Abstract
Objectives
To assess LRP5-/6 gene polymorphisms and its association with risk of abnormal bone mass (ABM) in postmenopausal women.
Methods
The study recruited 166 patients with ABM (case group) and 106 patients with normal bone mass (control group) based on bone mineral density (BMD) results. Multi-factor dimensionality reduction (MDR) was used to analyze the interaction between the Low-density lipoprotein receptor-related protein 5 (LRP5) gene (rs41494349, rs2306862) and the Low-density lipoprotein receptor-related protein 6 (LRP6) gene (rs10743980, rs2302685) and the subjects’ clinical characteristics of age and menopausal years.
Results
(1) Logistic regression analysis showed that the subjects with the CT or TT genotype at rs2306862 had a higher risk of ABM than those with the CC genotype (OR = 2.353, 95%CI = 1.039–6.186; OR = 2.434, 95%CI = 1.071, 5.531; P < 0.05). The subjects with the TC genotype at rs2302685 had a higher risk of ABM than those with the TT genotype (OR = 2.951, 95%CI = 1.030–8.457, P < 0.05). (2) When taking the three Single-nucleotide polymorphisms (SNPs) together, the accuracy was the highest with the cross-validation consistency of 10/10 (OR = 1.504, 95%CI:1.092–2.073, P < 0.05), indicating that the LRP5 rs41494349 and LRP6 rs10743980, rs2302685 were interactively associated with the risk of ABM. (3) Linkage disequilibrium (LD) results revealed that the LRP5 (rs41494349,rs2306862) were in strong LD (D′ > 0.9, r2 > 0.3). AC and AT haplotypes were significantly more frequently distributed in the ABM group than in the control group, indicating that subjects carrying the AC and AT haplotypes were associated with an increased risk of ABM (P < 0.01). (4) MDR showed that rs41494349 & rs2302685 & rs10743980 & age were the best model for ABM prediction. The risk of ABM in “high-risk combination” was 1.00 times that of “low-risk combination”(OR = 1.005, 95%CI: 1.002–1.008, P < 0.05). (5) MDR showed that there was no significant association between any of the SNPs and menopausal years and ABM susceptibility.
Conclusion
These findings indicate that LRP5-rs2306862 and LRP6-rs2302685 polymorphisms and gene–gene and gene–age interactions may increase the risk of ABM in postmenopausal women. There was no significant association between any of the SNPs and menopausal years and ABM susceptibility.
Similar content being viewed by others
Introduction
Osteoporosis (OP) is a metabolic disease, which is characterized by decreased bone density and damaged bone microstructure [1]. Postmenopausal women comprise about 1/3 of the 8.3 million people in China suffering from OP [2]. OP is a complex systemic disease resulting from genetic and environmental factors interacting. Biochemical markers of bone turnover (BTMs) are commonly used for therapy monitoring purposes for osteoporotic patients [3]. Currently, more than 400 gene loci associated with osteoporosis have been found [4, 5]. Among them, the effects of SNP of LRP5 and LRP6 genes on OP have attracted much attention. A study showed that LRP6 polymorphism may be associated with body composition and BMD in Iranian children [6]. Another study showed that there is a modest effect of the LRP5 rs3736228 C > T on the increased susceptibility of osteoporosis [7]. Moreover, the study [8] found that bone mass and bone strength decreased with age, indicating that age played an important role in BMD. In addition, estrogen is one of the important factors affecting OP, postmenopausal hormonal modification negatively impacts the quality of the bone [9]. Several studies have shown that with the increase in menopausal years, the risk of osteoporosis increases [10,11,12]. In our previous study, we investigated that the polymorphism of LRP5 gene was related to the decrease in BMD in postmenopausal women [13]. In order to further explore LRP5-/6 gene polymorphisms and its association with risk of abnormal bone mass in postmenopausal women, we performed a case–control study to investigate the role of LRP5-/6 gene–gene, gene-age and gene-menopausal years interactions on the risk of ABM in postmenopausal women.
Materials and methods
Study subjects
A total of 166 ABM patients and 106 age-matched healthy controls were included in this study. Patients with ABM were consecutively enrolled from the First Affiliated Hospital of Shihezi University School of Medicine between December 2018 and December 2019. The diagnostic criteria for OP refer to guidelines established by WHO in 1994 [14]. The controls were also registered from the same hospital during the same period. Based on the results of BMD, the study subjects were divided into the control group (normal bone mass group) and the ABM group (T-score ≤ -2.5SD for OP, − 2.5SD < T score < -1.0SD represents reduced bone mass; if T score < -1.0SD, represents abnormal bone mass (ABM). Inclusion criteria. (1) Women who have been in natural menopause for more than one year; (2) Ages 47 to 80 years; (3) All the participants were Han nationality living in Xinjiang Uygur Autonomous Region, without any genetic relationship, and had a complete medical history. Exclusion criteria: autoimmune diseases, thyroid, and parathyroid diseases, severe cardiac, hepatic, and renal diseases, and malignant tumors with underlying diseases affecting Ca and other bone metabolic indexes. This study was performed by the ethical guidelines of the Helsinki Declaration of 1975 (revised in 2008). It was approved by the Ethics Committee of the First Affiliated Hospital of Shihezi University School of Medicine (ethics number:2019–129-01). All participants signed informed consent forms.
Observation indicators
General descriptive data of the subjects: age, menopausal years, body mass index (BMI), and waist-to-hip ratio (WHR) of the included study subjects were collected and analyzed. The concentrations of peripheral serum calcium (Ca), phosphorus (P), and alkaline phosphatase (ALP) were measured by Roche automatic biochemical analyzer (Model Modular DPPH7600).
BMD of the lumbar spine 1–4 (L1-4) and femoral neck (FN) was measured by dual-energy X-ray (DEXA).
Genotyping
Genomic DNA was extracted from the peripheral blood of the participants using the DNeasy Blood & Tissue Kit(Thermo Fisher Scientific (China) Equipment Co) and stored at − 80 °C for future experiments. The Agena Mass ARRAY system was used for SNP genotyping. The primers for each locus are shown in Table 1.
Statistical analysis
Statistical analyses were performed using the SPSS 22.0 software. K-S method was used to test the normality of all data. The measurement data that do not conform to the normal distribution were expressed by quartiles, and rank sum test was used for comparison between two groups. The χ2 test was used to determine whether the LRP5 and LRP6 genes conformed to the Hardy–Weinberg equilibrium and the frequency of genotype and gene distribution between groups. Logistic regression analysis was used to analyze the association between gene polymorphism and ABM susceptibility. Multidimensional dimension reduction (MDR) was used to establish models and detect gene–gene, gene-age, and gene-menopausal years interactions. The linkage disequilibrium structure was constructed by SHEsis software, and the genetic association was examined at haplotype level. All tests were bilateral inspections, and a value of P < 0.05 was considered statistically significant.
Results
Characteristics of the research subjects
This study included 166 ABM patients (case group) and 106 healthy controls (control group). Compared with the control group, the ABM group had higher menopausal years (P < 0.05) and lower BMD (L1-4, FN) (P < 0.01) (Table 2).
Hardy–Weinberg genetic equilibrium and ABM susceptibility analysis
The Hardy–Weinberg equilibrium (HWE) test was performed in the case group and the control group. All four loci obeyed the HWE(P > 0.05).
Logistic regression analysis showed that the subjects with the CT or TT genotype at rs2306862 had a higher risk of ABM than those with the CC genotype (OR = 2.353, 95%CI = 1.039–6.186; OR = 2.434, 95%CI = 1.071, 5.531; P < 0.05). The subjects with the TC genotype at rs2302685 had a higher risk of ABM than those with the TT genotype (OR = 2.951, 95%CI = 1.030–8.457, P < 0.05) (Table 3).
LRP5-/6 gene locus interactions
Table 4 shows interaction analysis among the LRP5 rs41494349, rs2306862 and LRP6 rs10743980, rs2302685. When taking the three SNPs together, the accuracy was the highest with the cross-validation consistency of 10/10 (OR = 1.504, 95%CI:1.092–2.073, P < 0.05), indicating that the LRP5 rs41494349 and LRP6 rs10743980, rs2302685 were interactively associated with the risk of ABM.
Analysis of linkage disequilibrium
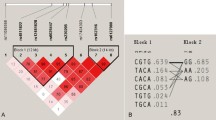
Linkage disequilibrium (LD) results revealed that the LRP5 (rs41494349, rs2306862) were in strong LD (D′ > 0.9, r2 > 0.3) (Fig. 1). AC and AT haplotypes were significantly more frequently distributed in the ABM group than in the control group, indicating that subjects carrying the AC and AT haplotypes were associated with an increased risk of ABM (P < 0.01) (Table 5).
Additionally, the LRP6(rs10743980, rs2302685) were in strong LD(D′ > 0.9, r2 > 0.3) (Fig. 2).There was no significant difference in the haplotype between two groups (Table 6).
Interaction between LRP5-/6 SNPs-age and menopausal years
LRP5-/6 SNPs-age interaction
The MDR was used to analyze the interaction between the alleles of LRP5 rs41494349 rs2306862 and LRP6 rs10743980, rs2302685, and the subjects’ age. We observed that rs41494349 & rs2302685 & rs10743980 & age were the best model for ABM prediction. The risk of ABM in “high-risk combination” was 1.00 times that of ‘low-risk combination’ (OR = 1.005, 95%CI: 1.002–1.008, P < 0.05) (Table 7).
LRP5-/6 SNP- menopausal years
The MDR was used to analyze the interaction between the alleles of LRP5 rs41494349 rs2306862 and LRP6 rs10743980, rs2302685 and the subjects’ menopausal years, and we did not observe any significant association between any of the SNPs and menopausal years and ABM susceptibility (P > 0.05) (Table 8).
Discussion
Postmenopausal osteoporosis is a long-term, progressive disease associated with a high risk of fractures, which seriously threatens people's health [15]. Currently, the most common prescription agent for postmenopausal osteoporosis is Denosuma, which reduces the frequency of non-vertebral fractures and increases bone formation [16, 17].
Wnt signaling pathway is one of the important signaling pathways to regulate bone metabolism [18]. LRP5-/6 receptor protein inhibits bone resorption and promotes bone formation by binding to downstream β-catenin [19], the mutation of LRP5-/6 gene leads to the loss of control of Wnt signal pathway, which affects bone metabolism [20, 21]. OP is not only influenced by genetic factors but also by other factors, such as age and menopause age [22, 23]. However, the pathogenesis of OP cannot be entirely explained by any of the genetic variables that have been found, therefore genetic interaction and gene-age/menopausal years interaction may be another significant cause of the disease.
In the present study, it was found that the risk of ABM in CT and CT/TT genotypes of LRP5 gene at rs2306862 locus was higher than that in CC genotype, indicating that the mutation of allele T increases the risk of ABM. At the rs2302685 locus of LRP6 gene, the risk of ABM in TC genotype was higher than that in TT genotype, indicating that the mutation of allele C increases the risk of ABM. AI et al. [24] found that uncommon loss-of-function mutations facilitate DKK1 competitive binding to the LRP5-/6 receptor in the LRP5 gene, which prevented Wnt/-catenin signaling. Riancho et al. [25] discovered that the polymorphisms at the LRP6 gene locus rs2302685 and rs11054704 were responsible for the decline in BMD in postmenopausal women in Europe. Additionally, Kokubu et al. [26] discovered that people with the CC genotype of the LRP6 gene rs2302685 loci had intercrural BMD that was considerably greater than those with the TT/CT genotype. Animal experiments also revealed that LRP5-/6 are functionally overlapping homologous receptors. In mouse embryonic development, both proteins stimulate postnatal bone acquisition via Wnt signaling stimulates postnatal bone acquisition [27, 28], and LRP6-/- mice were born to die at birth due to mesial skeletal and limb defects, whereas adult LRP5-/- mice result in OP [29]. This conclusion was in line with the present literature reports.
In the present study, we found that the Dʹ value between LRP5-rs41494349 and rs2306862 was more than 0.8; it showed a strong chain reaction. Thus, we also conducted haplotype analysis between rs41494349 and rs2306862. The results indicated that the haplotype containing the AC and AT haplotypes was associated with an increased OP risk. Kitjaroentham A et al. [30] found that the risk of osteopenia/osteoporosis was not correlated with either the rs41494349 or the rs2306862 SNPs. This contradicts our findings, and it may be due to the complex interactions between genes, which require further analysis. There was also a strong linkage disequilibrium between the two SNPs at the LRP6- rs10743980 and rs2302685, but haplotype analysis did not show a difference in haplotype frequency distribution between the two groups. This may be because different haplotype interactions can result in different phenotypic effects, which need further analysis.
At the same time, we investigated the interaction of each LRP5-/6 gene locus and found that the polymorphisms of rs2306862&rs10743980, rs41494349&rs2302685 & rs10743980 gene SNP were synergistic with the development of ABM and were risk factors for the development of ABM. Animal studies have shown that mice carrying both LRP5 and LRP6 heterozygous mutations could reduce BMD and cause limb deformities [31]. Van Meurs et al. [32] found that LRP5 and LRP6 genes played a role in bone metabolism and fracture. These findings imply that genetic variations may influence the occurrence of OP. Age and the polymorphisms at the rs2306862, rs2302685, rs41494349, rs2302685, and rs10743980 SNPs were risk factors for the development of ABM. It is generally known that being older is a risk factor in and of itself for the onset of OP and that OP prevalence rises yearly with age. However, this study did not find a correlation between the risk of ABM and menopausal years, which may be due to the following factors: (1) OP activation of Wnt/β catenin signaling was accompanied by stress activation of the OPG/RANK/RANKL-related osteoblast pathway, which compensatively stimulated osteoblast formation; (2) Different SNPs on the same gene responded differently to environmental factors.
Several limitations of this study should be considered: (1) More studies are needed to confirm our results, especially in larger and different ethnic groups; (2) The study should include more SNPs of LRP5 and LRP6 genes; (3) The mechanisms affecting the reduction of BMD are complex, and more environmental factors affecting BMD should be included in the further study.
Research on the occurrence of ABM has received considerable attention in recent years, our research will increase understanding of the etiology of OP and provide a theoretical basis for the pathogenesis of OP.
Conclusion
These findings indicate that LRP5-rs2306862 and LRP6-rs2302685 polymorphisms and gene–gene and gene-age interactions may increase the risk of ABM in postmenopausal women. There was no significant association between any of the SNPs and menopausal years and ABM susceptibility.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Abbreviations
- LRP5:
-
Low-density lipoprotein receptor-related protein 5
- LRP6:
-
Low-density lipoprotein receptor-related protein 6
- ABM:
-
Abnormal bone mass
- SNPs:
-
Single-nucleotide polymorphisms
- BMD:
-
Bone mineral density
- OP:
-
Osteoporosis
- BTMs:
-
Biochemical markers of bone turnover
- BMI:
-
Body mass index
- WHR:
-
Waist-to-hip ratio
- ALP:
-
Alkaline phosphatase
- FN:
-
Femoral neck
- DEXA:
-
Dual-energy X-ray
- MDR:
-
Multi-factor dimensionality reduction
References
Jordan KM, Cooper C. Epidemiology of osteoporosis. Best Pract Res Clin Rheumatol. 2002;16(5):795–806. https://doi.org/10.1053/berh.2002.0264.
Ralston SH, Fraser J. Diagnosis and management of osteoporosis. Practitioner. 2015;259(1788):15–22.
Migliorini F, Maffulli N, Spiezia F, Tingart M, Maria PG, Riccardo G. Biomarkers as therapy monitoring for postmenopausal osteoporosis: a systematic review. J Orthop Surg Res. 2021;16(1):318. https://doi.org/10.1186/s13018-021-02474-7.
Chiang KM, Chang HC, Yang HC, et al. Genome-wide association study of morbid obesity in Han Chinese. BMC Genet. 2019;20(1):97. https://doi.org/10.1186/s12863-019-0797-x.
Gregson CL, Newell F, Leo PJ, et al. Genome-wide association study of extreme high bone mass: Contribution of common genetic variation to extreme BMD phenotypes and potential novel BMD-associated genes. Bone. 2018;114:62–71. https://doi.org/10.1016/j.bone.2018.06.001.
Montazeri-Najafabady N, Dabbaghmanesh MH, Mohammadian Amiri R. The rs2302685 polymorphism in the LRP6 gene is associated with bone mineral density and body composition in Iranian children. J Gene Med. 2020;22(11): e3245. https://doi.org/10.1002/jgm.3245
Xu GY, Qiu Y, Mao HJ. Common polymorphism in the LRP5 gene may increase the risk of bone fracture and osteoporosis. Biomed Res Int. 2014; 2014:290531. https://doi.org/10.1155/2014/290531
Aspray TJ, Hill TR. Osteoporosis and the ageing skeleton. Subcell Biochem. 2019;91:453–76. https://doi.org/10.1007/978-981-13-3681-2_16.
Migliorini F, Giorgino R, Hildebrand F, et al. Fragility fractures: risk factors and management in the elderly. Medicina (Kaunas). 2021;57(10):1119. https://doi.org/10.3390/medicina57101119.
Chen CT, Liu CT, Chen GK, et al. Meta-analysis of loci associated with age at natural menopause in African-American women. Hum Mol Genet. 2014;23(12):3327–42. https://doi.org/10.1093/hmg/ddu041.
Tella SH, Gallagher JC. Prevention and treatment of postmenopausal osteoporosis. J Steroid Biochem Mol Biol. 2014;142:155–70. https://doi.org/10.1016/j.jsbmb.2013.09.008.
Kopiczko A. Bone mineral density in old age: the influence of age at menarche, menopause status and habitual past and present physical activity. Arch Med Sci. 2019;16(3):657–65. https://doi.org/10.5114/aoms.2019.81314.
Li J, Li S, Zhao H, Li J, Wang S, Shi Y. A study of the relationship between the polymorphism and mutation of rs682429 and rs3781590 in the LRP5 gene and bone metabolism in postmenopausal type 2 diabetic women in Xinjiang. J Diabetes Res. 2020;2020:3071217. https://doi.org/10.1155/2020/3071217.
Siris ES, Adler R, Bilezikian J, et al. The clinical diagnosis of osteoporosis: a position statement from the National Bone Health Alliance Working Group. Osteoporos Int. 2014;25(5):1439–43. https://doi.org/10.1007/s00198-014-2655-z.
Migliorini F, Colarossi G, Baroncini A, Eschweiler J, Tingart M, Maffulli N. Pharmacological management of postmenopausal osteoporosis: a level i evidence based—expert opinion. Expert Rev Clin Pharmacol. 2021;14(1):105–19. https://doi.org/10.1080/17512433.2021.1851192.
Migliorini F, Maffulli N, Colarossi G, Eschweiler J, Tingart M, Betsch M. Effect of drugs on bone mineral density in postmenopausal osteoporosis: a Bayesian network meta-analysis. J Orthop Surg Res. 2021;16(1):533. https://doi.org/10.1186/s13018-021-02678-x.
Migliorini F, Colarossi G, Eschweiler J, Oliva F, Driessen A, Maffulli N. Antiresorptive treatments for corticosteroid-induced osteoporosis: a Bayesian network meta-analysis. Br Med Bull. 2022;143(1):46–56. https://doi.org/10.1093/bmb/ldac017.
Kobayashi Y, Uehara S, Udagawa N, Takahashi N. Regulation of bone metabolism by Wnt signals. J Biochem. 2016;159(4):387–92. https://doi.org/10.1093/jb/mvv124.
Westendorf JJ, Kahler RA, Schroeder TM. Wnt signaling in osteoblasts and bone diseases. Gene. 2004;341:19–39. https://doi.org/10.1016/j.gene.2004.06.044.
Kang KS, Robling AG. New insights into Wnt-Lrp5/6-β-catenin signaling in mechanotransduction. Front Endocrinol (Lausanne). 2015;5:246. https://doi.org/10.3389/fendo.2014.00246.
Acebron SP, Niehrs C. β-catenin-independent roles of Wnt/LRP6 signaling. Trends Cell Biol. 2016;26(12):956–67. https://doi.org/10.1016/j.tcb.2016.07.009.
Vega EM, Egea MA, Mautalen CA. Influence of the menopausal age on the severity of osteoporosis in women with vertebral fractures. Maturitas. 1994;19(2):117–24. https://doi.org/10.1016/0378-5122(94)90061-2.
Bijelic R, Milicevic S, Balaban J. The influence of non-preventable risk factors on the development of osteoporosis in postmenopausal women. Mater Sociomed. 2019;31(1):62–5. https://doi.org/10.5455/msm.2019.31.62-65.
Ai M, Holmen SL, Van Hul W, Williams BO, Warman ML. Reduced affinity to and inhibition by DKK1 form a common mechanism by which high bone mass-associated missense mutations in LRP5 affect canonical Wnt signaling. Mol Cell Biol. 2005;25(12):4946–55. https://doi.org/10.1128/MCB.25.12.4946-4955.2005.
Riancho JA, Olmos JM, Pineda B, et al. Wnt receptors, bone mass, and fractures: gene-wide association analysis of LRP5 and LRP6 polymorphisms with replication. Eur J Endocrinol. 2011;164(1):123–31. https://doi.org/10.1530/EJE-10-0582.
Kokubu C, Heinzmann U, Kokubu T, et al. Skeletal defects in ringelschwanz mutant mice reveal that Lrp6 is required for proper somitogenesis and osteogenesis. Development. 2004;131(21):5469–80. https://doi.org/10.1242/dev.01405.
Rudnicki MA, Williams BO. Wnt signaling in bone and muscle. Bone. 2015;80:60–6. https://doi.org/10.1016/j.bone.2015.02.009.
Burgers TA, Williams BO. Regulation of Wnt/β-catenin signaling within and from osteocytes. Bone. 2013;54(2):244–9. https://doi.org/10.1016/j.bone.2013.02.022.
Pinson KI, Brennan J, Monkley S, Avery BJ, Skarnes WC. An LDL-receptor-related protein mediates Wnt signalling in mice. Nature. 2000;407(6803):535–8. https://doi.org/10.1038/35035124.
Kitjaroentham A, Hananantachai H, Phonrat B, Preutthipan S, Tungtrongchitr R. Low density lipoprotein receptor-related protein 5 gene polymorphisms and osteoporosis in Thai menopausal women. J Negat Results Biomed. 2016;15(1):16. https://doi.org/10.1186/s12952-016-0059-7.
Joeng KS, Schumacher CA, Zylstra-Diegel CR, Long F, Williams BO. Lrp5 and Lrp6 redundantly control skeletal development in the mouse embryo. Dev Biol. 2011;359(2):222–9. https://doi.org/10.1016/j.ydbio.2011.08.020.
van Meurs JB, Rivadeneira F, Jhamai M, et al. Common genetic variation of the low-density lipoprotein receptor-related protein 5 and 6 genes determines fracture risk in elderly white men. J Bone Miner Res. 2006;21(1):141–50. https://doi.org/10.1359/JBMR.050904.
Acknowledgements
Not applicable.
Funding
This work was supported by the funding of the International Science and Technology Cooperation and Promotion Program: Study on the role and mechanism of FOSL2 in T2DM through TGF-β1/Smad3 signaling pathway (GJHZ202206); Science and Technology Project of the Xinjiang Production and Construction Corps (2021AB031); the Achievement transformation and technology popularization project of Shihezi University (CGZH201911).
Author information
Authors and Affiliations
Contributions
JL—final approval of the article; ZL—data analysis and interpretation and writing the article; YR—data analysis and interpretation; HS—collection and assembly of data; SL–research concept and design. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was performed by the ethical guidelines of the Helsinki Declaration of 1975 (revised in 2008). It was approved by the Ethics Committee of the First Affiliated Hospital of Shihezi University School of Medicine (ethics number:2019-129-01). Informed consent was obtained from all subjects.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Li, J., Liu, Z., Ren, Y. et al. LRP5-/6 gene polymorphisms and its association with risk of abnormal bone mass in postmenopausal women. J Orthop Surg Res 18, 369 (2023). https://doi.org/10.1186/s13018-023-03829-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13018-023-03829-y