Abstract
Background
The determination of muscle pathologies in lumbar disc herniation (LDH) and other conditions with low back pain is important for understanding low back problems and determining appropriate treatment methods. In patients with lumbar disc herniation with radiculopathy, elucidating the effect of root compression on the severity of muscle degeneration may predict the importance of alleviating root compression. For this purpose, magnetic resonance imaging (MRI) was used to compare the degeneration and asymmetries of the lumbar musculus multifidus (MF) and lumbar musculus erector spinae (ES) muscles in patients with lumbar discopathy without root compression (radiculopathy) and in patients with lumbar discopathy with root compression (radiculopathy).
Methods
The patients were examined in two groups: 56 patients with lumbar discopathy and no radiculopathy (Non-rad group) and 51 patients with lumbar discopathy and radiculopathy (Rad group). On axial MRI sections passing through the centre of the disc at the L3-S1 level, the asymmetry, cross-sectional area (CSA), fat infiltration, and total CSA (TCSA = MF + ES) of the MF and ES muscles were measured and compared.
Results
No difference was seen between the groups with respect to the CSA values of the right and left MF and left ES, but a significant difference was found in the right ES CSA (p = 0.021). The CSA and TCSA of the MF and ES showed no asymmetry according to group. Severe fat infiltration of > 50% in the right and left MF and left ES was found in the Rad group at a higher rate than in the Non-rad group. Fat infiltration was significantly positively correlated with age, body mass index, and the duration of pain (p < 0.001, p < 0.001, p = 0.004, respectively).
Conclusions
The study results showed a correlation between LDH and paraspinal muscle degeneration, while no correlation was found with asymmetry. Severe (> 50%) fat infiltration is associated with root compression, and the severity of fat filtration increases in the presence of root compression. The development of more severe degeneration due to denervation associated with root compression plays a role in the emergence of this situation. Therefore, in patients with lumbar disc herniation with radiculopathy, it can be foreseen that to stop and correct severe fat infiltration and muscle degeneration, first, nerve root compression should be corrected with appropriate medical treatment methods, and in patients in whom there is no response, the pressure should be alleviated with appropriate surgical methods.
Similar content being viewed by others
Background
Lumbar disc herniation (LDH) is one of the causes of low back pain. Asymptomatic LDH is seen at a rate of 0.5–20%, and symptomatic LDH is seen at a rate of 1–3%. The male/female ratio of LDH is 2/1, and LDH is most often seen in patients between 30 and 50 years of age. In 95% of patients, LDH is at the level of L4-5 or L5-S1 [1,2,3,4]. The localised or widespread migration of disc material (nucleus pulposus or annulus fibrosis) to outside the intervertebral disc space is referred to as LDH [1, 5]. Symptoms and clinical findings can be seen as pain, muscle spasm, restricted movement, and sensory and motor neurological deficits depending on the size and level of disc herniation [6, 7]. In clinical practice, LDH is said to affect the paraspinal muscles, which are generally overlooked [7, 8].
The paraspinal muscles are responsible for the control of intersegmentary movements with movements of the spine and extremities, and they have unique architecture and design features [9, 10]. The MF and ES from the paraspinal muscles form the two main muscle groups that function in lumbar stabilisation and mobilisation [9, 11, 12]. In addition to these two muscles, the psoas muscle is appropriately placed to help stabilise the lumbar cylinder, in which the upper section is the diaphragm, the lower section is the pelvic floor, and the middle is the transversus abdominis muscle [13,14,15]. The psoas is referred to as a rod providing rigidity to and the stabilisation of the lumbar spine in the absence of posterior support [16]. In addition, the psoas contributes to anterior pelvic tilt balance and the rigidity of the lumbar spine as a compensatory mechanism [17].
The paraspinal muscles are of critical importance for spinal health. Paraspinal muscle dysfunction and failure are common in patients with low back pain [18]. Many spinal pathologies (LDH and other causes of low back pain) cause changes in the paraspinal muscles. These changes include an increase in fat and connective tissue in the muscle, fibre type changes, distorted cell populations, altered gene expression, low muscle resistance, low muscle strength, and muscle atrophy [19,20,21,22,23,24,25,26]. These findings are seen in parallel with the denervation and reinnervation of the paraspinal muscles in disc herniation and nerve root compression [18, 27].
Many studies have been conducted to reveal the status of the paraspinal muscles in patients with LDH and other causes of low back pain. Changes in muscle tissue composition, such as fat-fibrotic infiltration (as fatty tissue is not contractile, fat infiltration deteriorates the quality of the paraspinal muscles) [28, 29], muscle atrophy, and changes in fibre types, have been shown to be different at the point of asymmetry in patient populations and animal studies compared with control groups. Some of these studies have been clinical observational studies [26, 30,31,32,33,34,35,36,37], some have been histological studies [19, 38], and some have examined stem cells and performed gene analyses [22, 23, 39]. There are also publications in the literature of experimental animal model studies [24, 27, 40,41,42,43] and studies that have examined the changes occurring naturally in animals [44, 45].
Significant histological differences have been shown between the normal and disc hernia sides [27, 46, 47]. Previous studies have reported that a higher rate of fat-fibrotic infiltration is observed in the paraspinal muscles in patients with LDH; type I fibres (slow twitch and fatigue-resistant fibres) decrease, and type IIX fibres (fast twitch fibres less resistant to fatigue) increase. In addition, it has been shown that paraspinal muscles contain more stem cells in patients with LDH, and these stem cells have higher fibrogenic and adipogenic gene expression [19, 35, 48].
Studies examining the relationships between paraspinal muscle degeneration and dysfunction [23,24,25,26,27, 30, 34, 49,50,51,52,53,54] and pain in the structures in the spinal region (skin, fat tissue, fascia, ligaments, muscles) [55] could be of guidance in the prevention or treatment of spinal pathologies. Thus, the primary aim of this study was to compare the relationships between the CSA and fat infiltration of the MF and ES muscles in patients with low back pain with and without nerve root compression associated with LDH.
The secondary aims of the study were
-
a.
To reveal the relationship between muscle asymmetry and degeneration and the severity of radiculopathy,
-
b.
To reveal whether root compression affects the severity of muscle degeneration by determining the relationships among demographic/physical characteristics, segmentation and herniation.
Thus, the results obtained may guide clinicians in their application of conservative and/or surgical treatment.
Methods
Participants
This observational cross-sectional study included 107 patients who visited the Orthopaedics and Traumatology Clinic between February and May 2021 with complaints of low back pain and were diagnosed with LDH through magnetic resonance imaging (MRI). The patients were aged 20–65 years and described ongoing low back pain for the last 3 months. Two groups were formed of patients with low back pain with radiculopathy (Rad group) and without radiculopathy (Non-rad group). All the study participants signed informed consent forms. Approval for the study was granted by the Local Ethics Committee (YDU/2021/87-1273).
The Non-rad group included 56 patients with LDH but no degenerative disc changes (degenerative disc disease) or vertebral endplate changes (Modic changes) on MRI, no findings of nerve root compression in the neuromuscular examination, no radicular pain, and negativity in specific lumbar tests (straight leg raise, contralateral straight leg raise, Lasegue’s test, femoral nerve tension test). When nerve compression was suspected, EMG was requested. Patients with nerve compression determined on MRI and EMG and those with degenerative disc disorders were not included in the Non-rad group. The Rad group included 51 patients with radicular pain in the leg, positivity in the specific lumbar tests, and findings of nerve root compression on EMG and MRI.
MRI was performed to reveal the aetiology of the low back pain. The hernia types were classified as median, paramedian, foraminal, or extraforaminal according to the location of the protruding section of the disc and as bulging, protrusion, extrusion, or sequestration based on the degree of protrusion on MRI [38, 39]. The MRI-based nerve compression grading system was used to classify radiculopathy in this study [51, 52].
Low back pain was defined as pain between the inferior edge of the costae and the gluteal fold. Radicular pain was defined as pain radiating from below the gluteal fold to the thigh, leg, and foot. The pain was described by the patient and confirmed by the physician performing the examination. Pain intensity was evaluated using a standard 100 mm visual analogue scale (VAS).
Patients were excluded from the study if they had deformities (scoliosis, etc.) affecting the spine, malformations, pelvic disorders, limb length discrepancies, additional coxarthrosis, rheumatological diseases (rheumatoid arthritis, ankylosing spondylitis), infections, a history of lumbar surgery and/or low back treatment, vertebral fractures, spondylolisthesis, epidural injections, radiofrequency ablations, metabolic diseases (diabetes mellitus, etc.), obesity, pregnancy, or a history of malignancy.
Demographic, clinical, and disease-related data were obtained from the patients in face-to-face interviews. In patients where a differential diagnosis was necessary, C-reactive protein (CRP), antistreptolysin O (ASO), rheumatoid factor (RF), haemogram, erythrocyte sedimentation rate (ESR), and Salmonella and Brucella tests, as well as a full urine analysis, were requested. The examinations of the 107 patients were performed by two physicians with 25 years of experience in spinal surgery, and the lumbar spine MR images were analysed by an experienced consultant and radiology specialist blinded to the clinical history of the patients. The MR images were all taken by the same radiology technician.
Measurements
Magnetic resonance imaging
Imaging was performed with a 1.5 Tesla MR device (Signa Explorer SV25.3 16 channel, up-to-date software, General Electric, Milwaukee, WI, USA). The images were obtained with the patient in a supine position, with a routine protocol directed to the lumbar spine and with the measurement level between L3 and S1 (L3-4/L4-5/L5-S1) to be able to view the centre of the disc, parallel to the vertebral endplates. Turbo spin‒echo T1- and T2-weighted sagittal and turbo spin‒echo T2 axial 4-mm sections parallel to the disc spaces were taken. Evaluations were made on T2 axial sections. The asymmetry, fat content, CSA, and TCSA of the right and left sides of the MF and ES (m. iliocostalis and m. longissimus) were examined at the L3-S1 level. The technical values were TR 400–600, TE 15–25, thickness 4 mm, band width 25–150, and rotational angle 90° for T1 imaging and TR 3000–4000, TE 100–120, thickness 4 mm, band width 25–150, and rotational angle 130°–150° for T2 imaging, and the acquisition time was a mean of 10 min.
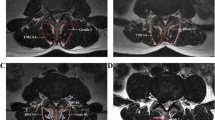
Assured reliability has been found in the evaluation of muscle-related changes on MRI [53, 54]. MRI has higher image resolution and provides better determination of muscle and soft tissues such as fat than ultrasonography and computed tomography [56,57,58]. Muscle degeneration is usually evaluated in axial MRI with T1- and T2-weighted sequences (Fig. 1) [54, 56, 58]. It has been reported that muscle degeneration can be observed on MRI scans as a decrease in muscle size or an increase in fat accumulation [20, 25].
T2 sagittal and axial sections of L5-S1 of a patient in the radiculopathy group, showing more evident narrowing of both neural foramens on the left side; an indentation in the anterior epidural space, with fat obliterating the left lateral recess; contact with the S1 nerve root; and the appearance of an extruding disc
In the objective evaluation of disc herniations, MRI and EMG are widely used methods. In the determination of disc diseases, MRI has become the gold standard. However, information about the functional and physiological status of neural and muscle tissues can be provided with the EMG method [59,60,61].
The indications for spinal MRI include degenerative disc disease and disc herniations and the evaluation of spinal deformities (scoliosis, kyphosis), spinal trauma, spinal instabilities, spinal stenosis, spinal infections, results of disc herniation surgery and instrumentation, implantation-related complications, and suspected cancer (primary and metastatic tumours of the spine) [62,63,64].
Generally, EMG is used in the diagnosis and differential diagnosis of peripheral neuropathy; motor neuron diseases; primary muscle diseases; and local (entrapment neuropathies), plexus (plexus brachialis), and radicular lesions. EMG has an important place in the differential diagnosis of radiculopathy in disc herniation and in the follow-up of prognosis [59,60,61, 65, 66]. Lower extremity sensory and motor nerve conduction tests were performed with EMG in the current study patients. The presence of denervation findings was evaluated with needle EMG in the muscles with L3, L4, L5, and S1 root innervation in both lower extremities.
In this study, fat infiltration was evaluated semiquantitatively. The CSA was measured by determining the fascial borders (epimysium) of the muscles and using the PACS (picture archive and communication system) report and manual drawing. In the evaluation, fat infiltration was defined as the replacement of the muscle mass with fat and fibrous tissue, with < 10% as normal, 10%–50% as moderate, and > 50% as severe (Fig. 2) [26, 31]. Asymmetry was calculated as “the largest edge—the smallest edge/the largest edge × 100” and presented as a percentage (%) [67].
For intra- and interobserver reliability, 15 randomly selected patients were evaluated again after 1 month by the same radiologist (K.T.) and by a different radiologist (H.E.). The intraobserver kappa agreement value obtained was 0.953, and the interobserver agreement kappa value obtained was 0.935.
Statistical analysis
Data obtained in the study were analysed statistically using IBM SPSS vn. 23 software (IBM, Chicago, IL, USA). Taking the CSA mean values into consideration, it was determined to be necessary to have a minimum sample size of 110 participants with 55 in each group for 95% confidence (1-α), 95% test power (1-β), and an effect size of f = 0.634. The study was completed with a total of 107 patients, 56 in the Non-rad group and 51 in the Rad group. According to the post hoc power analysis, the test power with 107 participants was determined to be 94.7% [68].
MANOVA was used to compare CSA and TCSA values according to group, segment, and sex. For multiple comparisons, the Duncan test was used. In the comparisons of age, BMI, the duration of low back pain, and VAS scores between the Rad and Non-rad groups, the Mann‒Whitney U test was used. The chi-square test was applied in the comparisons of categorical data. A value of p < 0.05 was accepted as statistically significant.
Results
The demographic characteristics of the patients are given in Table 1. No significant differences were seen between the groups with respect to the mean values of age, sex, BMI, and the duration of low back pain. Statistically significant differences were determined between the groups with respect to pain severity as resting and activity VAS scores (p < 0.001).
The comparisons of the CSA and TCSA values of the groups according to sex and segment are presented in Table 2. No difference was seen in the CSA of the right and left MF and the left ES between the groups, but a significant difference was seen in the right ES (p = 0.021). The mean CSA value of the Non-rad group was lower. A significant difference was seen in the mean right TCSA values between groups, with lower values obtained in the Non-rad group (p = 0.005).
A significant difference was seen in the right MF, right and left ES mean CSA values between groups, and a difference was seen in the right and left mean TCSA values according to sex, with higher values obtained in males than in females (p < 0.001, p < 0.001, p < 0.001, p < 0.001, and p = 0.003, respectively).
According to segments, the highest mean values for the right and left MF CSA were seen in the L5-S1 segment and the lowest mean values were seen in the L3-L4 segment (p < 0.001). The highest mean values for the right and left ES CSA were seen in the L3-L4 segment, and the lowest mean values were seen in the L5-S1 segment (p < 0.001). A significant difference was also seen in the mean TCSA values according to segments (p < 0.001).
No difference was observed between the mean values according to sex or segment. The mean right and left ES CSA values in the L5-S1 segment according to group and segment were determined to be lower in the Rad group than in the Non-rad group (p = 0.005, p = 0.043).
The results of the correlation analyses between the CSA values of the groups and age, BMI, pain duration, and pain severity (resting and activity VAS) scores are presented in Table 3. In the Non-rad group, a weak positive correlation was found between age and the left ES CSA and left TCSA (r = 0.270 and r = 0.271, respectively). In the Rad group, a weak negative correlation was found between age and the right MF CSA (r = − 0.281). In the Non-rad group, a moderate-level positive correlation was found between BMI and the right and left ES CSA and TCSA (r = 0.520, r = 0.482, r = 0.462, r = 0.422, respectively). No relationship was found between the duration and severity of pain and the CSA or TCSA.
The fat infiltration values according to group are presented in Table 4. A significant difference was seen between the groups in the fat infiltration of the right MF (p = 0.015). Grade 2 fat infiltration (10–50%) was found in 72% of the Non-rad group and in 56.9% of the Rad group. Grade 3 fat infiltration (> 50%) was seen at a higher rate in the right and left MF and left ES in the Rad group than in the Non-rad group.
A significant difference was found in the right and left MF fat infiltration according to age, BMI, and pain duration (p < 0.001, p < 0.001, p = 0.004, p < 0.001, p < 0.001, p = 0.003, respectively), but no difference was determined according to VAS activity or VAS resting scores (p = 0.129, p = 0.062, p = 0.423, p = 0.300, respectively).
A significant difference was found in the right and left ES fat infiltration according to age, BMI, and pain duration (p < 0.001, p < 0.001, p < 0.001, respectively), but no difference was found according to VAS activity or VAS resting scores (p = 0.251, p = 0.359, respectively).
The comparisons of categorical data according to fat infiltration are presented in Table 5. No difference in fat infiltration was seen according to herniation level or radiculopathy side. When examined according to segments, in the right and left MF and ES, the rate of Grade 1 (< 10%) fat infiltration was higher in the L3-L4 segment, and the rate of Grade 2 (10%-50%) fat infiltration was higher in the L4-L5 and L5-S1 segments. When examined according to sex, a significantly higher rate of fat infiltration was seen in females than in males (p < 0.001).
The distribution of fat infiltration in the MF and ES according to the number of herniations is presented in Table 6. A significant difference was seen in the right and left ES fat infiltration distributions (p < 0.001). A difference was seen in the distribution between patients with one herniation and those with three herniations. The rate of Grade 1 (< 10%) fat infiltration was higher in patients with a single herniation, and as the number of herniations increased, the rate and severity of fat infiltration increased.
The comparisons of the presence of asymmetry according to group are presented in Table 7. No difference was found between the groups with respect to MF and ES asymmetry. No difference was found between the groups with respect to TCSA asymmetry.
When the general levels of herniation were examined, in the Non-rad group, bilateral herniation was found in 85.7%, right-side herniation in 3.6%, and left-side herniation in 10.7% of patients. In the Rad group, bilateral herniation was found in 63.5%, right-side herniation in 38.5%, and left-side herniation in 61.5% of patients.
A difference in the distribution of herniation levels according to group was observed (p = 0.087). The distribution of levels of herniation was 46.4% in the L3-L4 segment, 88.1% in the L4-L5 segment, and 62.5% in the L5-S1 segment in the Non-rad group and 58.8% in the L3-L4 segment, 88.2% in the L4-L5 segment, and 80.4% in the L5-S1 segment in the Rad group (Table 8).
Discussion
In the results of this study, no significant difference was seen in the CSA values between the groups. Only the mean CSA value in the right ES was lower in the Non-rad group, and similarly, the mean right TCSA values were lower. In a study by Hyun et al. [69] of groups with disc herniation with and without radiculopathy, no significant difference was observed between the sides with respect to the TCSA or functional CSA (FCSA), but the FCSA values were higher in the group without radiculopathy than in the group with radiculopathy. In another study of patients operated on because of LDH, no difference was seen in the paraspinal muscle CSA values compared with the less affected side [70]. Some studies have reported the hypertrophy of the MF according to the CSA in LDH patients compared to control participants [34, 71]. In the current study, the right ES CSA and right TCSA values of the Non-rad group were lower than those of the Rad group, suggesting that increased CSA (pseudohypertrophy) was associated with severe fat infiltration of > 50% in the Rad group. Despite a decrease in FCSA when there is pseudohypertrophy, an increase is observed in TCSA because of the increasing fat tissue [25, 26]. The findings determined in the current study support this view.
In the current study, Grade 3 fat infiltration (> 50%) was found at a higher rate in the Rad group than in the Non-rad group. Ji Hye Min et al. [30] reported a relationship between radiculopathy and fat infiltration in the MF muscle associated with more severe and widespread atrophy. Kader et al. [72] determined that MF atrophy was associated with fat infiltration in 80% of patients with disc degeneration and nerve compression identified on MRI scans and showed that there was more severe and widespread MF atrophy in patients with radiculopathy than in those without radiculopathy. Chen et al. [23] showed that in patients with radiculopathy treated with microdiscectomy, markers for impaired muscle regeneration were associated with worse outcomes. In an experimental study of disc injury, Hodge et al. [73, 74] reported that after 6 months, there was an increase histologically in fat and connective tissue in the MF, but atrophy was not identified, and these findings were stated to be compatible with gene expression data from cytokines that play a role in both adipogenesis and fibrosis. It has been suggested that fat infiltration in muscles mediated by proinflammatory cytokines and muscle inhibition in the acute and subacute phases is associated with nonuse in later stages [24]. Similar to these findings in the literature, grade 2 and 3 fat infiltration and associated muscle atrophy were found in 73.3%–77.9% of the patients in both groups of the current study [53, 72]. As in other studies, atrophy associated with fat infiltration was evident, especially at the L5-S1 level [26, 30, 72]. As the number of herniations increased, the ratio of the involvement of the right–left ES muscles and the severity of fat infiltration significantly increased. Although the levels of fat infiltration seemed to be similar between the Rad and Non-rad groups, a higher rate of severe (> 50%) fat infiltration was observed in the Rad group. This suggests that nerve root compression originates from the increased severity of degeneration in the paraspinal muscles. When intervertebral foramens are under pressure (disc-related nerve root compression, spinal stenosis), denervation may explain fat infiltration and atrophy [75]. The results of the current study were consistent with findings in the literature.
No difference was found between the two groups with respect to asymmetry in the current study. Previous studies of healthy individuals have reported differences of 3 ± 4% [76] and 7.2 ± 9.6% [77] between the right and left sides compared to the largest edge. Based on these results, it has been reported that a difference of > 10% can be defined as asymmetry [26]. Kader et al. [72] stated that the majority of muscle degeneration in patients with unilateral radiculopathy at a single level was bilateral and multilevel. Ji Hye Min et al. [30] found no difference in MF asymmetry between bilateral and unilateral nerve root compression and bilateral MF atrophy in most patients with unilateral radiculopathy. In a study by Fortin et al. [35], there was no significant MF asymmetry at the upper or lower spinal segments of the level of disc herniation. The results of the current study showed that muscle degeneration not only occurred on the side of disc herniation and nerve compression but also developed on the contralateral side and/or in the upper and lower segments. In addition, it was thought that there was no difference between the groups with respect to asymmetry because muscle atrophy and fat infiltration were distributed equally on the right and left sides. Thus, it can be concluded that in patients with LDH with and without nerve compression, muscle degeneration developed after 3 months regardless of the herniation side, and as observed in patients with acute unilateral LDH [27], degeneration is not localised only on the side of the herniated disc but is widespread, involving the opposite segment and/or upper and lower segments. The current study results were consistent with the literature. However, in some studies, a relationship between paraspinal muscle asymmetry and unilateral low back pain with or without radiculopathy has been observed [31, 33, 36, 38, 76, 78, 79].
In the current study, the CSA and TCSA values of males were found to be higher than those of females. Previous studies have also shown a greater CSA and higher density of paraspinal muscles in males than in females [18, 26, 30, 72, 80, 81]. The current study results were consistent with the literature.
The results of the current study demonstrated a weak and moderate-level relationship between the CSA and TCSA values of the muscles and age and BMI. Studies in the literature have reported different results related to age. Some studies have stated that the CSA of muscles decreases with age [26, 30, 72, 81, 82], while others have shown no such relationship [68, 80, 83, 84]. Some studies have found a significant correlation between BMI and muscle values and have stated that paraspinal muscle changes are related to BMI [85], whereas others have stated that there is no relationship between BMI and CSA [53, 68, 82].
In the current study, age, sex, BMI, and the duration of pain were found to be related to fat infiltration. A higher rate of fat infiltration was found in females than in males. An increase in fat infiltration with ageing was seen in both groups, and as BMI increased, there was an increase in fat infiltration. As the duration of pain increased, the rate of fat infiltration increased in both groups. Fat infiltration of the paraspinal muscles was generally observed in the area adjacent to the vertebra. In the literature, a higher rate of fat infiltration in females than in males has been reported [53, 86, 87]. Kjaer et al. [53] suggested that the significant difference in fat infiltration in the MF muscles of males and females could be a result of differences in body composition, and the higher rate of fat infiltration in the MF in females could be a reflection of the higher percentage of body fat in females. Studies in the literature have stated that fat infiltration increases with ageing [86, 88,89,90]. Mcloughlin et al. [89] determined a relationship between paraspinal fat accumulation and age and the amount of subcutaneous fat and stated that this was a sign of paraspinal muscle atrophy in patients who had not undergone surgery. Some studies have shown a relationship between BMI and fat infiltration [21, 83], while others have found no relationship [53, 82]. In three studies by Ranger et al., which examined the relationship between MF fat infiltration and the duration of pain, there was limited evidence of no significant relationship between periods of less than or more than one year and fat infiltration [32]. According to the results of another study, there was a relationship between the duration of pain and the severity of MF muscle atrophy, although not at a statistically significant level, and it was stated that high pain scores tended to indicate an increase in muscle atrophy [26].
In the current study, fat infiltration was determined to be the lowest at L3-L4 and the greatest at L5-S1. According to the group–segment interaction, the right ES CSA was lowest in the L5-S1 segment in the Rad group, which was lower than that in the Non-rad group. The involvement of the MF was greater at L4-L5 and L5-S1, and in the ES, it was greater at L5-S1. The VAS resting and activity scores were found to be higher in the Rad group with severe (> 50%) fat infiltration than in the Non-rad group. In a recent study, it was reported that there was greater fat infiltration in the multifidus at the L4-L5 level in patients with a higher VAS score than in those with a lower VAS score, and to compensate for this, less fat infiltration formed in the psoas [16].
Muscles are tissues with the capacity to renew. Under various stimuli (neural activation, denervation, wounds and an inflammatory environment, exercise, flexion, and overloading), changes may occur in muscle measurements, and these changes affect the muscle strength capability [91]. In individuals with regular physical activity, the proinflammatory response against intervertebral disc degeneration is reduced, fibrosis is reduced, and through the regulation of the gene network in the MF, most harmful effects are prevented [39]. To reverse, reduce, or halt the degenerative changes in the paraspinal muscles, which are related to LDH, it is important that pharmacological treatment and appropriate exercise programmes determined according to the psychological and social status of the patient are prescribed and that patients visiting the clinic are informed about and referred to these exercise programmes.
A limitation of the current study was that there was no control group. Therefore, no comparison could be made with the fat infiltration level and asymmetry of healthy individuals. However, previous studies of healthy individuals have reported a relationship between fat infiltration and LDH and that there is asymmetry of > 10% difference between the right and left sides [20, 26, 30, 35]. Other limitations were that there were no evaluations of all the lumbar paraspinal muscles at all lumbar levels (L1-S1), disc degeneration, endplate changes (Modic changes), or facet joint degeneration [16, 92,93,94,95]. The facts that the patients were not classified according to decade age groups and the subcutaneous fat index was not used instead of BMI are other limitations of our study [96, 97].
Conclusion
In conclusion, there seems to be a relationship between widespread (2 or more lumbar segments) involvement of the lumbar paraspinal muscles together with severe (> 50%) fat infiltration and radiculopathy. Denervation in disc herniations, reinnervation, and nonuse are said to lead to atrophy in paraspinal muscles and increased fat infiltration in the muscles [98, 99]. By becoming more evident in the presence of root compression, this seems to cause more severe degeneration in the muscles and fat infiltration. This result shows the need for immediate correction of root compression in patients with radiculopathy, first with appropriate medical treatment methods (anti-inflammatory drugs, muscle relaxants, opioids when necessary, pain control with local applications and/or intense physiotherapy (massage, ultrasound, electric stimulation, exercises, magnets, manipulation) and then, if these are not successful, appropriate surgical methods to prevent the progression of denervation.
Availability of data and materials
The data obtained and analysed in this study are not available to the public because of ethical regulations and local management procedures but can be obtained on request from the corresponding author.
Abbreviations
- MF:
-
Lumbar musculus multifidus
- ES:
-
Lumbar musculus erector spinae
- MRI:
-
Magnetic resonance imaging
- CSA:
-
Cross-sectional area
- TCSA:
-
Total cross-sectional area
- FCSA:
-
Functional cross-sectional area
- VAS:
-
Visual analogue scale
- LDH:
-
Lumbar disc herniation
- BMI:
-
Body mass index
- EMG:
-
Electromyography
- CT:
-
Computed tomography
- Non-rad:
-
Lumbar disc herniation group without radiculopathy
- Rad:
-
Lumbar disc herniation group with radiculopathy
References
Jordan J, Konstantinou K, O’Dowd J. Herniated lumbar disc. BMJ Clin Evid. 2009. https://doi.org/10.1302/0301-620X.101B4.BJJ-2018-1184.R1.
Fjeld OR, Grøvle L, Helgeland J, Småstuen MC, Solberg TK, Zwart JA, et al. Complications, reoperations, readmissions, and length of hospital stay in 34 639 surgical cases of lumbar disc herniation. Bone Joint J. 2019;101(B(4)):470–7.
Schwarzer AC, Aprill CN, Derby R, Fortin J, Kine G, Bogduk N. The prevalence and clinical features of internal disc disruption in patients with chronic low back pain. Spine (Phila Pa 1976). 1995;20(17):1878–83.
Rubin DI. Epidemiology and risk factors for spine pain. Neurol Clin. 2007;25:353–71.
Brinjikji W, Luetmer PH, Comstock B, Bresnahan BW, Chen LE, Deyo RA, et al. Systematic literature review of imaging features of spinal degeneration in asymptomatic populations. AJNR Am J Neuroradiol. 2015;36(4):811–6.
Hoy D, March L, Brooks P, Blyth F, Woolf A, Bain C, et al. The global burden of low back pain: estimates from the Global Burden of Disease 2010 study. Ann Rheum Dis. 2014;73(6):968–74.
Kong WZ, Goel VK, Gilbertson LG, Weinstein JN. Effects of muscle dysfunction on lumbar spine mechanics. A finite element study based on a two motion segments model. Spine (Phila Pa 1996). 1996;21(19):2197–206.
Battié MC, Niemelainen R, Gibbons LE, Dhillon S. Is level- and side-specific multifidus asymmetry a marker for lumbar disc pathology? Spine J. 2012;12(10):932–9.
Fortin M, Gibbons LE, Videman T, Battié MC. Do variations in paraspinal muscle morphology and composition predict low back pain in men? Scand J MedSci Sports. 2015;25(6):880–7.
Rosatelli AL, Ravichandiran K, Agur AM. Three-dimensional study of the musculotendinous architecture of lumbar multifidus and its functional implications. Clin Anat. 2008;21(6):539–46.
Comerford MJ, Mottram SL. Movement and stability dysfunction–contemporary developments. Man Ther. 2001;6(1):15–26. https://doi.org/10.1054/math.2000.0388.
Davies R. Spinal control: the rehabilitation of back pain, state of the art and science. Physiother Can. 2015;67(4):394.
Hodges PW, Gandevia SC, Richardson CA. Contractions of specific abdominal muscles in postural tasks are affected by respiratory maneuvers. J Appl Physiol. 1985;83(3):753–60.
Sapsford RR, Hodges PW, Richardson CA, Cooper DH, Markwell SJ, Jull GA. Co-activation of the abdominal and pelvic floor muscles during voluntary exercises. Neurourol Urodyn. 2001;20(1):31–42.
Moseley GL, Hodges PW, Gandevia SC. Deep and superficial fibers of the lumbar multifidus muscle are differentially active during voluntary arm movements. Spine (Phila Pa 1976). 2002;27(2):29–36.
Özcan-Ekşi EE, Ekşi MŞ, Turgut VU, Canbolat Ç, Pamir MN. Reciprocal relationship between multifidus and psoas at L4–L5 level in women with low back pain. Br J Neurosurg. 2021;35(2):220–8.
Brinjikji W, Diehn FE, Jarvik JG, Carr CM, Kallmes DF, Murad MH, Luetmer PH. MRI findings of disc degeneration are more prevalent in adults with low back pain than in asymptomatic controls: a systematic review and meta-analysis. AJNR Am J Neuroradiol. 2015;36(12):2394–9.
Noonan AM, Brown SHM. Paraspinal muscle pathophysiology associated with low back pain and spine degenerative disorders. JOR Spine. 2021;4(3): e1171.
Agha O, Mueller-Immergluck A, Liu M, Zhang H, Theologis AA, Clark A, et al. Intervertebral disc herniation effects on multifidus muscle composition and resident stem cell populations. JOR Spine. 2020;3(2): e1091.
Danneels LA, Vanderstraeten GG, Cambier DC, Witvrouw EE, De Cuyper HJ. CT imaging of trunk muscles in chronic low back pain patients and healthy control subjects. Eur Spine J. 2000;9(4):266–72.
Lee SH, Park SW, Kim YB, Nam TK, Lee YS. The fatty degeneration of lumbar paraspinal muscles on computed tomography scan according to age and disc level. Spine J. 2017;17:81–7.
Shahidi B, Fisch KM, Gibbons MC, Ward SR. Increased fibrogenic gene expression in multifidus muscles of patients with chronic versus acute lumbar spine pathology. Spine. 2020;45(4):E189–95.
Chen X, Hodges PW, James G, Diwan AD. Do markers of inflammation and/or muscle regeneration in lumbar multifidus muscle and fat differ between individuals with good or poor outcome following microdiscectomy for lumbar disc herniation? Spine. 2021;46:678–86.
Hodges PW, Danneels L. Changes in structure and function of the back muscles in low back pain: different time points, observations, and mechanisms. J Orthop Sports Phys Ther. 2019;49(6):464–76.
Parkkola R, Rytokoski U, Kormano M. Magnetic resonance imaging of the discs and trunk muscles in patients with chronic low back pain and healthy control subjects. Spine. 1993;18:830–6.
Kalichman L, Carmeli E, Been E. The association between imaging parameters of the paraspinal muscles, spinal degeneration, and low back pain. Biomed Res Int. 2017. https://doi.org/10.1155/2017/2562957.
Hodges P, Holm AK, Hansson T, Holm S. Rapid atrophy of the lumbar multifidus follows experimental disc or nerve root injury. Spine. 2006;31(25):2926–33.
MacDonald D, Moseley LG, Hodges PW. Why do some patients keep hurting their back? Evidence of ongoing back muscle dysfunction during remission from recurrent back pain. Pain. 2009;142(3):183–8.
MacDonald D, Moseley GL, Hodges PW. People with recurrent low back pain respond differently to trunk loading despite remission from symptoms. Spine (Phila Pa 1976). 2010;35(7):818–24.
Min JH, Choi HS, Rhee WI, Lee JI. Association between radiculopathy and lumbar multifidus atrophy in magnetic resonance imaging. J Back Musculoskelet Rehabil. 2013;26:175–81.
Kim WH, Lee SH, Lee DY. Changes in the cross-sectional area of multifidus and psoas in unilateral sciatica caused by lumbar disc herniation. J Korean Neurosurg Soc. 2011;50(3):201.
Ranger TA, Cicuttini FM, Jensen TS, Peiris WL, Hussain SM, Fairley J, et al. Is the size and composition of the paraspinal muscles associated with low back pain? A systematic review. Spine J. 2017;17:1729–48.
Cooley JR, Walker BF, Ardakani EM, Kjaer P, Jensen TS, Hebert JJ. Relationships between paraspinal muscle morphology and neurocompressive conditions of the lumbar spine: a systematic review with meta-analysis. BMC Musculoskelet Disord. 2018;19(1):1–21.
Shahidi B, Hubbard JC, Gibbons MC, Ruoss S, Zlomislic V, Allen RT, et al. Lumbar multifidus muscle degenerates in individuals with chronic degenerative lumbar spine pathology. J Orthop Res. 2017;35(12):2700–6.
Fortin M, Lazary A, Varga PP, McCall I, Battie MC. Paraspinal muscle asymmetry and fat infiltration in patients with symptomatic disc herniation. Eur Spine J. 2016;25(5):1452–9.
Barker KL, Shamley DR, Jackson D. Changes in the cross-sectional area of multifidus and psoas in patients with unilateral back pain: the relationship to pain and disability. Spine. 2004;29:E515–9.
Ploumis A, Michailidis N, Christodoulou P, Kalaitzoglou I, Gouvas G, Beris A. Ipsilateral atrophy of paraspinal and psoas muscle in unilateral back pain patients with monosegmental degenerative disc disease. Br J Radiol. 2011;84(1004):709–13.
Agten A, Stevens S, Verbrugghe J, Timmermans A, Vandenabeele F. Biopsy samples from the erector spinae of persons with nonspecific chronic low back pain display a decrease in glycolytic muscle fibers. Spine J. 2020;20(2):199–206.
James G, Klyne DM, Millecamps M, Stone LS, Hodges PW. ISSLS prize in basic science 2019: physical activity attenuates fibrotic alterations to the multifidus muscle associated with intervertebral disc degeneration. Eur Spine J. 2019;28(5):893–904.
Cho TG, Park SW, Kim YB. Chronic paraspinal muscle injury model in rat. J Korean Neurosurg Soc. 2016;59(5):430.
Hu ZJ, Zhang JF, Xu WB, Zhao FD, Wang JY, Fan SW, et al. Effect of pure muscle retraction on multifidus injury and atrophy after posterior lumbar spine surgery with 24 weeks observation in a rabbit model. Eur Spine J. 2017;26(1):210–20.
James G, Sluka KA, Blomster L, Hall L, Schmid AB, Shu CC, et al. Macrophage polarization contributes to local inflammation and structural change in the multifidus muscle after intervertebral disc injury. Eur Spine J. 2018;27(8):1744–56.
Maas H, Noort W, Hodges PW, van Dieën J. Effects of intervertebral disc lesion and multifidus muscle resection on the structure of the lumbar intervertebral discs and paraspinal musculature of the rat. J Biomech. 2018;70:228–34.
Lerer A, Nykamp SG, Harriss AB, Gibson TW, Koch TG, Brown SH. MRI-based relationships between spine pathology, intervertebral disc degeneration, and muscle fatty infiltration in chondrodystrophic and non-chondrodystrophic dogs. Spine J. 2015;15(11):2433–9.
Bergknut N, Rutges JP, Kranenburg HJ, Smolders LA, Hagman R, Smidt HJ, et al. The dog as an animal model for intervertebral disc degeneration? Spine (Phila Pa 1976). 2012;37(5):351–8.
Zhao WP, Kawaguchi Y, Matsui H, Kanamori M, Kimura T. Histochemistry and morphology of the multifidus muscle in lumbar disc herniation: comparative study between diseased and normal sides. Spine (Phila Pa 1976). 2000;25(17):2191–9.
Yoshihara K, Shirai Y, Nakayama Y, Uesaka S. Histochemical changes in the multifidus muscle in patients with lumbar intervertebral disc herniation. Spine (Phila Pa 1976). 2001;26(6):622–6.
Zhu XZ, Parnianpour M, Nordin M, Kahanovitz N. Histochemistryand morphology of erector spinae muscle in lumbar disc herniation. Spine. 1989;14(4):391–7.
Fardon DF. Nomenclature and classification of lumbar disc pathology. Spine. 2001;26:461–2.
Mysliwiec LW, Cholewicki J, Winkelpleck MD, Eis GP. MSU classification for herniated lumbar discs on MRI: toward developing objective criteria for surgical selection. Eur Spine J. 2010;19(7):1087–93.
Pfirrmann CW, Dora C, Schmid MR, Zanetti M, Hodler J, Boos N. MR image-based grading of lumbar nerve root compromise due to disk herniation: reliability study with surgical correlation. Radiology. 2004;230(2):583–8.
Kaliya-Perumal AK, Ariputhiran-Tamilselvam SK, Luo CA, Thiagarajan S, Selvam U, Sumathi-Edirolimanian RP. Revalidating Pfirrmann’s magnetic resonance image-based grading of lumbar nerve root compromise by calculating reliability among orthopaedic residents. Clin Orthop Surg. 2018;10(2):210–5.
Kjaer P, Bendix T, Sorensen JS, Korsholm L, Leboeuf-Yde C. Are MRI-defined fat infiltrations in the multifidus muscles associated with low back pain? BMC Med. 2007;5:2.
Solgaard Sorensen J, Kjaer P, Jensen ST, Andersen P. Low- field magnetic resonance imaging of the lumbar spine: relia- bility of qualitative evaluation of disc and muscle parameters. Acta Radiol. 2006;47:947–53.
Kuslich SD, Ulstrom CL, Michael CJ. The tissue origin of low back pain and sciatica: a report of pain response to tissue stimulation during operations on the lumbar spine using local anesthesia. Orthop Clin North Am. 1991;22(2):181–7.
Dulor JP, Cambon B, Vigneron P, Reyne Y, Nougues J, Casteilla L, et al. Expression of specific white adipose tissue genes in denervation-induced skeletal muscle fatty degeneration. FEBS Lett. 1998;439:89–92.
Crawford RJ, Cornwall J, Abbott R, Elliott JM. Manually defining regions of interest when quantifying paravertebral muscles fatty infiltration from axial magnetic resonance imaging: a proposed method for the lumbar spine with anatomical cross-reference. BMC Musculoskelet Disord. 2017;18(1):25.
Upadhyay B, Toms A. CT and MRI evaluation of paraspinal muscle degeneration. Europen Cociety of Radiology
Lee JH, Lee SH. Physical examination, magnetic resonance image, and electrodiagnostic study in patients with lumbosacral disc herniation or spinal stenosis. J Rehabil Med. 2012;44:845–50.
Qie S, Li W, Li X, Chen X, Gong W, Xi J, Sun F, Yue S. Electromyography activities in patients with lower lumbar disc herniation. J Back Musculoskelet Rehabil. 2020;33(4):589–96.
Li W, Liu YC, Zheng CF, Miao J, Chen H, Quan HY, Yan SH, Zhang K. Diagnosis of compressed nerve root in lumbar disc herniation patients by surface electromyography. Orthop Surg. 2018;10(1):47–55.
Nudelman B, Mittal A, Rosinski A, Zaborovskii N, Wu S, Kondrashov D. Whole-spine magnetic resonance imaging: a review of suggested indications. JBJS Rev. 2021. https://doi.org/10.2106/JBJS.RVW.20.00267.
Millán Ortuondo E, Cabrera Zubizarreta A, Muñiz Saitua J, Sola Sarabia C, Arratibel JZ. Indicaciones de la resonancia magnética en la lumbalgia de adultos [Indications for magnetic resonance imaging for low back pain in adults]. Rev Calid Asist. 2014;29(1):51–7 (Spanish).
Nouh MR. Imaging of the spine: Where do we stand? World J Radiol. 2019;11(4):55–61.
Sarmast AH, Kirmani AR, Bhat AR. Evaluation of role of electrophysiological studies in patients with lumbar disc disease. Asian J Neurosurg. 2018;13(3):585–9.
Mills KR. The basics of electromyography. J Neurol Neurosurg Psychiatry. 2005;76(Suppl 2):ii32-5.
Hides JA, Richardson CA, Jull GA. Multifidus muscle recovery is not automatic after resolution of acute, first-episode low back pain. Spine. 1996;21(23):2763–9.
Kamaz M, Kiresi D, Oguz H, Emlik D, Levendoglu F. CT measurement of trunk muscle areas in patients with chronic low back pain. Diagn Interv Radiol. 2007;13(3):144–8.
Hyun JK, Lee JY, Lee SJ, Jeon JY. Asymmetric atrophy of mul- tifidus muscle in patients with unilateral lumbosacral radicu- lopathy. Spine. 2007;32:E598-602.
Ford D, Bagnall KM, McFadden KD, Greenhill B, Raso J. Analysis of vertebral muscle obtained during surgery for correction of a lumbar disc disorder. Cells Tissues Organs. 1983;116(2):152–7.
Bajek S, Bobinac D, Bajek G, Vranic TS, Lah B, Dragojevic DM. Muscle fiber type distribution in multifidus muscle in cases of lumbar disc herniation. Acta Med Okayama. 2000;54(6):235–42.
Kader DF, Wardlaw D, Smith FW. Correlation between the MRI changes in the lumbar multifidus muscles and leg pain. Clin Radiol. 2000;55:145–9.
Hodges PW, James G, Blomster L, Hall L, Schmid AB, Shu C, et al. Can proinflammatory cytokine gene expression explain multifidus muscle fiber changes after an intervertebral disc lesion? Spine (Phila Pa 1976). 2014;39(13):1010–7.
Hodges PW, James G, Blomster L, Hall L, Schmid A, Shu C, et al. Multifidus muscle changes after back injury are characterized by structural remodeling of muscle, adipose and connective tissue, but not muscle atrophy: molecular and morphological evidence. Spine (Phila Pa 1976). 2015;40(14):1057–71.
Yoshihara K, Nakayama Y, Fujii N, Aoki T, Ito H. Atrophy of the multifidus muscle in patients with lumbar disk herniation: histochemical and electromyographic study. Orthopedics. 2003;26:493–5.
Hides J, Stokes MJ, Saide M, Jull GA, Cooper DH. Evidence of lumbar multifidus muscles wasting ipsilateral to symptoms in patients with acute/subacute low back pain. Spine. 1994;19:165–72.
Stokes M, Rankin G, Newham DJ. Ultrasound imaging of lumbar multifidus muscle: normal reference ranges for mea- surements and practical guidance on the technique. Man Ther. 2005;10(2):116–26.
Hodges PW, Bailey JF, Fortin M, Battié MC. Paraspinal muscle imaging measurements for common spinal disorders: review and consensus-based recommendations from the ISSLS degenerative spinal phenotypes group. Eur Spine J. 2021;30(12):3428–41.
Rim DC. Quantitative Pfirrmann disc degeneration grading system to overcome the limitation of Pfirrmann disc degeneration grade. Korean J Spine. 2016;13(1):1–8.
Hides J, Gilmore C, Stanton W, Bohlscheid E. Multifidus size and symmetry among chronic LBP and healthy asymptomatic subjects. Man Ther. 2008;13(1):43–9.
Kalichman L, Hodges P, Li L, Guermazi A, Hunter DJ. Changes in paraspinal muscles and their association with low back pain and spinal degeneration: CT study. Eur Spine J. 2010;19(7):1136–44.
Parkkola R, Kormano M. Lumbar disc and back muscle degeneration on MRI: correlation to age and body mass. J Spinal Disord. 1992;5(1):86–92.
Takayama K, Kita T, Nakamura H, Kanematsu F, Yasunami T, Sakanaka H, et al. New predictive index for lumbar paraspinal muscle degeneration associated with aging. Spine. 2016;41(2):E84–90.
Cuellar WA, Wilson A, Blizzard CL, Otahal P, Callisaya ML, Hides JA, et al. The assessment of abdominal and multifidus muscles and their role in physical function in older adults: a systematic review. Physiotherapy. 2017;103(1):21–39.
Fortin M, Videman T, Gibbons LE, Battie MC. Paraspinal muscle morphology and composition: a 15-yr longitudinal magnetic resonance imaging study. Med Sci Sports Exerc. 2014;46(5):893–901.
Ogon I, Takebayashi T, Takashima H, Morita T, Yoshimoto M, Terashima Y, et al. Quantitative analysis concerning atrophy and fat infiltration of the multifidus muscle with magnetic resonance spectroscopy in chronic low back pain. Spine Surg Relat Res. 2018;3(2):163–70.
Crawford RJ, Volken T, Ni Mhuiris Á, Bow CC, Elliott JM, Hoggarth MA, et al. Geography of lumbar paravertebral muscle fatty infiltration: the influence of demographics, low back pain, and disability. Spine (Phila Pa 1976). 2019;44(18):1294–302.
Shahidi B, Gibbons MC, Esparza M, Zlomislic V, Allen RT, Garfin SR, et al. Cell populations and muscle fiber morphology associated with acute and chronic muscle degeneration in lumbar spine pathology. JOR Spine. 2020;3(2): e1087.
McLoughlin RF, D’Arcy EM, Brittain MM, Fitzgerald O, Masterson JB. The significance of fat and muscle areas in the lumbar paraspinal space: a CT study. J Comput Assisted Tomogr. 1994;18(2):275–8.
Crawford RJ, Filli L, Elliott JM, Nanz D, Fischer MA, Marcon M, et al. Age- and level-dependence of fatty infiltration in lumbar paravertebral muscles of healthy volunteers. AJNR Am J Neuroradiol. 2016;37(4):742–8.
Lieber RL. Skeletal Muscle Structure, Function, and Plasticity. Baltimore, MD: Lippincott Williams & Wilkins; 2008.
Sayed D, Naidu RK, Patel KV, Strand NH, Mehta P, Lam CM, et al. Best practice guidelines on the diagnosis and treatment of vertebrogenic pain with basivertebral nerve ablation from the American society of pain and neuroscience. J Pain Res. 2022;14(15):2801–19.
Ekşi MŞ, Özcan-Ekşi EE, Orhun Ö, Turgut VU, Pamir MN. Proposal for a new scoring system for spinal degeneration: Mo-Fi-Disc. Clin Neurol Neurosurg. 2020;198: 106120.
Özcan-Ekşi EE, Turgut VU, Küçüksüleymanoğlu D, Ekşi MŞ. Obesity could be associated with poor paraspinal muscle quality at upper lumbar levels and degenerated spine at lower lumbar levels: Is this a domino effect? J Clin Neurosci. 2021;94:120–7.
Özcan-Ekşi EE, Ekşi MŞ, Akçal MA. Severe lumbar intervertebral disc degeneration is associated with modic changes and fatty infiltration in the paraspinal muscles at all lumbar levels, except for L1–L2: a cross-sectional analysis of 50 symptomatic women and 50 age-matched symptomatic men. World Neurosurg. 2019;122:e1069–77.
Ekşi MŞ, Orhun Ö, Yaşar AH, Dursun AT, Berikol G, Börekci A, Özcan-Ekşi EE. At what speed does spinal degeneration gear up?: Aging paradigm in patients with low back pain. Clin Neurol Neurosurg. 2022;215: 107187.
Berikol G, Ekşi MŞ, Aydın L, Börekci A, Özcan-EkşI EE. Subcutaneous fat index: a reliable tool for lumbar spine studies. Eur Radiol. 2022. https://doi.org/10.1007/s00330-022-08775-7.
Aoki Y, Nakajima A, Ohtori S, Takahashi H, Watanabe F, Sonobe M, Terajima F, Saito M, Takahashi K, Toyone T, Watanabe A, Nakajima T, Takazawa M, Nakagawa K. Increase of nerve growth factor levels in the human herniated intervertebral disc: Can annular rupture trigger discogenic back pain? Arthritis Res Ther. 2014;16(4):R159.
Purslow PP. The structure and role of intramuscular connective tissue in muscle function. Front Physiol. 2020;11:495.
Acknowledgements
The authors would like to thank the radiology specialist Doctor Kerim Temiz for contributing to the MRI interpretations, technician Halil Ibrahim Efe for performing the MRI scans, and application specialist İbrahim Yeşilyurt for assisting with the adaptation of the MRI device to the study.
Funding
No funding for this study was provided by any source.
Author information
Authors and Affiliations
Contributions
AY and TY designed the study, interpreted the data, and made major contributions to the writing of the article. AY evaluated the suitability of the patients and referred potential participants to the polyclinics. All the authors examined the final draft of the manuscript, made changes, and approved the manuscript.
Corresponding author
Ethics declarations
Ethical approval and consent
The study was conducted in compliance with the principles of the Helsinki Declaration. Approval for the study was granted by the Ethics Committee of Near East University (YDU/2021/87-1273). All the participants provided informed consent.
Consent for publication
The patients consented to the publication of their pictures as well as their anonymous and clustered data.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yazici, A., Yerlikaya, T. The relationship between the degeneration and asymmetry of the lumbar multifidus and erector spinae muscles in patients with lumbar disc herniation with and without root compression. J Orthop Surg Res 17, 541 (2022). https://doi.org/10.1186/s13018-022-03444-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13018-022-03444-3