hydroxyvitamin D, cholesterol, and ultraviolet irradiation. Metabolism. 2008;57:741–8.
Cho HJ, Kang HC, Choi SA, Ju YC, Lee HS, Park HJ. The possible role of Ca2+ on the activation of microsomal triglyceride transfer protein in rat hepatocytes. Biol Pharm Bull. 2005;28:1418–23.
Christensen R, Lorenzen JK, Svith CR, Bartels EM, Melanson EL, Saris WH, Tremblay A, Astrup A. Effect of calcium from dairy and dietary supplements on faecal fat excretion: a meta-analysis of randomized controlled trials. Obes Rev. 2009;10:475–86.
Vaskonen T, Mervaala E, Sumuvuori V, Seppänen-Laakso T, Karppanen H. Effects of calcium and plant sterols on serum lipids in obese Zucker rats on a low-fat diet. Br J Nutr. 2002;87:239–45.
Song SJ, Si S, Liu J, Chen X, Zhou L, Jia G, Liu G, Niu Y, Wu J, Zhang W, Zhang J. Vitamin D status in Chinese pregnant women and their newborns in Beijing and their relationships to birth size. Public Health Nutr. 2013;16:687–92.
Zittermann A, Frisch S, Berthold HK, Götting C, Kuhn J, Kleesiek K, Stehle P, Koertke H, Koerfer R. Vitamin D supplementation enhances the beneficial effects of weight loss on cardiovascular disease risk markers. Am J Clin Nutr. 2009;89:1321–7.
Karnchanasorn R, Ou HY, Chiu KC. Plasma 25-hydroxyvitamin D levels are favorably associated with β-cell function. Pancreas. 2012;41:863–8.
Howard BV. Insulin resistance and lipid metabolism. Am J Cardiol. 1999;84:28j–32j.
Tai ES, Emmanuel SC, Chew SK, Tan BY, Tan CE. Isolated low HDL cholesterol: an insulin-resistant state only in the presence of fasting hypertriglyceridemia. Diabetes. 1999;48:1088–92.
Jiang W, Miyamoto T, Kakizawa T, Nishio SI, Oiwa A, Takeda T, Suzuki S, Hashizume K. Inhibition of LXRalpha signaling by vitamin D receptor: possible role of VDR in bile acid synthesis. Biochem Biophys Res Commun. 2006;351:176–84.
Christakos S, Dhawan P, Porta A, Mady LJ, Seth T. Vitamin D and intestinal calcium absorption. Mol Cell Endocrinol. 2011;347:25–9.
Karonova T, Belyaeva O, Jude EB, Tsiberkin A, Andreeva A, Grineva E, Pludowski P. Serum 25(OH)D and adipokines levels in people with abdominal obesity. J Steroid Biochem Mol Biol. 2018;175:170–6.
Duchaine CS, Diorio C. Association between intake of sugar-sweetened beverages and circulating 25-hydroxyvitamin D concentration among premenopausal women. Nutrients. 2014;6:2987–99.
Bouillon R, Marcocci C, Carmeliet G, Bikle D, White JH, Dawson-Hughes B, Lips P, Munns CF, Lazaretti-Castro M, Giustina A, Bilezikian J. Skeletal and extraskeletal actions of vitamin D: current evidence and outstanding questions. Endocr Rev. 2019;40:1109–51.
Acknowledgements
None.
Funding
The study was supported by the National Natural Science Foundation of China (CN) (82172441), the Elderly Health Research Project of Jiangsu Province (CN) (LKZ2022020), Suzhou Collaborative Innovation Research Project of Medical and Industrial Integration (CN) (SLJ2022023), Clinical Medical Science and Technology Development Fund of Jiangsu University (CN) (JLY2021048) and Suzhou Key Clinical Diagnosis and Treatment Technology Project (CN) (LCZX202024).
Author information
Authors and Affiliations
Contributions
Study design: KL, QS and CL. Study conduct: CL and KL. Data collection: SMX, KL, XFY, YWY, MZX, YQG. Data analysis: SMX and YQG. Data interpretation: SMX and KL. Drafting manuscript: SMX. Revising manuscript content: QS and KL. Approving final version of manuscript: CL, KL, QS and YQG. SMX and KL take responsibility for the integrity of the data analysis. SMX and KL contributed equally to this work and should be considered co-first authors. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This work is registered in the Chinese Clinical Trial Registry (ChiCTR2000036375).
Competing interests
All authors declare that they have no conflict of interest relevant to this work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Table S1 and Table S2.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Xu, Sm., Lu, K., Yang, Xf. et al. Association of 25-hydroxyvitamin D levels with lipid profiles in osteoporosis patients: a retrospective cross-sectional study. J Orthop Surg Res 18, 597 (2023). https://doi.org/10.1186/s13018-023-04079-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13018-023-04079-8