Abstract
Purpose/objective
Adjuvant whole breast radiotherapy and systemic therapy are part of the current evidence-based treatment protocols for early breast cancer, after breast-conserving surgery. Numerous randomized trials have investigated the therapeutic effects of partial breast irradiation (PBI) compared to whole breast irradiation (WBI), limiting the treated breast tissue. These trials were designed to achieve equal control of the disease with possible reduction in adverse events, improvements in cosmesis and quality of life (QoL). In this meta-analysis, we aimed to investigate the differences between PBI and WBI in side effects and QoL.
Material/methods
We performed a systematic literature review searching for randomized trials comparing WBI and PBI in early-stage breast cancer with publication dates after 2009. The meta-analysis was performed using the published event rates and the effect-sizes for available acute and late adverse events. Additionally, we evaluated cosmetic outcomes as well as general and breast-specific QoL using the EORTC QLQ-C30 and QLQ-BR23 questionnaires.
Results
Sixteen studies were identified (n = 19,085 patients). PBI was associated with a lower prevalence in any grade 1 + acute toxicity and grade 2 + skin toxicity (OR = 0.12; 95% CI 0.09–0.18; p < 0.001); (OR = 0.16; 95% CI 0.07–0.41; p < 0.001). There was neither a significant difference in late adverse events between the two treatments, nor in any unfavorable cosmetic outcomes, rated by either medical professionals or patients. PBI-technique using EBRT with twice-daily fractionation schedules resulted in worse cosmesis rated by patients (n = 3215; OR = 2.08; 95% CI 1.22–3.54; p = 0.007) compared to WBI. Maximum once-daily EBRT schedules (n = 2071; OR = 0.60; 95% CI 0.45–0.79; p < 0.001) and IORT (p = 0.042) resulted in better cosmetic results grade by medical professionals. Functional- and symptom-based QoL in the C30-scale was not different between PBI and WBI. Breast-specific QoL was superior after PBI in the subdomains of “systemic therapy side effects” as well as “breast-” and “arm symptoms”.
Conclusion
The analysis of multiple randomized trials demonstrate a superiority of PBI in acute toxicity as well breast-specific quality of life, when compared with WBI. Overall, late toxicities and cosmetic results were similar. PBI-technique with a fractionation of twice-daily schedules resulted in worse cosmesis rated by patients.
Similar content being viewed by others
Introduction
International guidelines recommend breast-conserving surgery (BCS), whole breast irradiation (WBI) and systemic therapy including endocrine therapy, HER2-targeted- and chemotherapy as the appropriate treatment for early-stage breast cancer. This multimodal approach has been shown to be equivalent to mastectomy in numerous randomized trials [1, 2]. Breast conserving surgery also seems to be associated with an improved quality of life (QoL) [3,4,5,6,7,8].
Scientific and structural advances including diagnostic imaging, high quality pathological testing, less invasive and morbid resection and effective systemic treatment have achieved very favorable oncologic results for most patients with early-stage disease. Women with localized breast cancer have a 99% chance of being alive after 5 years [9].
Several attempts have been put forward to de-escalate the treatment of early-stage breast cancer. Omission of adjuvant whole breast irradiation was studied in multiple randomized trials [10,11,12,13,14,15,16,17]. Published meta-analyses established that forgoing WBI does not impact overall survival in selected patients but is associated with a significantly higher rate of local recurrence [18,19,20]. Partial breast Irradiation (PBI) was suggested as a technique that limits radiotherapy to the tissue adjacent to the tumor bed which is the most frequent site of local recurrence. Studies of PBI investigated non-inferiority of local control, comparable or better cosmetic results and lower toxicities when compared to WBI. Additionally, PBI was designed to be delivered for shorter treatment durations, being more convenient for patients and less expensive for health care providers.
This analysis will review and analyze the current data on adverse events comparing WBI to PBI, their side effects and cosmesis. The results on survival as well as local control rates were published separately [21, 22].
Material and methods
On March 1st, 2023 we performed a literature review according to the published PRISMA guideline [23]. We searched the PubMed library with the search terms (“radiation therapy” or “radiotherapy” or “irradiation”) AND ("breast cancer" or "carcinoma of the breast") AND ("partial" or "targeted") AND (“randomized” OR “randomized” OR “randomly”). In addition, we screened the major meetings (e.g. ASTRO-, ESTRO-, ESMO-, ASCO annual meetings) for published abstracts. The search was supplemented by hand searches. We included randomized controlled trials including patients suffering from early-stage invasive breast cancer comparing PBI to WBI with a language restriction to English. Trials had to be published after the year 2009 in order allow for comparable modern techniques.
Selection of endpoints
All available toxicity and QoL analyses were retrieved from the published literature and filtered for matching scales and follow-up time. In order to include the highest possible number of trials we chose the endpoint and scoring used in the majority of trials. In the toxicity and QoL analyses, the longest available follow-up time was used to insure adequate capture of adverse events. In the evaluation of cosmetic events, we stratified by follow-up time. In order to compare different PBI techniques and external beam radiation therapy (EBRT) fractionation schedules, we performed an analysis using all available data that included non-standard protocol-specific endpoints. We hierarchically used the grade 2 + toxicities, or when unavailable, grade 3 + and subsequently grade 1 + toxicities.
Toxicities
Acute and late toxicities were generally reported on the EORTC/RTOG or LENT-SOMA scales which are 6 and 5 point Likert-scales (0–4/5) in increments of 1 with the main difference of death from toxicity (5) only scorable in the EORTC/RTOG scoring system. We also included other interval scales like the four point used in the IMPORT LOW-trial reporting dichotomized when we were investigating relative effect sizes in the study arms.
The cosmetic results were obtained at the latest available time point according to a four point scale (excellent/good/fair/poor) in most trials [24,25,26,27,28,29,30,31,32,33,34]. The cosmetic assessment by the physicians from the IMPORT LOW trial was based on the photographic assessment in a three-point scale (no change [none], mild, or marked change) and reported for the mild and marked change at the five year timepoint [35]. Cosmetic results evaluated by the patients were scored in 4-point scale. The item “Breast appearance changed” was dichotomized in the 4-point scale (not at all, a little, quite a bit, or very much) and used from the IMPORT LOW trial at the five years follow-up timepoint. The DBCG PBI trial assessed patient rated cosmesis in the item “Patient satisfaction, compared with contralateral breast” on a 4-point scale [36]. When the objective assessment included both physicians and nurses, we used the evaluation of the trained nurses as the might provide a more unbiased assessment [26, 34].
Quality of life analysis
Three trials used the EORTC QLQ-C30 questionnaire for general quality of life and the QLQ-BR23 questionnaire for breast- cancer specific quality of life. Both scales are subdivided into functional and symptomatic scales.
The IMPORT LOW trials also assessed QoL with the QLQ-BR23 questionnaire and protocol specific questions. However, the published analysis was restricted to the dichotomized values for moderate or marked responses. The NSABP B-39 trial reported several patient assessments including QoL measured by the BCTOS scale. Due to these differences, we were unable to include both trials in the assessment. The predefined threshold for minimal clinically meaningful difference was set at values of 5 or above [37].
Endpoints
We extracted the provided hazard ratios, odds ratios or event numbers from the identified trials to estimate the effect sizes comparing WBI to PBI in the endpoints of acute adverse events including any acute toxicities, acute skin toxicity, pneumonitis and breast pain. Late adverse events included any late toxicities, late skin toxicity, late subcutaneous/fibrosis/induration, telangiectasia, breast pain, chest wall pain, breast edema, fat necrosis and pulmonary toxicity. Bone toxicity according to RTOG scale was pooled with chest wall pain from the Common Terminology Criteria for Adverse Events (CTCAE) Version 3.0 [26, 33].
Subgroups
In order to compare different techniques, we grouped the trials according to PBI technique into external beam radiation (QD = a maximum of once daily treatment and BID = twice daily therapy), intraoperative radiotherapy using electrons or photons and any interstitial or applicator-based brachytherapy. If a trial included more than one technique, the trials were reported as the combination of the two techniques, except when separate data were available. The respective PBI techniques in Figs. 2, 4, 5, 6 and 7 are: mixed (green), EBRT BID (light blue), darker blue (EBRT QD), red (IORT), orange (BT).
Statistics
The comparison of acute and late adverse events and cosmetic results was obtained using odds ratios. QoL subcategories from the EORTC QLQ-C30 and EORTC QLQ-BR23 scales were compared with weighted mean differences (WMD). We used the inverse variance heterogeneity model (ivhet) to pool effect sizes, as this model uses a more conservative estimate of the confidence limits, has less observed variances and favors larger trials compared to the commonly used random effects model [38]. Zero event correction was applied where appropriate [39]. Statistical significance limit was set at p-values lower than 0.05. Significant values are marked in bold letters for better visibility. Heterogeneity within the meta-analysis was obtained with Cochran’s Q-test with the corresponding p values. The I2 statistics were also described, with values above 25% identified as considerable heterogeneity, triggering a subgroup analysis by technique [40]. Funnel plots were created to assess publication bias. For statistical analysis, Microsoft Excel add-in MetaXL 5.3 was used (EpiGear International, Sunrise Beach, Australia). Plots were created using Microsoft Excel for Microsoft Office 365 Pro Plus (Redmond, Washington, U.S.).
Results
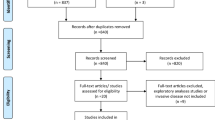
The literature analysis (Appendix Fig. 8) identified 51 publications reporting 16 different randomized trials. These included an overall number of 19,085 patients. Median follow-up for the primary endpoints was 8.6 years. Appendix Tables 1 and 2 show an overview of the included trials with important patient characteristics, treatment details and toxicity endpoints.
Acute toxicity
The comparison in acute toxicities between PBI and WBI is described in Fig. 1. PBI was associated with a significant decrease in acute adverse events equal or higher than grade I (n = 678; OR = 0.12; 95% CI 0.09–0.18; p < 0.001) and a reduction in acute skin toxicity, specifically grade II + radiodermatitis (n = 7348; OR = 0.16; 95% CI 0.07–0.41; p < 0.001). Grade II + acute skin toxicity occurred in 5.5% (95% CI 2.1–9.5%) of patients treated with PBI compared to 29.5% (95% CI 18.1–41.6%) with WBI. There were no statistically significant differences regarding grade II + acute pneumonitis (OR = 0.26; 95% CI 0.06–1.06; p = 0.060), or grade II + breast pain (OR = 0.92; 95% CI 0.65–1.30; p = 0.632) between the two modalities. In addition to the shown endpoints, PBI decreased acute breast edema, as analyzed in the RAPID trial (n = 2135; OR = 0.68; 95% CI 0.49–0.95; p = 0.023). Acute fatigue was not statistically different between the two groups (n = 2135; OR = 0.90; 95% CI 0.71–1.16; p = 0.423).
The analysis of relative acute skin toxicity by subgroup is shown in Fig. 2. A relative reduction of acute toxicity was shown for all PBI methods, except for IORT (p = 0.104).
Late toxicity
The combined analysis of all assessed late toxicity endpoints separated by grading is presented in Fig. 3. Any late toxicity, late skin toxicity, late subcutaneous fibrosis/induration, late telangiectasia, late breast pain, fat necrosis and lung toxicity were not different between PBI and WBI as shown in Fig. 3. PBI resulted in more late chest wall pain in all analyzed grades.
When the PBI technique was analyzed separately (Appendix Fig. 9), there was no difference in any late adverse events between the trial groups (OR = 2.05’; 95% CI 0.45–9.39; p = 0.357). Numerically, EBRT BID had higher rates of late adverse events (OR = 2.05; 95% CI 0.39–24.13; p = 0.285), but without reaching the threshold of statistical significance. There was also no difference in late skin adverse events like fibrosis, by either radiation modality or technique (Appendix Fig. 10). In the sub-analysis of late subcutaneous tissue toxicity by different radiation techniques, the overall comparison likewise did not detect a significant difference (OR = 2.00; 95% CI 0.89–4.51; p = 0.094) (Appendix Fig. 11) Patients treated with brachytherapy suffered more subcutaneous tissue toxicity (OR = 1.66; 95% CI 1.03–2.67; p = 0.037).
Cosmesis
Unfavorable cosmetic outcome (fair or poor on the 4-point scale) rated by medical professionals (physicians or nurses) was not different between the treatments after 1, 3, 5, 10 years and maximal follow-up, but reached statistical significance at the 20th year time point. However, this was based on data from a single trial (Fig. 4). The analysis by technique using all available maximal follow-up time intervals showed a significantly worse cosmesis for EBRT BID/BT (n = 604; rate PBI: 25.9%; rate WBI: 30.9%; OR = 2.14; 95% CI 1.41–3.24; p < 0.001), while once a day EBRT resulted in less cosmetic deterioration (n = 2071; rate PBI: 7.7%; rate WBI: 14.7%; OR = 0.60; 95% CI 0.45–0.79; p < 0.001). There was a trend towards worse cosmesis using the point estimates for EBRT BID, while numerically BT and IORT were associated with improved cosmesis.
When using the patients’ assessment of breast cosmesis we obtained similar results. Overall, unfavorable cosmesis was not different between PBI and WBI at the timepoints 1y, 3y, 5y, 10y and maximal follow-up. Both EBRT BID/BT (n = 675; OR = 1.63; 95% CI 1.14–2.34; p = 0.007) and EBRT BID (n = 3215; OR = 2.08; 95% CI 1.22–3.54; p = 0.007) resulted in significantly worse cosmetic results. Patients receiving IORT reported better results (n = 68; OR = 0.24; 95% CI 0.06–0.95; p = 0.042) (Fig. 5).
QoL assessment
Quality of life was scored by the EORTC QLQ-C30 and QLQ-BR23 questionnaires divided into functional and symptom scales. The pooled QLQ-C30 functional items (global, physical function, role function, emotional function, cognitive function and social function) did not show any differences between PBI and WBI (Appendix Fig. 12). Notably, all comparisons had significant heterogeneity with superior QoL in all items in the assessment of the Florence trial.
The analysis of the QLQ-C30 symptom scales (Fig. 6) did not show a significant improvement in any subscale when analyzing all PBI techniques together. Numerically, all point estimates were superior in the PBI arm. Clinically meaningful differences are reported for the PBI arm in the Florence trial for fatigue, pain and appetite loss.
Comparison of QLQ-C30 scores between partial breast irradiation and whole breast irradiation in different subdomains using weighted mean differences. Lower symptom scores represent better QoL. FA = fatigue, NV = nausea and vomiting, PA = pain, DY = dyspnea, SL = insomnia, AP = appetite loss, CO = constipation, DI = diarrhea, FI = financial difficulties
The effect of PBI versus WBI on breast-specific quality of life was compared using the EORTC QLQ-BR23. The functional scales on body image, sexual functioning, sexual enjoyment and future perspective were not significantly different between PBI and WBI (Appendix Fig. 13). CMDs were present in the PBI arm of the Florence trial in the items body image, sexual enjoyment, and future perspective.
The analysis of the EORTC QLQ-BR23 symptom domains showed that patients receiving PBI reported significantly fewer systemic side effects, breast and arm symptoms (BRST: WMD = − 3.4; 95% CI − 6.5 to 0.3; p = 0.031) (BRBS: WMD = − 6.6; 95% CI − 11.4 to 1.9; p = 0.007); (BRAS: WMD = − 5.9; 95% CI − 8.5 to 3.3; p < 0.001) (Fig. 7). Notably, the pooled differences did not exceed the threshold for CMD.
Discussion
This meta-analysis compares the side effects of partial and whole breast radiotherapy. While PBI seems to be associated with less acute toxicity and better breast-specific QoL, the effects on late and cosmetic events are similar to whole breast radiation. However, when analyzing the pooled estimates, it is important to consider that the radiation fractionation schedule, influenced the late adverse events as well as the cosmetic breast appearance.
The reduction in treated breast volume led to a noticeable relative decrease in acute skin toxicity by 83%. With an estimation of grade 2+ acute skin toxicity of around 29.5% in WBI and 5.5% in PBI, this difference should be clinically meaningful and might be considered by the treating physicians. However, PBI did not result in a reduction in grade 3 skin toxicity which occurred in less than 4% of patients.
Acute side effects appear to be heavily influenced by the treated volume while PBI reported a lower incidence of acute skin reactions of well as strong tendencies in pneumonitis and any acute side effects. The lack of statistical significance in some of the acute toxicity endpoints might be explained by the conservative comparative model used in this investigation. When the alternatives of fixed effect or random effect models were used, the results showed significant differences between the two treatment modalities. The improvement in acute toxicities was similar in all used radiation techniques and was consistent with the reported prospective and retrospective data as well as the published systematic reviews [41,42,43,44,45,46,47,48]. Therefore, different PBI procedures did not seem to have a relevant impact on acute adverse events as all point estimates favored PBI.
The assessment of late adverse events and cosmetic outcomes showed no overall significant differences. Unfavorable cosmetic results were detected after PBI in about 17% by both medical professionals and patients whereas WBI resulted in impaired cosmesis in 15% and 14% respectively. However, significant heterogeneity in the comparison suggested an association with the radiation technique and the fractionation schedules used. Partial breast treatment with once daily EBRT, BT and IORT might be associated with improved cosmesis. The analyses showed a consistent harmful effect of twice-daily external beam radiotherapy in any late adverse outcome measures and cosmesis as previously hypothesized [49]. Five trials used twice daily radiation schedules [24, 26, 30, 50, 51]. A proposed explanation for this observation is an insufficient normal tissue recovery time between fractions, which was initially anticipated to be less than 6 h. Other authors suggested that an inhomogeneous dose distribution with excessive hotspots could contribute to this finding. However, the published trial protocols did not allow non-standard radiation dose maxima in the target volume. Moreover, other techniques of BT and KV-based IORT also apply inhomogeneous doses and reported favorable cosmesis.
Younger age, larger breast size/surgical deficit, lymph node positivity and higher levels of anxiety/depression have been reported as adverse outcome predictors [52], while previous breast infection/surgical complication, seroma, higher age, smoking status, larger breast volume, greater volume of breast excised, central or inner tumor location, application of a tumor bed boost and use of a conventionally fractionated treatment schedule for whole breast irradiation [53,54,55,56,57] are predictors of poor cosmetic results.
Patients receiving a radiation boost of the tumor bed have higher incidence of tissue fibrosis. The use of a boost doubled the cumulative incidence of moderate or severe fibrosis from 15 to 30% after 20 years of follow-up [55] and it was likely a contributing factor for induration when PBI and WBI were compared. The criteria and frequency of the treatment in the included trials were not standardized, therefore any imbalance in these risk factors could contribute to the obtained results, even though unlikely, due to the randomized distribution of patients to the treatment arms.
Numerous publications have investigated the effects of different radiation schedules on the volume of breast fibrosis and cosmesis [58, 59]. Peterson and colleagues analysed predictors of poor cosmesis in the RAPID trial [53]. Their multivariable model did not demonstrate a significant impact of high dose treatment volume on adverse cosmesis. A detailed analysis from the DBCG-PBI trial that used 40 Gy in 15 fractions in both treatment arms demonstrated that the volume of breast treated with 40 Gy (V40Gy) was closely linked to a diagnosis of breast induration. This observation would support the use of PBI in larger breast sizes. Recently, the DBCG group showed that this correlation is true for women that are older than 65 years (Thomsen et al. ESTRO 2023, Vienna).
A substudy of the TARGIT-A trial focusing on adverse events with the use of IORT, reported better cosmetic results and less acute and late side effects. However, this was a single center outcome analysis within the trial, limiting a widespread applicability of their results. Further, the differences in the experimental arm between patients receiving TARGIT alone and TARGIT with additional whole breast radiation raise concerns regarding the long-term effects of the combined treatment approach [60].
Biologically effective doses (BED) used in PBI arms of the included trials differed significantly. Assuming an alpha/beta ratio of 4 the PBI techniques delivered the following dose ranges: EBRT QD (66.8–75.6 Gy), EBRT BID (62.9–75.6 Gy), IORT (120–131.2 Gy) and BT (62.9–83.7 Gy). The observed changes in the cosmetic outcome between the two EBRT techniques albeit almost no differences in BEDs as well as no relevant differences in the IORT arms despite much higher BEDs given suggest that other factors are mainly contributing to these findings.
The detectable impact of the addition of whole breast radiotherapy compared to endocrine therapy alone on QoL appears to be small. In the PRIME I trial only “breast symptoms” were more pronounced in the radiotherapy arm and resolved after 3 years [11]. This was confirmed by another prospective assessment of QoL during radiotherapy compared to no WBI [61]. Age, socioeconomic status of the patient, administration of chemotherapy or endocrine therapy, BMI and higher baseline anxiety scores are well known factors associated with poor QoL [7, 62,63,64,65,66]. Randomization should help decrease bias related to group allocation. With a threshold of 5 points for clinically meaningful difference, we detected improved QoL only in the Florence study, using EBRT with IMRT and treatment in QD schedules. Other trials and the pooled results did not reach a statistically significant threshold. The analysis was limited by the number of trials and patients included to detect smaller differences. Moreover, pooling of the results might be influenced by the different follow-up times of the trials, as a phenomenon called the “response shift” could influence the QoL scores explained by an adaption of the individual’s QoL assessment [67, 68]. However, other studies have already demonstrated that even a numerical small change in QoL scores could result in a clinically meaningful difference for the individual patient [69].
Our results are consistent with a prospective evaluation of different cohorts receiving IORT, PBI (EBRT QD) and various WBI regimes showed small differences in QoL. Breast symptoms were better after IORT and EBRT compared to WBI, in addition to decreased fatigue, global health status and role functioning over time. These differences were limited to a 2 years-period.
The comparison to other published meta-analyses is difficult because of different data pooling methods, outcome measures and selected endpoints [47, 48, 70, 71]. The scientific evidence for partial breast radiotherapy is also supplemented by numerous prospective single arm trials which also endorsed the same approach as in the trials included in our paper. BID EBRT with total doses of 38 Gy or above seemed to provide excellent/good cosmesis in less than 90% and a substantial rate of induration or fibrosis [59, 72,73,74,75]. When the total dose was reduced to 34–38 Gy, the treatment appeared to be better tolerated [42, 76]. It is possible that a reduction in single fraction and overall dose in the BID EBRT treatment might reduce breast induration and mitigate the observed higher toxicity rates. In the small trial from India included in this analysis, this approach showed early favorable results [51].
The other analyzed PBI techniques, the trials using once daily EBRT trials reported excellent/good cosmesis above 88% [41, 44, 45, 76, 77]. Brachytherapy using “Mammosite” also described good cosmetic results (excellent/good above 90%) [78, 79], whereas results with interstitial brachytherapy caused more diverse results (excellent/good: 68–94%) [43, 46, 80,81,82]. In low-risk cohorts receiving KV-based IORT, the reports are stating satisfactory cosmetic results (excellent/good: 89–97%) [83,84,85,86].
The interpretation of randomized trials comparing PBI and WBI is often difficult due to several factors changed in the PBI arms. Investigators changed not only the treated breast volume, but also the fractionation schedule, number of daily treatments and radiation technique which interferes with the genuine study query. Only two trials used the same technique and fractionation schedule and randomized only to the treated breast volume [35, 36]. Both of them reported favourable point estimates for all evaluated toxicities, patient-reported outcomes, cosmetic results and QoL analyses.
Data from multiple randomized trials suggest that the difference in oncologic endpoints between partial- and whole breast radiation therapy is very limited [21, 22, 48, 71]. This observation strengthens the necessity of an analysis of adverse events as well as quality of life. Comparative research suggests that patients’ priorities when weighing side effects and QoL compared to oncologic cure are similarly important [87]. In addition to equal recurrence and survival outcomes, Shah and colleagues demonstrated that multiple PBI regimes are cost effective, both per cost-effectiveness ratio analysis and cost per quality adjusted life year compared to hypofractionated WBI [88, 89].
Limitations
A limitation of our study is the use of published data rather than individual patient data which would be generally preferable. However, meta-analyses of aggregated patient data have been also shown to provide valuable conclusions [90]. Pooling of different toxicity scales can introduce bias in the analysis [91]. Yet, a good correlation between the LENT-SOMA and the RTOG/EORTC toxicity scales has been reported [92]. The strategy of using the last available time point during follow-up reduced the number of patients, and ensured the detection of possible toxicities. Further, the prevalence of breast hardness, pain, oversensitivity, edema, and skin changes is reduced over follow-up whereas breast shrinkage increased [52]. The use of conventionally fractionated instead of hypofractionated radiation therapy (HFX) in the standard arm of most trials introduces a bias towards PBI, especially regarding skin toxicity. The pooled analysis of the UK START trials, a Cochrane meta-analysis as well as other randomized trials demonstrated reduction in the adverse events as well as improved QoL and cosmetic results with hypofractionated WBI [52, 93,94,95,96,97].
A noticeable strength of our analysis is the use of multiple toxicity endpoints, separated by grading and different follow-up intervals as well as the differentiation by PBI technique. A follow-up period with a median of 8.6 years should be adequate to capture the majority of adverse events.
Conclusion
A reduction of the breast volume treated by adjuvant radiotherapy reduces acute skin toxicity and improves breast symptom-related quality of life. Twice-daily fractionation leads to higher fibrosis and worse cosmesis.
Abbreviations
- ASCO:
-
American Society for Clinical Oncology
- ASTRO:
-
American Society for Radiation Oncology
- BCS:
-
Breast conserving surgery
- BCTOS:
-
Breast Cancer Treatment Outcome Scale
- BED:
-
Biologically effective dose
- BID:
-
Twice a day radiation therapy
- BMI:
-
Body mass index
- BT:
-
Brachytherapy
- CFX:
-
Conventionally fractionated radiation therapy
- CI:
-
Confidence interval
- CTCAE:
-
Common Terminology Criteria for Adverse Events
- DBCG:
-
Danish Breast Cancer Cooperative Group
- EBRT:
-
External beam radiation therapy
- ESTRO:
-
European Society for Radiation Oncology
- ESMO:
-
European Society for Medical Oncology
- EORTC:
-
European Organization for Research and Treatment of Cancer
- HFX:
-
Hypofractionated radiation therapy
- IMRT:
-
Intensity-modulated radiation therapy
- KV:
-
Kilovoltage
- ivhet:
-
Inverse variance heterogeneity model
- LENT-SOMA:
-
Late effects of normal tissues-subjective objective management analytic
- OR:
-
Odds ratio
- PBI:
-
Partial breast irradiation
- PRISMA:
-
Preferred Reporting Items for Systematic reviews and Meta-analyses
- QoL:
-
Quality of life
- QD:
-
Once a day radiation therapy
- QLQ:
-
Quality of life questionnaire
- QLQ-BR23:
-
Questionnaire for measuring the quality of life in patients with breast cancerQLQ-C30: quality of life of cancer patient’s questionnaire
- RAPID trial:
-
Randomized Trial of Accelerated Partial Breast Irradiation
- RTOG:
-
Radiation Therapy Oncology Group
- START:
-
Standardization of breast radiotherapy
- TARGIT:
-
Targeted intraoperative radiotherapy
- UK:
-
United Kingdom
- WBI:
-
Whole breast irradiation
- WMD:
-
Weighted mean differences
References
Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347:1233–41.
Veronesi U, Marubini E, Mariani L, et al. Radiotherapy after breast-conserving surgery in small breast carcinoma: long-term results of a randomized trial. Ann Oncol. 2001;12:997–1003.
Janz NK, Mujahid M, Lantz PM, et al. Population-based study of the relationship of treatment and sociodemographics on quality of life for early stage breast cancer. Qual Life Res Int J Qual Asp Treat Care Rehabil. 2005;14:1467–79.
Ng ET, Ang RZ, Tran BX, et al. Comparing quality of life in breast cancer patients who underwent mastectomy versus breast-conserving surgery: a meta-analysis. Int J Environ Res Public Health. 2019;16:4970.
Dominici LS, Hu J, King TA, et al. Abstract GS6-06: local therapy and quality of life outcomes in young women with breast cancer. Cancer Res. 2019;79:GS6-06.
Engel J, Kerr J, Schlesinger-Raab A, Sauer H, Hölzel D. Quality of life following breast-conserving therapy or mastectomy: results of a 5-year prospective study. Breast J. 2004;10:223–31.
Bantema-Joppe EJ, de Bock GH, Woltman-van Iersel M, et al. The impact of age on changes in quality of life among breast cancer survivors treated with breast-conserving surgery and radiotherapy. Br J Cancer. 2015;112:636–43.
Tsai H-Y, Kuo RNC, Chung K-P. Quality of life of breast cancer survivors following breast-conserving therapy versus mastectomy: a multicenter study in Taiwan. Jpn J Clin Oncol. 2017;47:909–18.
Noone A-M, Cronin KA, Altekruse SF, et al. Cancer Incidence and Survival Trends by Subtype Using Data from the Surveillance Epidemiology and End Results Program, 1992–2013. Cancer Epidemiol Biomarkers Prev. 2017;26:632–41.
Kunkler IH, Williams LJ, Jack WJL, Cameron DA, Dixon JM. Breast-conserving surgery with or without irradiation in women aged 65 years or older with early breast cancer (PRIME II): a randomised controlled trial. Lancet Oncol. 2015;16:266–73.
Williams L, Kunkler I, King C, Jack W, van der Pol M. A randomised controlled trial of post-operative radiotherapy following breast-conserving surgery in a minimum-risk population. Quality of life at 5 years in the PRIME trial. Clinical Governance International Journal 2011;16.
Pötter R, Gnant M, Kwasny W, et al. Lumpectomy plus tamoxifen or anastrozole with or without whole breast irradiation in women with favorable early breast cancer. Int J Radiat Oncol Biol Phys. 2007;68:334–40.
Fisher B, Bryant J, Dignam JJ, et al. Tamoxifen, radiation therapy, or both for prevention of ipsilateral breast tumor recurrence after lumpectomy in women with invasive breast cancers of one centimeter or less. J Clin Oncol. 2002;20:4141–9.
Blamey R, Bates T, Chetty U, et al. Radiotherapy or tamoxifen after conserving surgery for breast cancers of excellent prognosis: British Association of Surgical Oncology (BASO) II trial. Eur J Cancer. 2013;49:2294–302.
Winzer K-J, Sauerbrei W, Braun M, et al. Radiation therapy and tamoxifen after breast-conserving surgery: updated results of a 2× 2 randomised clinical trial in patients with low risk of recurrence. Eur J Cancer. 2010;46:95–101.
Hughes KS, Schnaper LA, Bellon JR, et al. Lumpectomy plus tamoxifen with or without irradiation in women age 70 years or older with early breast cancer: long-term follow-up of CALGB 9343. J Clin Oncol. 2013;31:2382.
Hughes KS, Schnaper LA, Berry D, et al. Lumpectomy plus tamoxifen with or without irradiation in women 70 years of age or older with early breast cancer. N Engl J Med. 2004;351:971–7.
Matuschek C, Bölke E, Haussmann J, et al. The benefit of adjuvant radiotherapy after breast conserving surgery in older patients with low risk breast cancer- a meta-analysis of randomized trials. Radiat Oncol. 2017;12:60.
Chesney TR, Yin JX, Rajaee N, et al. Tamoxifen with radiotherapy compared with Tamoxifen alone in elderly women with early-stage breast cancer treated with breast conserving surgery: a systematic review and meta-analysis. Radiother Oncol. 2017;123:1–9.
Kunkler IH, Williams LJ, Jack WJL, Cameron DA, Dixon JM. Breast-conserving surgery with or without irradiation in early breast cancer. N Engl J Med. 2023;388:585–94.
Haussmann J, Budach W, Corradini S, et al. No difference in overall survival and non-breast cancer deaths after partial breast radiotherapy compared to whole breast radiotherapy-a meta-analysis of randomized trials. Cancers. 2020;12:2309.
Haussmann J, Budach W, Strnad V, et al. Comparing Local and Systemic Control between Partial- and Whole-Breast Radiotherapy in Low-Risk Breast Cancer-A Meta-Analysis of Randomized Trials. Cancers. 2021;13:2967.
Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535.
Boutrus R, El Hossieny H, El Sherif S, et al. Two-year results of once daily accelerated partial breast irradiation: a randomized controlled study. Int J Radiat Oncol Biol Phys. 2018;102:S81.
White JR, Winter K, Cecchini RS, et al. Cosmetic outcome from post lumpectomy whole breast irradiation (WBI) versus partial breast irradiation (PBI) on the NRG oncology/NSABP B39-RTOG 0413 phase III Clinical trial. Int J Radiat Oncol Biol Phys. 2019;105:S3–4.
Whelan TJ, Julian JA, Berrang TS, et al. External beam accelerated partial breast irradiation versus whole breast irradiation after breast conserving surgery in women with ductal carcinoma in situ and node-negative breast cancer (RAPID): a randomised controlled trial. Lancet. 2019;394:2165–72.
Polgár C, Major T, Takácsi-Nagy Z, Fodor J. Breast-conserving surgery followed by partial or whole breast irradiation: twenty-year results of a phase 3 clinical study. Int J Radiat Oncol Biol Phys. 2020;109:998.
Schafer R, Strnad V, Polgar C, et al. Quality-of-life results for accelerated partial breast irradiation with interstitial brachytherapy versus whole-breast irradiation in early breast cancer after breast-conserving surgery (GEC-ESTRO): 5-year results of a randomised, phase 3 trial. Lancet Oncol. 2018;19:834–44.
Offersen B, Nielsen HM, Thomsen M, et al. SP-0315: Partial breast radiotherapy after breast conservation for breast cancer: early results from the randomised DBCG PBI trial. Radiother Oncol. 2017;123:S163–4.
Rodriguez N, Sanz X, Dengra J, et al. Five-year outcomes, cosmesis, and toxicity with 3-dimensional conformal external beam radiation therapy to deliver accelerated partial breast irradiation. Int J Radiat Oncol Biol Phys. 2013;87:1051–7.
Veronesi U, Orecchia R, Maisonneuve P, et al. Intraoperative radiotherapy versus external radiotherapy for early breast cancer (ELIOT): a randomised controlled equivalence trial. Lancet Oncol. 2013;14:1269–77.
Meattini I, Marrazzo L, Saieva C, et al. Accelerated partial-breast irradiation compared with whole-breast irradiation for early breast cancer: long-term results of the randomized phase III APBI-IMRT-florence trial. J Clin Oncol. 2020;38:4175.
Meduri B, Baldissera A, Galeandro M, et al. OC-0568: Accelerated PBI VS standard radiotherapy (IRMA trial): interim cosmetic and toxicity results. Radiother Oncol. 2017;123:S303.
Corica T, Nowak AK, Saunders CM, et al. Cosmetic outcome as rated by patients, doctors, nurses and BCCT.core software assessed over 5 years in a subset of patients in the TARGIT-A Trial. Radiat Oncol. 2018;13:68.
Coles CE, Griffin CL, Kirby AM, et al. Partial-breast radiotherapy after breast conservation surgery for patients with early breast cancer (UK IMPORT LOW trial): 5-year results from a multicentre, randomised, controlled, phase 3, non-inferiority trial. Lancet. 2017;390:1048–60.
Offersen BV, Alsner J, Nielsen HM, et al. Partial breast irradiation versus whole breast irradiation for early breast cancer patients in a randomized phase III trial: the danish breast cancer group partial breast irradiation trial. J Clin Oncol Offic J Am Soc Clin Oncol. 2022;40:4189–97.
Osoba D, Rodrigues G, Myles J, Zee B, Pater J. Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol Offic J Am Soc Clin Oncol. 1998;16:139–44.
Doi SA, Barendregt JJ, Khan S, Thalib L, Williams GM. Advances in the meta-analysis of heterogeneous clinical trials I: the inverse variance heterogeneity model. Contemp Clin Trials. 2015;45:130–8.
Friedrich JO, Adhikari NK, Beyene J. Inclusion of zero total event trials in meta-analyses maintains analytic consistency and incorporates all available data. BMC Med Res Methodol. 2007;7:5.
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60.
Braunstein LZ, Thor M, Flynn J, et al. Daily fractionation of external beam accelerated partial breast irradiation to 40 Gy is well tolerated and locally effective. Int J Radiat Oncol Biol Phys. 2019;104:859–66.
Lei RY, Leonard CE, Howell KT, et al. Four-year clinical update from a prospective trial of accelerated partial breast intensity-modulated radiotherapy (APBIMRT). Breast Cancer Res Treat. 2013;140:119–33.
Ott OJ, Hildebrandt G, Pötter R, et al. Accelerated partial breast irradiation with multi-catheter brachytherapy: local control, side effects and cosmetic outcome for 274 patients: results of the German-Austrian multi-centre trial. Radiother Oncol J Eur Soc Ther Radiol Oncol. 2007;82:281–6.
Ott OJ, Strnad V, Stillkrieg W, Uter W, Beckmann MW, Fietkau R. Accelerated partial breast irradiation with external beam radiotherapy : first results of the German phase 2 trial. Strahlentherapie und Onkologie : Organ der Deutschen Rontgengesellschaft. 2017;193:55–61.
Vinante L, Avanzo M, Furlan C, et al. Ten daily fractions for partial breast irradiation: long-term results of a prospective phase II trial. Breast J. 2019;25:243–9.
Strnad V, Hildebrandt G, Pötter R, et al. Accelerated partial breast irradiation: 5-year results of the German-Austrian multicenter phase II trial using interstitial multicatheter brachytherapy alone after breast-conserving surgery. Int J Radiat Oncol Biol Phys. 2011;80:17–24.
Shah C, Jia X, Hobbs BP, et al. Outcomes with partial breast irradiation vs. whole breast irradiation: a meta-analysis. Ann Surg Oncol. 2021;28:4985.
Goldberg M, Bridhikitti J, Khan AJ, McGale P, Whelan TJ. A meta-analysis of trials of partial breast irradiation. Int J Radiat Oncol Biol Phys. 2023;115:60–72.
Bentzen SM, Yarnold JR. Reports of unexpected late side effects of accelerated partial breast irradiation–radiobiological considerations. Int J Radiat Oncol Biol Phys. 2010;77:969–73.
Vicini FA, Cecchini RS, White JR, et al. Long-term primary results of accelerated partial breast irradiation after breast-conserving surgery for early-stage breast cancer: a randomised, phase 3, equivalence trial. Lancet. 2019;394:2155–64.
Yadav BS, Loganathan S, Sharma SC, Singh R, Dahiya D. Comparison of toxicity and cosmetic outcomes after accelerated partial breast irradiation or whole breast irradiation using 3-dimensional conformal external beam radiation therapy. Adv Radiat Oncol. 2019;5:171–9.
Bhattacharya IS, Haviland JS, Kirby AM, et al. Patient-reported outcomes over 5 years after whole- or partial-breast radiotherapy: longitudinal analysis of the IMPORT LOW (CRUK/06/003) phase III randomized controlled trial. J Clin Oncol Offic J Am Soc Clin Oncol. 2019;37:305–17.
Peterson D, Truong PT, Parpia S, et al. Predictors of adverse cosmetic outcome in the RAPID trial: an exploratory analysis. Int J Radiat Oncol Biol Phys. 2015;91:968–76.
Vrieling C, Collette L, Fourquet A, et al. The influence of the boost in breast-conserving therapy on cosmetic outcome in the EORTC “boost versus no boost” trial: EORTC radiotherapy and breast cancer cooperative groups: European Organization for research and treatment of cancer. Int J Radiat Oncol Biol Phys. 1999;45:677–85.
Bartelink H, Maingon P, Poortmans P, et al. Whole-breast irradiation with or without a boost for patients treated with breast-conserving surgery for early breast cancer: 20-year follow-up of a randomised phase 3 trial. Lancet Oncol. 2015;16:47–56.
Schmeel LC, Koch D, Schmeel FC, et al. Acute radiation-induced skin toxicity in hypofractionated vs conventional whole-breast irradiation: an objective, randomized multicenter assessment using spectrophotometry. Radiother Oncol. 2020;146:172–9.
Romestaing P, Lehingue Y, Carrie C, et al. Role of a 10-Gy boost in the conservative treatment of early breast cancer: results of a randomized clinical trial in Lyon, France. J Clin Oncol Offic J Am Soc Clin Oncol. 1997;15:963–8.
Jagsi R, Ben-David MA, Moran JM, et al. Unacceptable cosmesis in a protocol investigating intensity-modulated radiotherapy with active breathing control for accelerated partial-breast irradiation. Int J Radiat Oncol Biol Phys. 2010;76:71–8.
Leonard KL, Hepel JT, Hiatt JR, Dipetrillo TA, Price LL, Wazer DE. The effect of dose-volume parameters and interfraction interval on cosmetic outcome and toxicity after 3-dimensional conformal accelerated partial breast irradiation. Int J Radiat Oncol Biol Phys. 2013;85:623–9.
Welzel G, Boch A, Sperk E, et al. Radiation-related quality of life parameters after targeted intraoperative radiotherapy versus whole breast radiotherapy in patients with breast cancer: results from the randomized phase III trial TARGIT-A. Radiat Oncol. 2013;8:9.
Xiao C, Miller AH, Felger J, Mister D, Liu T, Torres MA. A prospective study of quality of life in breast cancer patients undergoing radiation therapy. Adv Radiat Oncol. 2016;1:10–6.
Chen Q, Li S, Wang M, Liu L, Chen G. Health-related quality of life among women breast cancer patients in eastern China. Biomed Res Int. 2018;2018:1452635.
Lavdaniti M, Owens DA, Liamopoulou P, et al. Factors influencing quality of life in breast cancer patients six months after the completion of chemotherapy. Diseases. 2019;7:26.
Ganz PA, Petersen L, Bower JE, Crespi CM. Impact of adjuvant endocrine therapy on quality of life and symptoms: observational data over 12 months from the mind-body study. J Clin Oncol Offic J Am Soc Clin Oncol. 2016;34:816–24.
Ferreira AR, Di Meglio A, Pistilli B, et al. Differential impact of endocrine therapy and chemotherapy on quality of life of breast cancer survivors: a prospective patient-reported outcomes analysis. Ann Oncol. 2019;30:1784–95.
Daldoul A, Khechine W, Bhiri H, et al. Factors predictive of quality of life among breast cancer patients. Asian Pac J Cancer Prev. 2018;19:1671–5.
Schwartz CE, Sprangers MA. Methodological approaches for assessing response shift in longitudinal health-related quality-of-life research. Soc Sci Med. 1982;1999(48):1531–48.
Sprangers MA, Schwartz CE. Integrating response shift into health-related quality of life research: a theoretical model. Soc Sci Med. 1982;1999(48):1507–15.
Ousmen A, Conroy T, Guillemin F, et al. Impact of the occurrence of a response shift on the determination of the minimal important difference in a health-related quality of life score over time. Health Qual Life Outcomes. 2016;14:167.
Korzets Y, Fyles A, Shepshelovich D, Amir E, Goldvaser H. Toxicity and clinical outcomes of partial breast irradiation compared to whole breast irradiation for early-stage breast cancer: a systematic review and meta-analysis. Breast Cancer Res Treat. 2019;175:531–45.
Hickey BE, Lehman M. Partial breast irradiation versus whole breast radiotherapy for early breast cancer. Cochr Database Syst Rev. 2021;8:CD007077.
Chafe S, Moughan J, McCormick B, et al. Late toxicity and patient self-assessment of breast appearance/satisfaction on RTOG 0319: a phase 2 trial of 3-dimensional conformal radiation therapy-accelerated partial breast irradiation following lumpectomy for stages I and II breast cancer. Int J Radiat Oncol Biol Phys. 2013;86:854–9.
Rabinovitch R, Moughan J, Vicini F, et al. Long-term update of NRG Oncology RTOG 0319: a phase 1 and 2 trial to evaluate 3-dimensional conformal radiation therapy confined to the region of the lumpectomy cavity for stage I and II breast carcinoma. Int J Radiat Oncol Biol Phys. 2016;96:1054–9.
Vicini F, Winter K, Wong J, et al. Initial efficacy results of RTOG 0319: three-dimensional conformal radiation therapy (3D-CRT) confined to the region of the lumpectomy cavity for stage I/II breast carcinoma. Int J Radiat Oncol Biol Phys. 2010;77:1120–7.
Berrang TS, Olivotto I, Kim DH, et al. Three-year outcomes of a Canadian multicenter study of accelerated partial breast irradiation using conformal radiation therapy. Int J Radiat Oncol Biol Phys. 2011;81:1220–7.
Horst KC, Fasola C, Ikeda D, et al. Five-year results of a prospective clinical trial investigating accelerated partial breast irradiation using 3D conformal radiotherapy after lumpectomy for early stage breast cancer. Breast. 2016;28:178–83.
Livi L, Buonamici FB, Simontacchi G, et al. Accelerated partial breast irradiation with IMRT: new technical approach and interim analysis of acute toxicity in a phase III randomized clinical trial. Int J Radiat Oncol Biol Phys. 2010;77:509–15.
Belkacémi Y, Chauvet MP, Giard S, et al. Partial breast irradiation as sole therapy for low risk breast carcinoma: early toxicity, cosmesis and quality of life results of a MammoSite brachytherapy phase II study. Radiother Oncol J Eur Soc Ther Radiol Oncol. 2009;90:23–9.
Vicini FA, Keisch M, Shah C, et al. Factors associated with optimal long-term cosmetic results in patients treated with accelerated partial breast irradiation using balloon-based brachytherapy. Int J Radiat Oncol Biol Phys. 2012;83:512–8.
Rabinovitch R, Winter K, Kuske R, et al. RTOG 95–17, a Phase II trial to evaluate brachytherapy as the sole method of radiation therapy for Stage I and II breast carcinoma–year-5 toxicity and cosmesis. Brachytherapy. 2014;13:17–22.
White J, Winter K, Kuske RR, et al. Long-term cancer outcomes from study NRG oncology/RTOG 9517: a phase 2 study of accelerated partial breast irradiation with multicatheter brachytherapy after lumpectomy for early-stage breast cancer. Int J Radiat Oncol Biol Phys. 2016;95:1460–5.
Polgár C, Major T, Fodor J, et al. Accelerated partial-breast irradiation using high-dose-rate interstitial brachytherapy: 12-year update of a prospective clinical study. Radiother Oncol J Eur Soc Ther Radiol Oncol. 2010;94:274–9.
Laplana M, Garcia-Marqueta M, Sanchez-Fernandez JJ, et al. Effectiveness and safety of intraoperative radiotherapy (IORT) with low-energy X-rays (INTRABEAM((R))) for accelerated partial breast irradiation (APBI). Clin Transl Oncol Offic Publ Feder Span Oncol Soc Natl Cancer Inst Mexico. 2022;24:1732–43.
Lemanski C, Bourgier C, Draghici R, et al. Intraoperative partial irradiation for highly selected patients with breast cancer: results of the INTRAOBS prospective study. Cancer Radiother. 2020;24:114–9.
Valente SA, Tendulkar RD, Cherian S, et al. TARGIT-R (Retrospective): 5-Year follow-up evaluation of intraoperative radiation therapy (IORT) for breast cancer performed in North America. Ann Surg Oncol. 2021;28:2512–21.
Grobmyer SR, Lightsey JL, Bryant CM, et al. Low-kilovoltage, single-dose intraoperative radiation therapy for breast cancer: results and impact on a multidisciplinary breast cancer program. J Am Coll Surg. 2013;216:617–23.
Shrestha A, Martin C, Burton M, Walters S, Collins K, Wyld L. Quality of life versus length of life considerations in cancer patients: a systematic literature review. Psychooncology. 2019;28:1367–80.
Shah C, Lanni TB, Saini H, et al. Cost-efficacy of acceleration partial-breast irradiation compared with whole-breast irradiation. Breast Cancer Res Treat. 2013;138:127–35.
Shah C, Ward MC, Tendulkar RD, Cherian S, Vicini F, Singer ME. Cost and cost-effectiveness of image guided partial breast irradiation in comparison to hypofractionated whole breast irradiation. Int J Radiat Oncol Biol Phys. 2019;103:397–402.
Tudur Smith C, Marcucci M, Nolan SJ, et al. Individual participant data meta-analyses compared with meta-analyses based on aggregate data. Cochr Database Syst Rev. 2016;9:MR000007.
Hoeller U, Tribius S, Kuhlmey A, Grader K, Fehlauer F, Alberti W. Increasing the rate of late toxicity by changing the score? A comparison of RTOG/EORTC and LENT/SOMA scores. Int J Radiat Oncol Biol Phys. 2003;55:1013–8.
Anacak Y, Yalman D, Özsaran Z, Haydaroğlu A. Late radiation effects to the rectum and bladder in gynecologic cancer patients: the comparison of LENT/SOMA and RTOG/EORTC late-effects scoring systems. Int J Radiat Oncol Biol Phys. 2001;50:1107–12.
Offersen BV, Alsner J, Nielsen HM, et al. Hypofractionated versus standard fractionated radiotherapy in patients with early breast cancer or ductal carcinoma in situ in a randomized phase III trial: the DBCG HYPO trial. J Clin Oncol. 2020;38:3615–25.
Versmessen H, Vinh-Hung V, Van Parijs H, et al. Health-related quality of life in survivors of stage I-II breast cancer: randomized trial of post-operative conventional radiotherapy and hypofractionated tomotherapy. BMC Cancer. 2012;12:495.
Haviland JS, Owen JR, Dewar JA, et al. The UK Standardisation of Breast Radiotherapy (START) trials of radiotherapy hypofractionation for treatment of early breast cancer: 10-year follow-up results of two randomised controlled trials. Lancet Oncol. 2013;14:1086–94.
Whelan TJ, Pignol J-P, Levine MN, et al. Long-term results of hypofractionated radiation therapy for breast cancer. N Engl J Med. 2010;362:513–20.
Hickey BE, James ML, Lehman M, et al. Fraction size in radiation therapy for breast conservation in early breast cancer. Cochr Database Syst Rev. 2016;7:CD003860.
Vicini F, Cecchini R, White J, et al. Abstract GS4–04: Primary results of NSABP B-39/RTOG 0413 (NRG Oncology): a randomized phase III study of conventional whole breast irradiation (WBI) versus partial breast irradiation (PBI) for women with stage 0, I, or II breast cancer. Cancer Res. 2019;79:GS4-04.
Ganz PA, Cecchini RS, White JR, et al. Patient-reported outcomes (PROs) in NRG oncology/NSABP B-39/RTOG 0413: a randomized phase III study of conventional whole breast irradiation (WBI) versus partial breast irradiation (PBI) in stage 0, I, or II breast cancer. J Clin Oncol. 2019;37:508.
Polgar C, Major T, Fodor J, et al. High-dose-rate brachytherapy alone versus whole breast radiotherapy with or without tumor bed boost after breast-conserving surgery: seven-year results of a comparative study. Int J Radiat Oncol Biol Phys. 2004;60:1173–81.
Polgar C, Fodor J, Major T, et al. Breast-conserving treatment with partial or whole breast irradiation for low-risk invasive breast carcinoma–5-year results of a randomized trial. Int J Radiat Oncol Biol Phys. 2007;69:694–702.
Lövey K, Fodor J, Major T, et al. Fat necrosis after partial-breast irradiation with brachytherapy or electron irradiation versus standard whole-breast radiotherapy—4-year results of a randomized trial. Int J Radiat Oncol Biol Phys. 2007;69:724–31.
Polgar C, Fodor J, Major T, Sulyok Z, Kasler M. Breast-conserving therapy with partial or whole breast irradiation: ten-year results of the Budapest randomized trial. Radiother Oncol J Eur Soc Ther Radiol Oncol. 2013;108:197–202.
Polgar C, Major T, Sulyok Z, Takacsi-Nagy Z, Fodor J. Long-term toxicity and cosmetic results of partial versus whole breast irradiation: 10-Year results of a phase III APBI Trial. Int J Radiat Oncol Biol Phys. 2014;90:S133–4.
Olivotto IA, Whelan TJ, Parpia S, et al. Interim cosmetic and toxicity results from RAPID: a randomized trial of accelerated partial breast irradiation using three-dimensional conformal external beam radiation therapy. J Clin Oncol Offic J Am Soc Clin Oncol. 2013;31:4038–45.
Whelan T, Julian J, Levine M, et al. Abstract GS4–03: RAPID: a randomized trial of accelerated partial breast irradiation using 3-dimensional conformal radiotherapy (3D-CRT). Cancer Res. 2019;79:GS4-03.
Meduri B, Baldissera A, Stam M, et al. OC-0611: APBI with 3D-CRT vs. WBI: cosmetic and toxicity results of the prospective randomised IRMA trial. Radiother Oncol. 2020;152:S347.
Meduri B, Baldissera A, Iotti C, et al. Cosmetic results and side effects of accelerated partial-breast irradiation versus whole-breast irradiation for low-risk invasive carcinoma of the breast: the randomized phase III IRMA trial. J Clin Oncol Offic J Am Soc Clin Oncol. 2023;41:2201.
Li X, Sanz J, Foro P, et al. Long-term results of a randomized partial irradiation trial compared to whole breast irradiation in the early stage and low-risk breast cancer patients after conservative surgery. Clin Transl Oncol Offic Publ Feder Span Oncol Soc Natl Cancer Inst Mexico. 2021;23:2127.
Livi L, Meattini I, Marrazzo L, et al. Accelerated partial breast irradiation using intensity-modulated radiotherapy versus whole breast irradiation: 5-year survival analysis of a phase 3 randomised controlled trial. Eur J Cancer. 1990;2015(51):451–63.
Meattini I, Saieva C, Miccinesi G, et al. (1990) Accelerated partial breast irradiation using intensity modulated radiotherapy versus whole breast irradiation: health-related quality of life final analysis from the Florence phase 3 trial. Eur J Cancer. 2017;76:17–26.
Meattini I, Saieva C, Lucidi S, et al. Abstract GS4–06: accelerated partial breast or whole breast irradiation after breast conservation surgery for patients with early breast cancer: 10-year follow up results of the APBI IMRT Florence randomized phase 3 trial. Cancer Res. 2020;80:GS4-06.
Bhattacharya IS, Haviland JS, Hopwood P, et al. Can patient-reported outcomes be used instead of clinician-reported outcomes and photographs as primary endpoints of late normal tissue effects in breast radiotherapy trials? Results from the IMPORT LOW trial. Radiother Oncol J Eur Soc Therap Radiol Oncol. 2019;134:220–30.
Bhattacharya IS, Haviland JS, Perotti C, et al. Is breast seroma after tumour resection associated with patient-reported breast appearance change following radiotherapy? Results from the IMPORT HIGH (CRUK/06/003) trial. Radiother Oncol J Eur Soc Therap Radiol Oncol. 2019;136:190–6.
Franceschini D, Loi M, Chiola I, et al. Preliminary results of a randomized study on postmenopausal women with early stage breast cancer: adjuvant hypofractionated whole breast irradiation versus accelerated partial breast irradiation (HYPAB trial). Clin Breast Cancer. 2021;21:231.
Song YC, Sun GY, Fang H, et al. Quality of life after partial or whole-breast irradiation in breast-conserving therapy for low-risk breast cancer: 1-year results of a phase 2 randomized controlled trial. Front Oncol. 2021;11:738318.
Orecchia R, Veronesi U, Maisonneuve P, et al. Intraoperative irradiation for early breast cancer (ELIOT): long-term recurrence and survival outcomes from a single-centre, randomised, phase 3 equivalence trial. Lancet Oncol. 2021;22:597.
Vaidya JS, Joseph DJ, Tobias JS, et al. Targeted intraoperative radiotherapy versus whole breast radiotherapy for breast cancer (TARGIT-A trial): an international, prospective, randomised, non-inferiority phase 3 trial. Lancet. 2010;376:91–102.
Andersen KG, Gartner R, Kroman N, Flyger H, Kehlet H. Persistent pain after targeted intraoperative radiotherapy (TARGIT) or external breast radiotherapy for breast cancer: a randomized trial. Breast. 2012;21:46–9.
Sperk E, Welzel G, Keller A, et al. Late radiation toxicity after intraoperative radiotherapy (IORT) for breast cancer: results from the randomized phase III trial TARGIT A. Breast Cancer Res Treat. 2012;135:253–60.
Keshtgar MR, Williams NR, Bulsara M, et al. Objective assessment of cosmetic outcome after targeted intraoperative radiotherapy in breast cancer: results from a randomised controlled trial. Breast Cancer Res Treat. 2013;140:519–25.
Vaidya JS, Wenz F, Bulsara M, et al. Risk-adapted targeted intraoperative radiotherapy versus whole-breast radiotherapy for breast cancer: 5-year results for local control and overall survival from the TARGIT-A randomised trial. Lancet. 2014;383:603–13.
Vaidya JS, Wenz F, Bulsara M, et al. An international randomised controlled trial to compare TARGeted Intraoperative radioTherapy (TARGIT) with conventional postoperative radiotherapy after breast-conserving surgery for women with early-stage breast cancer (the TARGIT-A trial). Health Technol Assess. 2016;20:1–188.
Corica T, Nowak AK, Saunders CM, et al. Cosmesis and breast-related quality of life outcomes after intraoperative radiation therapy for early breast cancer: a substudy of the TARGIT-A trial. Int J Radiat Oncol Biol Phys. 2016;96:55–64.
Vaidya JS, Bulsara M, Saunders C, et al. Effect of delayed targeted intraoperative radiotherapy vs whole-breast radiotherapy on local recurrence and survival: long-term results from the targit-a randomized clinical trial in early breast cancer. JAMA Oncol. 2020;6:e200249.
Vaidya JS, Bulsara M, Baum M, et al. Long term survival and local control outcomes from single dose targeted intraoperative radiotherapy during lumpectomy (TARGIT-IORT) for early breast cancer: TARGIT-A randomised clinical trial. BMJ. 2020;370:m2836.
Ott OJ, Strnad V, Hildebrandt G, et al. GEC-ESTRO multicenter phase 3-trial: accelerated partial breast irradiation with interstitial multicatheter brachytherapy versus external beam whole breast irradiation: early toxicity and patient compliance. Radiother Oncol J Eur Soc Therap Radiol Oncol. 2016;120:119–23.
Strnad V, Ott OJ, Hildebrandt G, et al. 5-year results of accelerated partial breast irradiation using sole interstitial multicatheter brachytherapy versus whole-breast irradiation with boost after breast-conserving surgery for low-risk invasive and in-situ carcinoma of the female breast: a randomised, phase 3, non-inferiority trial. Lancet. 2016;387:229–38.
Polgar C, Ott OJ, Hildebrandt G, et al. Late side-effects and cosmetic results of accelerated partial breast irradiation with interstitial brachytherapy versus whole-breast irradiation after breast-conserving surgery for low-risk invasive and in-situ carcinoma of the female breast: 5-year results of a randomised, controlled, phase 3 trial. Lancet Oncol. 2017;18:259–68.
Strnad V, Polgár C, Ott OJ, et al. Accelerated partial breast irradiation using sole interstitial multicatheter brachytherapy compared with whole-breast irradiation with boost for early breast cancer: 10-year results of a GEC-ESTRO randomised, phase 3, non-inferiority trial. Lancet Oncol. 2023;24:262–72.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
JH, CM wrote the main manuscript, EB, WB helped to design the study and wrote part of the manuscript, JH prepared the figures, WB, KK, SC, DJ, DK BT, AP did the literature research and prepared the data for analysis, all authors reviewed the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
For a meta-analysis of published trials an ethics approval declaration in the manuscript is not necessary. A consent to participate declaration in the manuscript is not necessary because this is a meta-analysis of published trials.
Consent for publication
Human Ethics declaration: not applicable because this is an meta-analysis of published trials.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
See Figs. 8, 9, 10, 11, 12, 13 and Tables 1 and 2.
Comparison of late subcutaneous tissue/fibrosis toxicity by technique using odds ratios with the respective 95% confidence intervals between partial breast radiotherapy and whole breast radiotherapy. The green line represents the comparison of EBRT BID/BT to WBI. The red line represents the comparison of BT–WBI. The green line represents the comparison of EBRT BID/BT to WBI. The orange line represents the comparison of BT to WBI
Comparison of QLQ-C30 functional scores between partial breast irradiation and whole breast irradiation in different subdomains using weighted mean differences. Higher functional scores represent better QoL. PF = physical functioning, RF = role functioning, EF = emotional functioning, CF = cognitive functioning, SF = social functioning
Comparison of QLQ-BR23 functional scores between partial breast irradiation and whole breast irradiation in different subdomains using weighted mean differences. Higher functional scores represent better QoL. BRBI = body image, BRSEF = sexual functioning, BRSEE = sexual enjoyment, BRFU = future perspective
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Haussmann, J., Budach, W., Corradini, S. et al. Comparison of adverse events in partial- or whole breast radiotherapy: investigation of cosmesis, toxicities and quality of life in a meta-analysis of randomized trials. Radiat Oncol 18, 181 (2023). https://doi.org/10.1186/s13014-023-02365-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13014-023-02365-7