Abstract
Background
Acute mesenteric ischaemia (AMI) is a disease with different pathophysiological mechanisms, leading to a life-threatening condition that is difficult to diagnose based solely on clinical signs. Despite widely acknowledged need for biomarkers in diagnosis of AMI, a broad systematic review on all studied biomarkers in different types of AMI is currently lacking. The aim of this study was to estimate the diagnostic accuracy of all potential biomarkers of AMI studied in humans.
Methods
A systematic literature search in PubMed, The Cochrane Library, Web of Science and Scopus was conducted in December 2022. Studies assessing potential biomarkers of AMI in (at least 10) adult patients and reporting their diagnostic accuracy were included. Meta-analyses of biomarkers’ sensitivity, specificity, and positive and negative likelihood ratios were conducted. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were followed, and the study quality was assessed with the QUADAS-2 tool.
Results
Seventy-five studies including a total of 9914 patients assessed 18 different biomarkers in serum/plasma and one in urine (each reported in at least two studies), which were included in meta-analyses. None of the biomarkers reached a conclusive level for accurate prediction. The best predictive value overall (all studies with any type and stage of AMI pooled) was observed for Ischaemia-modified albumin (2 studies, sensitivity 94.7 and specificity 90.5), interleukin-6 (n = 4, 96.3 and 82.6), procalcitonin (n = 6, 80.1 and 86.7), and intestinal fatty acid-binding protein (I-FABP) measured in serum (n = 16, 73.9 and 90.5) or in urine (n = 4, 87.9 and 78.9). In assessment of transmural mesenteric ischaemia, urinary I-FABP (n = 2, 92.3 and 85.2) and D-dimer (n = 3, 87.6 and 83.6) showed moderate predictive value. Overall risk of bias was high, mainly because of selected study populations and unclear timings of the biomarker measurements after onset of symptoms. Combinations of biomarkers were rarely studied, not allowing meta-analyses.
Conclusions
None of the studied biomarkers had sufficient sensitivity and specificity to diagnose AMI, although some biomarkers showed moderate predictive accuracy. Future studies should focus on timing of measurements of biomarkers, distinguishing between early stage and transmural necrosis, and between different types of AMI. Additionally, studies on combinations of biomarkers are warranted.
PROSPERO registration: CRD42022379341.
Similar content being viewed by others
Background
Acute mesenteric ischemia (AMI) is a rare disease with a very high reported mortality (50–70%) showing only a modest improvement during the past few decades, with above 50% of patients still dying during the index hospitalization [1]. Such a small improvement in mortality despite widely available computed tomography, vascular surgery and interventional radiology is most likely explained by insufficient awareness and difficulties in diagnosis. AMI has different forms, which are encountered and managed by different medical specialties (e.g. emergency care physicians, vascular surgeons, interventional radiologists, visceral surgeons, gastroenterologists, intensivists), potentially complicating a uniform approach. A recent survey distributed amongst different medical specialists individually as well as in teams within different hospitals demonstrated that diagnosis of AMI is often delayed and that management is widely variable [2]. It has been shown that improved awareness (clinical suspicion) and focusing on the problem may improve outcomes [3, 4]. However, the lack of both specific symptoms and reliable biomarkers to diagnose AMI remains major factors limiting progress. Identification of reliable biomarkers is considered a priority. Previous systematic reviews assessing diagnostic accuracy of novel serum and haematological markers of AMI were published in 2017 and 2019 [5, 6]. A broad systematic review on all studied biomarkers in different types of AMI is currently lacking, and combinations of biomarkers have rarely been studied, giving a strong rationale to this study.
The aim of our study was to assess the diagnostic accuracy of all potential biomarkers for the diagnosis of AMI in adult patients. Additionally, any combinations of biomarkers that have been studied in this population were also to be assessed.
Methods
In this systematic review and meta-analysis, we assess diagnostic accuracy of all potential biomarkers of AMI studied in adult patients. Any clinical studies including at least 10 adult patients were included, and any publications not presenting original data (e.g. reviews, editorials), case reports, cohort studies with < 10 patients, animal studies, studies in neonates and children, and studies published only as abstracts were excluded.
The population of interest was adult (> 18 years of age) patients with suspected AMI regardless of pathophysiological mechanism (occlusive arterial thrombosis or embolism, mesenteric venous thrombosis, non-occlusive mesenteric ischemia, mesenteric ischaemia due to strangulated bowel disease/obstruction—SBO).
Studies were considered eligible if:
-
1.
A potential biomarker was measured in patients in whom AMI was suspected;
-
2.
The diagnosis of AMI was confirmed either at surgery, CT-angiography, mesenteric angiography, endoscopy, or histopathological examination (incl. autopsy); and
-
3.
Diagnostic accuracy of a potential biomarker was reported as sensitivity and specificity, or as true-positive (TP), true-negative (TN), false-positive (FP) and false-negative (FN) cases.
The list of pertinent biomarkers was predefined based on scoping literature searches. However, we did not exclude (studies on) other potential novel biomarkers.
Review questions
What is the diagnostic accuracy of the following biomarkers in diagnosing AMI in adult patients?
-
1.
Serum/plasma
-
2.
Intestinal fatty acid-binding protein (I-FABP)
-
3.
Alpha glutathione S transferase (alpha-GST)
-
4.
Ischaemia-modified albumin (IMA)
-
5.
Smooth muscle protein 22 (SM22)
-
6.
Cobalt-albumin binding assay
-
7.
Citrulline
-
8.
Adropin
-
9.
Intestinal ileal bile acid binding protein (I-BABP)
-
10.
Hypoxia-inducible factor 1-alpha (HIF-1-alfa)
-
11.
Fibroblast growth factor 23 (FGF-23)
-
12.
Apelin
-
13.
D-lactate
-
14.
L-lactate
-
15.
Metabolic acidosis
-
16.
D-dimers
-
17.
Neutrophil–lymphocyte ratio
-
18.
Platelet–lymphocyte ratio
-
19.
White blood cell count
-
20.
C-reactive protein
-
21.
Troponin
-
22.
Creatinine
-
23.
Urine
-
24.
Urinary long non-coding RNA (lncRNA) H19
-
25.
Urinary I-FABP
-
26.
Urinary intestinal ileal bile acid binding protein (I-BABP)
What is the diagnostic accuracy of any other serum or urine biomarker in diagnosing AMI in adult patients?
What is the accuracy of any combination of biomarkers in diagnosing AMI in adult patients?
This systematic review was registered in PROSPERO registry (CRD42022379341, “Diagnostic accuracy of biomarkers to detect acute mesenteric ischaemia in adult patients: a systematic review and meta-analysis”) and performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.
Searches
Literature searches were performed on 19th of December, searching PubMed, The Cochrane Library, Web of Science and Scopus since their inception until December 2022. The searches were not restricted to date or language. Additional studies were searched by screening of cross references of relevant articles, including existing systematic reviews. All search strategies are presented in Additional file 1.
Main outcomes
-
Accuracy of diagnosis of AMI
-
Threshold value of positive or negative test result
Data extraction
Titles and abstracts of studies identified utilizing the developed search strategy and from additional sources were screened independently by two reviewers to identify studies for full-text review. The selected full texts were independently assessed by two reviewers. For any disagreements during the title/abstract and full-text review, consensus was reached, involving a third reviewer if necessary. Animal, in vitro and paediatric studies, duplicates, studies that were not original or included less than 10 patients, were excluded during the title/abstract review. During the full-text review, we excluded studies that did not report biomarkers measured in blood, serum, plasma or urine; did not report extractable data of diagnostic accuracy of studied biomarkers; and studies where the reference standard for diagnosis of AMI was not applicable. Our per-protocol predefined applicable reference standards included surgery, computed tomography, angiography, endoscopy or histopathological examination. The following information was extracted independently by two reviewers from assessed full texts: study setting, patient selection, age, gender, studied biomarker and any combination of biomarkers, measurement method with reference values, timing of biomarker measurement, number of patients with AMI and without AMI, sensitivity and specificity of the biomarker for diagnosis of AMI with TP, TN, FP and FN cases, determined biomarker cut-off, diagnostic criteria used for AMI, and progression, type and localisation of AMI if available.
Risk of bias (quality) assessment
The QUADAS-2 tool was used to assess risk of bias and applicability of included studies and completed by two research team members in parallel. Decisions were made after reaching consensus, or by involving a third reviewer if necessary. If a study was judged as “low” on all domains relating to bias/applicability, it was judged as having “low risk of bias” / “low concern regarding applicability”. If a study was judged "high" or "unclear" on one or more domains, it was judged as being “at risk of bias” / having “concerns regarding applicability”.
Strategy for data synthesis and analysis
We constructed two-by-two contingency tables for all biomarkers. We calculated sensitivity and specificity with 95% confidence intervals (CI) based on the data (TP, TN, FP, and FN) extracted from each of the included studies. If TP, TN, FP and FN were not provided, we calculated these based on given sensitivity, specificity, sample size and AMI prevalence.
Random-effects meta-analyses were used to pool the sensitivities, specificities, positive and negative likelihood ratios in subgroups. For sensitivity and specificity analyses, we used logit-transformation in R software (V.4.1.0, R Foundation for Statistical Computing, Vienna, Austria) package meta. The confidence intervals were calculated using the Clopper–Pearson method [7].
The pooled likelihood ratios were obtained based on the bivariate model for diagnostic test accuracy in R package mada. It applies a sampling-based approach proposed by Zwinderman and Bossuyt that uses the parameters of a fit to the bivariate model to generate samples for observed sensitivities and false-positive rates [8].
The results are presented in tables with estimates and their 95% CI or in forest plots along with I2 statistic, τ2 and Cochran’s Q-test to describe the heterogeneity.
Youden index (sensitivity + specificity − 1) was used to rank the biomarkers [9].
Positive likelihood ratio > 10 and negative likelihood ratio < 0.1 were considered as high diagnostic accuracy confirming the accurate performance of a biomarker. Positive likelihood ratio > 5 and negative likelihood ratio < 0.2 were considered as moderate diagnostic accuracy showing potential for usage without being confirmative [10].
Analysis of subgroups or subsets
We predefined the following subgroups:
-
1.
Different types of AMI (occlusive arterial, mesenteric venous thrombosis, non-occlusive mesenteric ischaemia, mesenteric ischaemia due to strangulated bowel disease/obstruction – SBO).
-
2.
Different progression of AMI (non-transmural / transmural intestinal ischaemia)
-
3.
Different time points of the measurement of the biomarker (immediately at admission to hospital, perioperatively, within first 6h / 24h / > 24h of suspicion of AMI).
Results
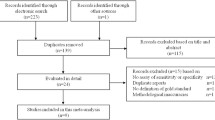
The search identified 2026 titles, and 16 additional studies. Among those, 250 studies were selected for full-text review (Fig. 1).
It was possible to extract TP, TN, FP, FN in 83 papers, and among them, 75 (with 9914 participants) provided data for quantitative analysis [11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85]. Assessment of risk of bias of all studies included in qualitative analysis is presented in Additional file 2: Table S1. All studies were judged to have some risk of bias and/or some concerns regarding applicability, and thus, it was not possible to perform the planned sensitivity analyses, excluding studies with lower quality.
It was not possible to differentiate studies/patients with early non-transmural AMI; therefore, we adapted our subgroups to “any stage” (including studies with any stage of AMI, possibly containing transmural; but excluding studies where only patients with transmural AMI were assessed) and “transmural” (including only studies on transmural AMI). Accordingly, these results need to be interpreted with caution as the proportion of “transmural” within the “any stage” is not clear.
All biomarkers included in meta-analyses with the number of studies and patients, as well as predictive values in subgroups for any stage, and “transmural” for all these biomarkers are presented in Table 1. Table 2 presents the 12 best-performing biomarkers: “overall”, “any stage” and “transmural”, ranked based on Youden index.
Forest plots for analysed biomarkers are presented in Figs. 2–5 (analyses including > 10 studies) and in Additional file 3: Figures S1–S15 (analyses including < 10 studies). Next to overall diagnostic accuracy (pooling all studies on a specific biomarker), we present studies assessing only non-occlusive mesenteric ischaemia (NOMI) or ischaemia due to strangulating bowel disease (SBO) separately on these figures, where applicable.
Sensitivity (panel A) and specificity (panel B) of serum intestinal fatty acid-binding protein (I-FABP) predicting AMI. AMI—acute mesenteric ischaemia; NOMI—non-occlusive mesenteric ischaemia; SBO—strangulated bowel disease. Any stage—studies assessing different stages of AMI, including but not limited to non-transmural and transmural; Transmural—studies assessing transmural AMI, with control group including non-transmural AMI. Comment: Uzun 2014 included healthy volunteers as control. Hycult—Hycult Biotech measurement kit from Uden, the Netherlands. Osaka—D.S. Pharma Biomedical measurement kit from Osaka, Japan. R&D—R&D Systems measurement kit from Minneapolis, USA
For most of the biomarkers, different thresholds/cut-offs were used in individual studies, making interpretation of results difficult. It was not possible to analyse biomarkers separately in vascular AMI and SBO, because most of the studies either included SBO under the broad group of “any type of AMI” (see Table 1) or did not specify the exclusion of SBO.
Diagnostic accuracy of the biomarkers
None of the studied biomarkers demonstrated high diagnostic accuracy, whereas a few showed modest diagnostic accuracy (Table 1).
The inflammatory markers demonstrated relatively high predictive values (Tables 1 and 2), with IL-6 showing the best prediction. Figure 3 provides an overview of the performance of the white blood cell count—as a more commonly used inflammatory marker, while other inflammatory markers are presented in Additional file 3: Figures S2–S4.
Sensitivity (panel A) and specificity (panel B) of white blood cell count (WBC) predicting AMI. AMI—acute mesenteric ischaemia; NOMI—non-occlusive mesenteric ischaemia; SBO—strangulated bowel disease. Any stage—studies assessing different stages of AMI, including but not limited to non-transmural and transmural; Transmural—studies assessing transmural AMI, with control group including non-transmural AMI
Measurement of D-dimers had insufficient predictive value for AMI at any stage but performed better in studies assessing transmural ischaemia (Fig. 4 and Table 1). Heterogeneous cut-offs complicate the interpretation of results, but it appears that patients with AMI do not present with normal values of D-dimers.
Sensitivity (panel A) and specificity (panel B) of serum D-dimers predicting AMI. AMI—acute mesenteric ischaemia; NOMI—non-occlusive mesenteric ischaemia; SBO—strangulated bowel disease. Any stage—studies assessing different stages of AMI, including but not limited to non-transmural and transmural; Transmural—studies assessing transmural AMI, with control group including non-transmural AMI
I-FABP (Fig. 2), the most studied novel biomarker, reached moderate diagnostic accuracy, although several recent studies showed rather disappointing results [56, 64]. Interpretation of data for this biomarker is further complicated by the multiple methods of laboratory analytics as well as highly variable thresholds for abnormality. Our analysis suggests that urinary I-FABP may perform better for transmural AMI (Additional file 3: Figure S1 and Tables 1 and 2), but this result is based on only two studies.
L-lactate (Fig. 5), probably the most studied biomarker of AMI, did not show sufficient diagnostic accuracy in our analysis and should not be considered an early biomarker of AMI. Some additional value of this biomarker in diagnosing transmural AMI is not excluded, because only inflammatory markers that are also not specific, and I-FABP which is not promptly available in clinical practice, performed better in our analysis (Table 2).
Sensitivity (panel A) and specificity (panel B) of blood L-lactate predicting AMI. AMI—acute mesenteric ischaemia; NOMI—non-occlusive mesenteric ischaemia; SBO—strangulated bowel disease. Any stage—studies assessing different stages of AMI, including but not limited to non-transmural and transmural; Transmural—studies assessing transmural AMI, with control group including non-transmural AMI
For a number of biomarkers, the sensitivity and specificity were reported (or could be calculated) in only one study and meta-analysis was not possible. These biomarkers were:
Stromal cell-derived factor-1 (SDF-1) (sensitivity and specificity 91 and 95%, respectively) [86].
Serum long-coding RNA H19 (94 and 100%) [87].
Serum IL-8 (88 and 100%) [71].
Serum creatine kinase BB isoenzyme (CK-BB) (63 and 100%) [88].
Plasma presepsin (89 and 85%) [89].
Serum creatinine with a cut-off of 200 micromol/L (58 and 97%) [90].
Serum L-FABP (59 and 88%, respectively) [75].
Serum hypoxia-induced factor alpha (HIF1-α) (75 and 70%) [91].
Serum smooth muscle actin (54 and 100%) [40].
Serum endothelin-1 (51 and 94%) [92].
Serum adropin (65 and 70%) [91].
Serum I-BABP (64 and 63%) [75].
Cell-free plasma DNA (54 and 84%) [93].
Urinary long-coding RNA H19 (80 and 100%) [87].
Urinary I-BABP (70 and 89%) [75].
Urinary L-FABP (80 and 78%) [75].
Other biomarkers assessed in individual studies as potential biomarkers of AMI are not presented as they were not considered novel and had been excluded from our predefined list of interest. These were haemoglobin, haematocrit, erythrocyte volume fraction, immature granulocytes, delta neutrophil index, fibrinogen, prothrombin, blood urea nitrogen, creatine phosphokinase, amylase, ASAT, ALAT and phosphate.
It was not possible to perform subgroup analyses based on timing of biomarker measurements. The time elapsed from the onset of symptoms until biomarker measurement was generally not reported in studies, and the exact times after hospital admission also remained unclear.
Scores/combinations of biomarkers were assessed in only 5 studies [23, 35, 42, 85, 94], mainly combining laboratory biomarkers with radiological or clinical markers and thus not permitting any meta-analysis.
Discussion
In this systematic review, a considerable number of studies assessing biomarkers of AMI were identified. Despite this increasing body of evidence, no biomarker currently provides sufficient diagnostic accuracy to be recommended for clinical use. Available evidence is hard to interpret due to:
-
Different cut-offs and laboratory methods in different studies;
-
A lack of differentiation between different stages of AMI (non-transmural vs transmural) in most of the studies;
-
A lack of differentiation between different types of AMI in most of the studies;
-
Missing data on timing of biomarker measurement after development of symptoms.
Compared to previous systematic reviews, more studies on existing and novel biomarkers were included. No breakthrough in defining a reliable biomarker with acceptable sensitivity and specificity was observed however [5, 6]. Novel biomarkers such as IMA and alpha glutathione S transferase (alpha-GST) were associated with great hope a few years ago, but no newer studies were identified than those in the systematic review by Treskes in 2017 [5]. Newer studies assessing I-FABP have been published, but do not confirm the initial enthusiasm [21, 40, 56, 64, 68]. Accordingly, a moderate diagnostic accuracy may be considered disappointing for I-FABP, as the hope was that I-FABP was specific and would provide good diagnostic accuracy [5, 95,96,97]. However, the diagnostic value of I-FABP may be dependent on timing of its measurement [56].
Although nonspecific, inflammatory biomarkers such as IL-6, CRP and PCT performed relatively well in our analysis when compared to the novel and supposedly specific biomarkers, probably because of systemic inflammation from ischaemic injury to the bowel occurring in the later stages of AMI. Our analyses support this rationale, showing that inflammatory biomarkers may perform better in predicting transmural AMI. At the same time, inflammatory biomarkers may not be able to distinguish between severe inflammation in the bowel/peritoneal cavity of other causes vs. mesenteric ischaemia [71]. Ideally, a biomarker should be specific and diagnostic in the early phases of AMI, to enable salvage of the threatened bowel and these criteria are probably not fulfilled with inflammatory markers. Additionally, it is difficult to interpret inflammatory markers in patients with NOMI who usually have an active inflammatory state due to their severe underlying illness and its treatment (e.g. ICU patients) [98, 99]. Of note, there was no study assessing IL-6 in NOMI.
Lactate is often used in clinical practice today; however, it clearly should not be used for exclusion of AMI [100]. Lactate can be effectively metabolized in the liver, explaining why it does not serve as an early marker of AMI. Increased metabolism may cover increased production, whereas decreased metabolism may lead to elevated values without a relevant increase in production [101, 102]. However, elevated lactate should still call for our attention and maybe trigger further investigation in patients with suspected AMI [103].
As one biomarker is currently insufficient to diagnose AMI, possible combinations of different biomarkers should be studied hoping for an additive value in diagnosis. At the same time, a rapid turn-round in laboratory analytics is an important factor necessary for any future biomarker of AMI.
Strengths and limitations
The main strength of our study is the updated synthesis of evidence on diagnostic accuracy of the potential biomarkers of AMI. To the best of our knowledge, it is the first systematic review attempting separation of transmural ischaemia from earlier stages of AMI.
The limitations of our study are mainly related to the original studies that are heterogeneous regarding patient populations (incl. control groups), types of AMI, laboratory methods and cut-offs of biomarkers and often do not report the time from development of symptoms to measurement of biomarkers. Thus, all the studies in our review were judged as being at risk of bias and/or having concerns regarding applicability. However, uncovering the need to set certain methodological standards for studies on AMI biomarkers could also be considered a strength of our study.
Additionally, we were not able to clearly separate non-transmural from transmural AMI and vascular AMI from SBO in our analyses.
Conclusions
Currently, based on available evidence, no single biomarker enables accurate diagnosis of AMI, whereas combinations of these biomarkers have rarely been studied. Available evidence carries considerable risk of bias, is very heterogeneous and does not allow precise distinctions between different types and stages of AMI. Inflammatory markers and D-dimers may be considered to assist in diagnosis of transmural ischaemia. Future studies should focus on timing of measurements of biomarkers, considering different biomarkers for diagnosis of early stage of AMI and transmural necrosis.
Availability of data and materials
Template data collection forms and data used for analyses can be made available on request.
Abbreviations
- ABSU:
-
Absorbance units
- ALAT:
-
Alanine transaminase
- Alpha-GST:
-
Alpha glutathione S transferase
- AMI:
-
Acute mesenteric ischemia
- ASAT:
-
Aspartate aminotransferase
- BE:
-
Base excess
- CABA:
-
Cobalt-albumin binding assay
- CI:
-
Confidence interval
- CK-BB:
-
Serum creatine kinase BB isoenzyme
- CRP:
-
C-reactive protein
- FGF-23:
-
Fibroblast growth factor 23
- FN:
-
False negative
- FP:
-
False positive
- HIF1-α:
-
Hypoxia-inducible factor 1-alpha
- I-BABP:
-
Intestinal ileal bile acid binding protein
- ICU:
-
Intensive care unit
- I-FABP:
-
Intestinal fatty-acid-binding protein
- IL-6:
-
Interleukin 6
- IL-8:
-
Interleukin 8
- IMA:
-
Ischaemia-modified albumin
- IQR:
-
Interquartile range
- LDH:
-
Lactate dehydrogenase
- L-FABP:
-
Liver fatty acid-binding protein
- RDW:
-
Red cell distribution width
- lncRNA H19:
-
Long non-coding RNA
- LR-:
-
Negative likelihood ratio
- LR+:
-
Positive likelihood ratio
- MPV:
-
Mean platelet volume
- NLR:
-
Neutrophil–lymphocyte ratio
- NOMI:
-
Non-occlusive mesenteric ischemia
- NPV:
-
Negative predictive value
- PCT:
-
Procalcitonin
- PLR:
-
Platelet–lymphocyte ratio
- PPV:
-
Positive predictive value
- SBO:
-
Strangulated bowel disease/obstruction
- SDF-1:
-
Stromal cell-derived factor-1
- SM22:
-
Smooth muscle protein 22
- TN:
-
True negative
- TP:
-
True positive
- WBC:
-
White blood cells count
References
Tamme K, Reintam Blaser A, Laisaar KT, Mändul M, Kals J, Forbes A, Kiss O, Acosta S, Björck M, Starkopf J. Incidence and outcomes of acute mesenteric ischaemia: a systematic review and meta-analysis. BMJ Open. 2022;12:e062846. https://doi.org/10.1136/bmjopen-2022-062846.
Hess B, Cahenzli M, Forbes A, Burgos R, Coccolini F, Corcos O, Holst M, Irtun Ø, Klek S, Pironi L, Rasmussen HH, Serlie MJ, Thibault R, Gabe S, Reintam BA. ESPEN Special Interest Group on Acute Intestinal Failure ESPEN (European Society for Clinical Nutrition and Metabolism). Management of acute mesenteric ischaemia: results of a worldwide survey. Clin Nutr ESPEN. 2023;54:194–205. https://doi.org/10.1016/j.clnesp.2022.12.022.
Tolonen M, Lemma A, Vikatmaa P, Peltola E, Mentula P, Björkman P, Leppäniemi A, Sallinen V. The implementation of a pathway and care bundle for the management of acute occlusive arterial mesenteric ischemia reduced mortality. J Trauma Acute Care Surg. 2021;91(3):480–8. https://doi.org/10.1097/TA.0000000000003305.
Najdawi M, Garzelli L, Nuzzo A, Huguet A, Raynaud L, Paulatto L, Panis Y, Ben Abdallah I, Castier Y, Sibert A, Vilgrain V, Corcos O, Ronot M. Endovascular revascularization of acute arterial mesenteric ischemia: report of a 3-year experience from an intestinal stroke center unit. Eur Radiol. 2022;32(8):5606–15. https://doi.org/10.1007/s00330-022-08660-3.
Treskes N, Persoon AM, van Zanten ARH. Diagnostic accuracy of novel serological biomarkers to detect acute mesenteric ischemia: a systematic review and meta-analysis. Intern Emerg Med. 2017;12(6):821–36. https://doi.org/10.1007/s11739-017-1668-y.
Khan SM, Emile SH, Wang Z, Agha MA. Diagnostic accuracy of hematological parameters in Acute mesenteric ischemia-A systematic review. Int J Surg. 2019;66:18–27. https://doi.org/10.1016/j.ijsu.2019.04.005.
Shim SR, Kim SJ, Lee J. Diagnostic test accuracy: application and practice using R software. Epidemiol Health. 2019;41:e2019007. https://doi.org/10.4178/epih.e2019007.
Zwinderman AH, Bossuyt PM. We should not pool diagnostic likelihood ratios in systematic reviews. Stat Med. 2008;27(5):687–97. https://doi.org/10.1002/sim.2992.
Acosta S, Nilsson TK, Björck M. Preliminary study of D-dimer as a possible marker of acute bowel ischaemia. Br J Surg. 2001;88(3):385–8. https://doi.org/10.1046/j.1365-2168.2001.01711.x.
Acosta S, Nilsson TK, Björck M. D-dimer testing in patients with suspected acute thromboembolic occlusion of the superior mesenteric artery. Br J Surg. 2004;91(8):991–4. https://doi.org/10.1002/bjs.4645.
Aktimur R, Cetinkunar S, Yildirim K, Aktimur SH, Ugurlucan M, Ozlem N. Neutrophil-to-lymphocyte ratio as a diagnostic biomarker for the diagnosis of acute mesenteric ischemia. Eur J Trauma Emerg Surg. 2016;42(3):363–8. https://doi.org/10.1007/s00068-015-0546-4.
Akyildiz H, Akcan A, Oztürk A, Sozuer E, Kucuk C, Karahan I. The correlation of the D-dimer test and biphasic computed tomography with mesenteric computed tomography angiography in the diagnosis of acute mesenteric ischemia. Am J Surg. 2009;197(4):429–33. https://doi.org/10.1016/j.amjsurg.2008.02.011.
Arif R, Farag M, Zaradzki M, Reissfelder C, Pianka F, Bruckner T, Kremer J, Franz M, Ruhparwar A, Szabo G, Beller CJ, Karck M, Kallenbach K, Weymann A. Ischemic colitis after cardiac surgery: can we foresee the threat? PLoS ONE. 2016;11(12):e0167601. https://doi.org/10.1371/journal.pone.0167601.
Bandea D, Halmaciu I, Suciu BA, Bandea N, Tilinca M, Godja D, Brinzaniuc K, Voidazan S. The role of lymphocyte-to-monocyte ratio and platelet-to-lymphocyte ratio reports as a predictive factor for the occurrence of intestinal necrosis in complicated incisional hernias. Medical-Surgical Journal-Revista Medico-Chirurgicala. 2019;123(4):682–8.
Beng Fuh R, Eisele R. Acute disturbance of the mesenterial circulation. What is the diagnostic value of easily performed preoperative tests? Chirurgische Praxis. 2004;63(4):573–83.
Bjørnestad E, Lie RT, Janssen CW Jr. The diagnostic potential of some routine laboratory tests. Off Br J Clin Pract. 1993;47(5):243–5.
Block T, Nilsson TK, Björck M, Acosta S. Diagnostic accuracy of plasma biomarkers for intestinal ischaemia. Scand J Clin Lab Invest. 2008;68(3):242–8. https://doi.org/10.1080/00365510701646264.
Bogusevicius A, Grinkevicius A, Maleckas A, Pundzius J. The role of D-dimer in the diagnosis of strangulated small-bowel obstruction. Medicina (Kaunas). 2007;43(11):850–4.
Bourcier S, Ulmann G, Jamme M, Savary G, Paul M, Benghanem S, Lavillegrand JR, Schmidt M, Luyt CE, Maury E, Combes A, Pène F, Neveux N, Cariou A. A multicentric prospective observational study of diagnosis and prognosis features in ICU mesenteric ischemia: the DIAGOMI study. Ann Intensive Care. 2022;12(1):113. https://doi.org/10.1186/s13613-022-01092-8.
Brillantino A, Iacobellis F, Renzi A, Nasti R, Saldamarco L, Grillo M, Romano L, Castriconi M, Cittadini A, De Palma M, Scaglione M, Di Martino N, Grassi R, Paladino F. Diagnostic value of arterial blood gas lactate concentration in the different forms of mesenteric ischemia. Eur J Trauma Emerg Surg. 2018;44(2):265–72. https://doi.org/10.1007/s00068-017-0805-7.
Calame P, Winiszewski H, Doussot A, Malakhia A, Grillet F, Verdot P, Vuitton L, Ronot M, Pili-Floury S, Heyd B, Delabrousse E, Piton G. Evaluating the risk of irreversible intestinal necrosis among critically ill patients with nonocclusive mesenteric ischemia. Am J Gastroenterol. 2021;116(7):1506–13. https://doi.org/10.14309/ajg.0000000000001274.
Chiu YH, Huang MK, How CK, Hsu TF, Chen JD, Chern CH, Yen DH, Huang CI. D-dimer in patients with suspected acute mesenteric ischemia. Am J Emerg Med. 2009;27(8):975–9. https://doi.org/10.1016/j.ajem.2009.06.006.
Collange O, Lopez M, Lejay A, Pessaux P, Ouattara A, Dewitte A, Rimmele T, Girardot T, Arnaudovski D, Augustin P, Chakfe N, Tacquard C, Oulehri W, Zieleskiewicz L, Severac F, Leone M, Mertes PM. Serum lactate and acute mesenteric ischaemia: an observational, controlled multicentre study. Anaesth Crit Care Pain Med. 2022;41(6):101141. https://doi.org/10.1016/j.accpm.2022.101141.
Cosse C, Regimbeau JM, Fuks D, Mauvais F, Scotte M. Serum procalcitonin for predicting the failure of conservative management and the need for bowel resection in patients with small bowel obstruction. J Am Coll Surg. 2013;216(5):997–1004. https://doi.org/10.1016/j.jamcollsurg.2012.12.051.
Cossé C, Sabbagh C, Fumery M, Zogheib E, Mauvais F, Browet F, Rebibo L, Regimbeau JM. Serum procalcitonin correlates with colonoscopy findings and can guide therapeutic decisions in postoperative ischemic colitis. Dig Liver Dis. 2017;49(3):286–90. https://doi.org/10.1016/j.dld.2016.12.003.
Cronk DR, Houseworth TP, Cuadrado DG, Herbert GS, McNutt PM, Azarow KS. Intestinal fatty acid binding protein (I-FABP) for the detection of strangulated mechanical small bowel obstruction. Curr Surg. 2006;63(5):322–5. https://doi.org/10.1016/j.cursur.2006.05.006.
Degerli V, Ergin I, Duran FY, Ustuner MA, Duran O. Could mean platelet volume be a reliable indicator for acute mesenteric ischemia diagnosis? A case-control study. Biomed Res Int. 2016;2016:9810280. https://doi.org/10.1155/2016/9810280.
Delaney CP, O’Neill S, Manning F, Fitzpatrick JM, Gorey TF. Plasma concentrations of glutathione S-transferase isoenzyme are raised in patients with intestinal ischaemia. Br J Surg. 1999;86(10):1349–53. https://doi.org/10.1046/j.1365-2168.1999.01245.x.
Durak D, Turhan VB, Alkurt EG, Tutan MB, Şahiner I. The role of immature granulocyte count and delta neutrophil index in the early prediction of mesenteric ischemia. Eur Rev Med Pharmacol Sci. 2022;26(12):4238–43. https://doi.org/10.26355/eurrev_202206_29060.
Edwards M, Sidebotham D, Smith M, Leemput JV, Anderson B. Diagnosis and outcome from suspected mesenteric ischaemia following cardiac surgery. Anaesth Intensive Care. 2005;33(2):210–7. https://doi.org/10.1177/0310057X0503300209.
Eyvaz K, Dincer OI, Kazim Kazan M, Dincer A, Aslaner A, Acar A, Cakir T. Neutrophil to C-reactive protein ratio: an estimating factor for intestinal ischemia before the surgery of incarcerated inguinal hernia. North Clin Istanb. 2021;8(6):575–80. https://doi.org/10.14744/nci.2021.26878.
Ferrada P, Callcut R, Bauza G, O’Bosky KR, Luo-Owen X, Mansfield NJ, Inaba K, Pasley J, Bugaev N, Pereira B, Moore FO, Han J, Pasley A, DuBose J. AAST Multi-institutional Trials Committee. Pneumatosis intestinalis predictive evaluation study: a multicenter epidemiologic study of the american association for the surgery of trauma. J Trauma Acute Care Surg. 2017;82(3):451–60. https://doi.org/10.1097/TA.0000000000001360.
Gearhart SL, Delaney CP, Senagore AJ, Banbury MK, Remzi FH, Kiran RP, Fazio VW. Prospective assessment of the predictive value of alpha-glutathione S-transferase for intestinal ischemia. Am Surg. 2003;69(4):324–9.
Grotelueschen R, Miller V, Heidelmann LM, Melling N, Ghadban T, Grupp K, Reeh M, Welte MN, Uzunoglu FG, Izbicki JR, Bachmann KA. Acute mesenteric infarction: the chameleon of acute abdomen evaluating the quality of the diagnostic parameters in acute mesenteric ischemia. Dig Surg. 2021;38(2):149–57. https://doi.org/10.1159/000512779.
Gün B, Yolcu S, Değerli V, Elçin G, Tomruk Ö, Erdur B, Parlak İ. Multi-detector angio-CT and the use of D-dimer for the diagnosis of acute mesenteric ischemia in geriatric patients. Ulus Travma Acil Cerrahi Derg. 2014;20(5):376–81. https://doi.org/10.5505/tjtes.2014.57639.
Gunduz A, Turedi S, Mentese A, Karahan SC, Hos G, Tatli O, Turan I, Ucar U, Russell RM, Topbas M. Ischemia-modified albumin in the diagnosis of acute mesenteric ischemia: a preliminary study. Am J Emerg Med. 2008;26(2):202–5. https://doi.org/10.1016/j.ajem.2007.04.030.
Güzel M, Sözüer EM, Salt Ö, İkizceli İ, Akdur O, Yazıcı C. Value of the serum I-FABP level for diagnosing acute mesenteric ischemia. Surg Today. 2014;44(11):2072–6. https://doi.org/10.1007/s00595-013-0810-3.
Hong J, Gilder E, Blenkiron C, Jiang Y, Evennett NJ, Petrov MS, Phillips ARJ, Windsor JA, Gillham M. Nonocclusive mesenteric infarction after cardiac surgery: potential biomarkers. J Surg Res. 2017;211:21–9. https://doi.org/10.1016/j.jss.2016.12.001.
Hot S, Duraker N, Sarı A, Çetin K. The value of d-dimer in diagnosis of acute mesenteric ischemia and differential diagnosis from acute pancreatitis and acute cholecystitis. Dicle Tıp Dergisi. 2016;43(1):88–92.
Huang X, Fang G, Lin J, Xu K, Shi H, Zhuang L. A prediction model for recognizing strangulated small bowel obstruction. Gastroenterol Res Pract. 2018;2018:7164648. https://doi.org/10.1155/2018/7164648.
Icoz G, Makay O, Sozbilen M, Gurcu B, Caliskan C, Firat O, Kurt Z, Ersin S. Is D-dimer a predictor of strangulated intestinal hernia? World J Surg. 2006;30(12):2165–9. https://doi.org/10.1007/s00268-006-0138-x.
Jancelewicz T, Vu LT, Shawo AE, Yeh B, Gasper WJ, Harris HW. Predicting strangulated small bowel obstruction: an old problem revisited. J Gastrointest Surg. 2009;13(1):93–9. https://doi.org/10.1007/s11605-008-0610-z.
Kanda T, Fujii H, Tani T, Murakami H, Suda T, Sakai Y, Ono T, Hatakeyama K. Intestinal fatty acid-binding protein is a useful diagnostic marker for mesenteric infarction in humans. Gastroenterology. 1996;110(2):339–43. https://doi.org/10.1053/gast.1996.v110.pm8566578.
Kanda T, Tsukahara A, Ueki K, Sakai Y, Tani T, Nishimura A, Yamazaki T, Tamiya Y, Tada T, Hirota M, Hasegawa J, Funaoka H, Fujii H, Hatakeyama K. Diagnosis of ischemic small bowel disease by measurement of serum intestinal fatty acid-binding protein in patients with acute abdomen: a multicenter, observer-blinded validation study. J Gastroenterol. 2011;46(4):492–500. https://doi.org/10.1007/s00535-011-0373-2.
Karadeniz E, Bayramoğlu A, Atamanalp SS. Sensitivity and specificity of the platelet-lymphocyte ratio and the neutrophil-lymphocyte ratio in diagnosing acute mesenteric ischemia in patients operated on for the diagnosis of mesenteric ischemia: a retrospective case-control study. J Invest Surg. 2020;33(8):774–81. https://doi.org/10.1080/08941939.2019.1566418.
Kintu-Luwaga R, Galukande M, Owori FN. Serum lactate and phosphate as biomarkers of intestinal ischemia in a Ugandan tertiary hospital: a cross-sectional study. Int J Emerg Med. 2013;6(1):44. https://doi.org/10.1186/1865-1380-6-44.
Kisaoglu A, Bayramoglu A, Ozogul B, Atac K, Emet M, Atamanalp SS. Sensitivity and specificity of red cell distribution width in diagnosing acute mesenteric ischemia in patients with abdominal pain. World J Surg. 2014;38(11):2770–6. https://doi.org/10.1007/s00268-014-2706-9.
Kittaka H, Akimoto H, Takeshita H, Funaoka H, Hazui H, Okamoto M, Kobata H, Ohishi Y. Usefulness of intestinal fatty acid-binding protein in predicting strangulated small bowel obstruction. PLoS ONE. 2014;9(6):e99915. https://doi.org/10.1371/journal.pone.0099915.
Klingele M, Bomberg H, Poppleton A, Minko P, Speer T, Schäfers HJ, Groesdonk HV. Elevated procalcitonin in patients after cardiac surgery: a hint to nonocclusive mesenteric ischemia. Ann Thorac Surg. 2015;99(4):1306–12. https://doi.org/10.1016/j.athoracsur.2014.10.064.
Koami H, Isa T, Ishimine T, Kameyama S, Matsumura T, Yamada KC, Sakamoto Y. Risk factors for bowel necrosis in patients with hepatic portal venous gas. Surg Today. 2015;45(2):156–61. https://doi.org/10.1007/s00595-014-0941-1.
Kulu R, Akyildiz H, Akcan A, Oztürk A, Sozuer E. Plasma citrulline measurement in the diagnosis of acute mesenteric ischaemia. ANZ J Surg. 2017;87(9):E57–60. https://doi.org/10.1111/ans.13524.
Lange H, Toivola A. Varningssignal vid akuta magåkommor. Laktat bästa markören vid mesenteriell ischemi [Warning signals in acute abdominal disorders. Lactate is the best marker of mesenteric ischemia]. Lakartidningen. 1997;94(20):1893–6.
Li H, Sun D, Sun D, Xiao Z, Zhuang J, Yuan C. The diagnostic value of coagulation indicators and inflammatory markers in distinguishing between strangulated and simple intestinal obstruction. Surg Laparosc Endosc Percutan Tech. 2021;31(6):750–5. https://doi.org/10.1097/SLE.0000000000000982.
Ludewig S, Jarbouh R, Ardelt M, Mothes H, Rauchfuß F, Fahrner R, Zanow J, Settmacher U. Bowel ischemia in ICU patients: diagnostic value of I-FABP depends on the interval to the triggering event. Gastroenterol Res Pract. 2017;2017:2795176. https://doi.org/10.1155/2017/2795176.
Markogiannakis H, Memos N, Messaris E, Dardamanis D, Larentzakis A, Papanikolaou D, Zografos GC, Manouras A. Predictive value of procalcitonin for bowel ischemia and necrosis in bowel obstruction. Surgery. 2011;149(3):394–403. https://doi.org/10.1016/j.surg.2010.08.007.
Matsumoto S, Sekine K, Funaoka H, Yamazaki M, Shimizu M, Hayashida K, Kitano M. Diagnostic performance of plasma biomarkers in patients with acute intestinal ischaemia. Br J Surg. 2014;101(3):232–8. https://doi.org/10.1002/bjs.9331.
Matsumoto S, Shiraishi A, Kojima M, Funaoka H, Funabiki T, Saida F, Kitano M. Comparison of diagnostic accuracy for nonocclusive mesenteric ischemia in models with biomarkers including intestinal fatty acid-binding protein in addition to clinical findings. J Trauma Acute Care Surg. 2019;86(2):220–5. https://doi.org/10.1097/TA.0000000000002100.
Mothes H, Wickel J, Sponholz C, Lehmann T, Kaluza M, Zanow J, Doenst T. Monitoring of the progression of the perioperative serum lactate concentration improves the accuracy of the prediction of acute mesenteric ischemia development after cardiovascular surgery. J Cardiothorac Vasc Anesth. 2021;35(6):1792–9. https://doi.org/10.1053/j.jvca.2021.02.007.
Murray MJ, Gonze MD, Nowak LR, Cobb CF. Serum D(-)-lactate levels as an aid to diagnosing acute intestinal ischemia. Am J Surg. 1994;167(6):575–8. https://doi.org/10.1016/0002-9610(94)90101-5.
Nagata J, Kobayashi M, Nishikimi N, Komori K. Serum procalcitonin (PCT) as a negative screening test for colonic ischemia after open abdominal aortic surgery. Eur J Vasc Endovasc Surg. 2008;35(6):694–7. https://doi.org/10.1016/j.ejvs.2007.11.014.
Nuzzo A, Maggiori L, Ronot M, Becq A, Plessier A, Gault N, Joly F, Castier Y, Vilgrain V, Paugam C, Panis Y, Bouhnik Y, Cazals-Hatem D, Corcos O. Predictive factors of intestinal necrosis in acute mesenteric ischemia: prospective study from an intestinal stroke center. Am J Gastroenterol. 2017;112(4):597–605. https://doi.org/10.1038/ajg.2017.38.
Nuzzo A, Guedj K, Curac S, Hercend C, Bendavid C, Gault N, Tran-Dinh A, Ronot M, Nicoletti A, Bouhnik Y, Castier Y, Corcos O, Peoc’h K. SURVI (Structure d’URgences Vasculaires Intestinales) Research Group (French Intestinal Stroke Center). Accuracy of citrulline, I-FABP and D-lactate in the diagnosis of acute mesenteric ischemia. Sci Rep. 2021;11(1):18929. https://doi.org/10.1038/s41598-021-98012-w.
Poeze M, Froon AH, Greve JW, Ramsay G. D-lactate as an early marker of intestinal ischaemia after ruptured abdominal aortic aneurysm repair. Br J Surg. 1998;85(9):1221–4. https://doi.org/10.1046/j.1365-2168.1998.00837.x.
Polk JD, Rael LT, Craun ML, Mains CW, Davis-Merritt D, Bar-Or D. Clinical utility of the cobalt-albumin binding assay in the diagnosis of intestinal ischemia. J Trauma. 2008;64(1):42–5. https://doi.org/10.1097/TA.0b013e31815b846a.
Sadot E, Telem DA, Cohen L, Arora M, Divino CM. Nonocclusive ischemic colitis: analysis of risk factors for severity. Am Surg. 2014;80(5):454–60.
Salim SY, Young PY, Churchill TA, Khadaroo RG. Urine intestinal fatty acid-binding protein predicts acute mesenteric ischemia in patients. J Surg Res. 2017;209:258–65. https://doi.org/10.1016/j.jss.2016.07.017.
Sekino M, Funaoka H, Sato S, Okada K, Inoue H, Yano R, Matsumoto S, Ichinomiya T, Higashijima U, Matsumoto S, Hara T. Intestinal fatty acid-binding protein level as a predictor of 28-day mortality and bowel ischemia in patients with septic shock: A preliminary study. J Crit Care. 2017;42:92–100. https://doi.org/10.1016/j.jcrc.2017.07.012.
Schoettler JJ, Kirschning T, Hagmann M, Hahn B, Fairley AM, Centner FS, Schneider-Lindner V, Herrle F, Tzatzarakis E, Thiel M, Krebs J. Maintaining oxygen delivery is crucial to prevent intestinal ischemia in critical ill patients. PLoS ONE. 2021;16(7):e0254352. https://doi.org/10.1371/journal.pone.0254352.
Sgourakis G, Papapanagiotou A, Kontovounisios C, Karamouzis MV, Lanitis S, Konstantinou C, Karaliotas C, Papavassiliou AG. The value of plasma neurotensin and cytokine measurement for the detection of bowel ischaemia in clinically doubtful cases: a prospective study. Exp Biol Med (Maywood). 2013;238(8):874–80. https://doi.org/10.1177/1535370213494663.
Shi H, Wu B, Wan J, Liu W, Su B. The role of serum intestinal fatty acid binding protein levels and D-lactate levels in the diagnosis of acute intestinal ischemia. Clin Res Hepatol Gastroenterol. 2015;39(3):373–8. https://doi.org/10.1016/j.clinre.2014.12.005.
Sutherland F, Cunningham H, Pontikes L, Parsons L, Klassen J. Elevated serum interleukin 6 levels in patients with acute intestinal ischemia. Hepatogastroenterology. 2003;50(50):419–21.
Tanrıkulu Y, Şen Tanrıkulu C, Sabuncuoğlu MZ, Temiz A, Köktürk F, Yalçın B. Diagnostic utility of the neutrophil-lymphocyte ratio in patients with acute mesenteric ischemia: a retrospective cohort study. Ulus Travma Acil Cerrahi Derg. 2016;22(4):344–9. https://doi.org/10.5505/tjtes.2015.28235.
Thuijls G, van Wijck K, Grootjans J, Derikx JP, van Bijnen AA, Heineman E, Dejong CH, Buurman WA, Poeze M. Early diagnosis of intestinal ischemia using urinary and plasma fatty acid binding proteins. Ann Surg. 2011;253(2):303–8. https://doi.org/10.1097/SLA.0b013e318207a767.
Türkoğlu A, Gül M, Oğuz A, Bozdağ Z, Ülger BV, Yılmaz A, Aldemir M. Mean platelet volume: is it a predictive parameter in diagnosis of acute mesenteric ischemia? Int Surg. 2015;100(5):962–5. https://doi.org/10.9738/INTSURG-D-14-00268.1.
Uzun O, Turkmen S, Eryigit U, Mentese A, Turkyilmaz S, Turedi S, Karahan SC, Gunduz A. Can intestinal fatty acid binding protein (I-FABP) be a marker in the diagnosis of abdominal pathology? Turk J Emerg Med. 2014;14(3):99–103. https://doi.org/10.5505/1304.7361.2014.15679.
van der Voort PH, Westra B, Wester JP, Bosman RJ, van Stijn I, Haagen IA, Loupatty FJ, Rijkenberg S. Can serum L-lactate, D-lactate, creatine kinase and I-FABP be used as diagnostic markers in critically ill patients suspected for bowel ischemia. BMC Anesthesiol. 2014;14:111. https://doi.org/10.1186/1471-2253-14-111.
Vermeulen Windsant IC, Hellenthal FA, Derikx JP, Prins MH, Buurman WA, Jacobs MJ, Schurink GW. Circulating intestinal fatty acid-binding protein as an early marker of intestinal necrosis after aortic surgery: a prospective observational cohort study. Ann Surg. 2012;255(4):796–803. https://doi.org/10.1097/SLA.0b013e31824b1e16.
Wan J, Zang Z, Li S, Guojian MAY, Zang G, Du L. Clinical significance of d-dimer and intestinal fatty acid binding protein in patients with acute superior mesenteric vein thrombosis. Chinese General Practice. 2019;22(24):2933–6.
Woodford EP, Woodford HM, Hort AR, Pang TC, Lam VWT, Nahm CB. Neutrophil-lymphocyte ratio and platelet-lymphocyte ratio use in detecting bowel ischaemia in adhesional small bowel obstruction. ANZ J Surg. 2022;92(11):2915–20. https://doi.org/10.1111/ans.18073.
Yamamoto T, Umegae S, Kitagawa T, Matsumoto K. The value of plasma cytokine measurement for the detection of strangulation in patients with bowel obstruction: a prospective, pilot study. Dis Colon Rectum. 2005;48(7):1451–9. https://doi.org/10.1007/s10350-005-0019-7.
Yang S, Fan X, Ding W, Liu B, Meng J, Wang K, Wu X, Li J. D-dimer as an early marker of severity in patients with acute superior mesenteric venous thrombosis. Medicine (Baltimore). 2014;93(29):e270. https://doi.org/10.1097/MD.0000000000000270.
Zielinski MD, Eiken PW, Bannon MP, Heller SF, Lohse CM, Huebner M, Sarr MG. Small bowel obstruction-who needs an operation? A multivariate prediction model. World J Surg. 2010;34(5):910–9. https://doi.org/10.1007/s00268-010-0479-3.
Zogheib E, Cosse C, Sabbagh C, Marx S, Caus T, Henry M, Nader J, Fumery M, Bernasinski M, Besserve P, Trojette F, Renard C, Duhaut P, Kamel S, Regimbeau JM, Dupont H. Biological scoring system for early prediction of acute bowel ischemia after cardiac surgery: the PALM score. Ann Intensive Care. 2018;8(1):46. https://doi.org/10.1186/s13613-018-0395-5.
Kim KY, Lee HK, Kim H, Kim Y, Kim Y, Choi HH, Kim SW, Kim HK, Chae HS. Stromal cell-derived factor-1 as a serologic biomarker for the diagnosis of colon ischemia with chronic cardiovascular disease. Medicine (Baltimore). 2020;99(23):e20539. https://doi.org/10.1097/MD.0000000000020539.
Dai Y, Yan L, Fan J, Zou Q. Urinary long non-coding RNA H19 may serve as a biomarker for early diagnosis of acute intestinal necrosis. Nan Fang Yi Ke Da Xue Xue Bao. 2018;38(7):867–72. https://doi.org/10.3969/j.issn.1673-4254.2018.07.16.
Fried MW, Murthy UK, Hassig SR, Woo J, Oates RP. Creatine kinase isoenzymes in the diagnosis of intestinal infarction. Dig Dis Sci. 1991;36(11):1589–93. https://doi.org/10.1007/BF01296402.
Stroeder J, Bomberg H, Wagenpfeil S, Buecker A, Schaefers HJ, Katoh M, Groesdonk HV, Minko P. Presepsin and inflammatory markers correlate with occurrence and severity of nonocclusive mesenteric ischemia after cardiovascular surgery. Crit Care Med. 2018;46(6):e575–83. https://doi.org/10.1097/CCM.0000000000003091.
Nilsson J, Hansson E, Andersson B. Intestinal ischemia after cardiac surgery: analysis of a large registry. J Cardiothorac Surg. 2013;8:156. https://doi.org/10.1186/1749-8090-8-156.
Kurt E, Tekin E, Kurt N, Bayramoglu A. The role of adropin, HIF-1α and apelin biomarkers in the diagnosis of acute mesentaric ischemia. Am J Emerg Med. 2022;51:223–7. https://doi.org/10.1016/j.ajem.2021.10.058.
Groesdonk HV, Raffel M, Speer T, Bomberg H, Schmied W, Klingele M, Schäfers HJ. Elevated endothelin-1 level is a risk factor for nonocclusive mesenteric ischemia. J Thorac Cardiovasc Surg. 2015;149(5):1436-42.e2. https://doi.org/10.1016/j.jtcvs.2014.12.019.
Arnalich F, Maldifassi MC, Ciria E, Quesada A, Codoceo R, Herruzo R, Garcia-Cerrada C, Montoya F, Vazquez JJ, López-Collazo E, Montiel C. Association of cell-free plasma DNA with perioperative mortality in patients with suspected acute mesenteric ischemia. Clin Chim Acta. 2010;411(17–18):1269–74. https://doi.org/10.1016/j.cca.2010.05.017.
Zhuang X, Chen F, Zhou Q, Zhu Y, Yang X. A rapid preliminary prediction model for intestinal necrosis in acute mesenteric ischemia: a retrospective study. BMC Gastroenterol. 2021;21(1):154. https://doi.org/10.1186/s12876-021-01746-0.
Acosta S, Nilsson T. Current status on plasma biomarkers for acute mesenteric ischemia. J Thromb Thrombolysis. 2012;33(4):355–61. https://doi.org/10.1007/s11239-011-0660-z.
Sun DL, Cen YY, Li SM, Li WM, Lu QP, Xu PY. Accuracy of the serum intestinal fatty-acid-binding protein for diagnosis of acute intestinal ischemia: a meta-analysis. Sci Rep. 2016;6:34371. https://doi.org/10.1038/srep34371.
Montagnana M, Danese E, Lippi G. Biochemical markers of acute intestinal ischemia: possibilities and limitations. Ann Transl Med. 2018;6(17):341. https://doi.org/10.21037/atm.2018.07.22.
Chen Z, Liu X, Shou C, Yang W, Yu J. Advances in the diagnosis of non-occlusive mesenteric ischemia and challenges in intra-abdominal sepsis patients: a narrative review. PeerJ. 2023;11:e15307. https://doi.org/10.7717/peerj.15307.
Yu B, Ko RE, Yoo K, Gil E, Choi KJ, Park CM. Non-occlusive mesenteric ischemia in critically ill patients. PLoS ONE. 2022;17(12):e0279196. https://doi.org/10.1371/journal.pone.0279196.
Acosta S, Block T, Björnsson S, Resch T, Björck M, Nilsson T. Diagnostic pitfalls at admission in patients with acute superior mesenteric artery occlusion. J Emerg Med. 2012;42(6):635–41. https://doi.org/10.1016/j.jemermed.2011.03.036.
Bakker J, Nijsten MW, Jansen TC. Clinical use of lactate monitoring in critically ill patients. Ann Intensive Care. 2013;3(1):12. https://doi.org/10.1186/2110-5820-3-12.
Jakob SM, Merasto-Minkkinen M, Tenhunen JJ, Heino A, Alhava E, Takala J. Prevention of systemic hyperlactatemia during splanchnic ischemia. Shock. 2000;14(2):123–7. https://doi.org/10.1097/00024382-200014020-00008.
Bala M, Catena F, Kashuk J, De Simone B, Gomes CA, Weber D, Sartelli M, Coccolini F, Kluger Y, Abu-Zidan FM, Picetti E, Ansaloni L, Augustin G, Biffl WL, Ceresoli M, Chiara O, Chiarugi M, Coimbra R, Cui Y, Damaskos D, Di Saverio S, Galante JM, Khokha V, Kirkpatrick AW, Inaba K, Leppäniemi A, Litvin A, Peitzman AB, Shelat VG, Sugrue M, Tolonen M, Rizoli S, Sall I, Beka SG, Di Carlo I, Ten Broek R, Mircea C, Tebala G, Pisano M, van Goor H, Maier RV, Jeekel H, Civil I, Hecker A, Tan E, Soreide K, Lee MJ, Wani I, Bonavina L, Malangoni MA, Koike K, Velmahos GC, Fraga GP, Fette A, de’Angelis N, Balogh ZJ, Scalea TM, Sganga G, Kelly MD, Khan J, Stahel PF, Moore EE. Acute mesenteric ischemia: updated guidelines of the World Society of Emergency Surgery. World J Emerg Surg. 2022;17(1):54. https://doi.org/10.1186/s13017-022-00443-x.
Acknowledgements
None.
Funding
This study was funded by Grant PRG1255 from Estonian Research Council.
Author information
Authors and Affiliations
Contributions
ARB, JS, MB, AF, KTL, EK and KT designed the study. EK designed and performed all searches. ARB, JS, AF, KK, VM, MMu, ALV and KT conducted assessment of literature and data extraction. MMä performed all analyses. ARB and MMä designed figures. ARB drafted the manuscript. ARB, MMu, MMä, ALV and KT designed and drafted all tables. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors have no conflicts of interest regarding this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Search strategies.
Additional file 2:
Table S1. Risk of bias assessment.
Additional file 3:
Figures S1-S15. Forest plots.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Reintam Blaser, A., Starkopf, J., Björck, M. et al. Diagnostic accuracy of biomarkers to detect acute mesenteric ischaemia in adult patients: a systematic review and meta-analysis. World J Emerg Surg 18, 44 (2023). https://doi.org/10.1186/s13017-023-00512-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13017-023-00512-9