Abstract
Background
Definitive radiotherapy plus concurrent chemotherapy has been a standard treatment for esophagus patients who are unfit to undergo surgery. However, there are a variety of concurrent chemotherapy regimens with varying efficacy. In this phase II prospective study, we compared the efficacy and toxicity of DP (docetaxel and cisplatin) and PF (cisplatin and 5-fluorouracil) regimens with concurrent chemoradiotherapy (CCRT) in patients with esophageal squamous cell carcinoma (ESCC) and analyzed the 5-year overall survival (OS) and progression free survival (PFS). We also summarized the salvage treatments and late toxicities.
Methods
We enrolled 86 patients with clinical stage II-IVA from the Sun Yat-sen University Cancer Center. The patients were divided into two groups: PF group (41) and DP group (45). Statistics were analyzed using SPSS version 19.0.
Results
The 5-year OS rates were 62.9% ± 7.6% in PF group, and 52.7% ± 7.5% in DP group (P = 0.131), respectively. The 5-year PFS rates were 43.9% ± 7.8% for PF group, and 40.0% ± 7.3% for DP group (P = 0.398), respectively. Sixteen patients in the DP group and thirteen in the PF group received salvage treatment. For those patients with local residual or local recurrent disease, the median survival time after salvage treatment was 13.5 months and the 1, 2, and 3-year survival rates were 79.0%, 50.3%, and 43.1%, respectively. For all patients, thirteen (15.1%) had Grade 2 late cardiac toxicities. One patient had Grade 2 pleural effusion and required diuretic. Most patients with pneumonia are mild, and only one patient in PF group had Grade 2 pneumonia. One patient in the DP group developed tracheoesophageal fistula.
Conclusions
The 5-year follow-up confirmed that definitive CCRT with the DP regimen did not improve the treatment response, OS, or PFS in patients with ESCC compared to the PF regimen. The PF regimen remains the standard regimen for definitive CCRT for patients with locally advanced ESCC. Long-term follow-up also suggested that appropriate and active salvage treatment has a survival benefit for some patients, and late cardiopulmonary toxicities should be noticed during follow-up.
Trial registration
The trial was registered at https://clinicaltrials.gov (ClinicalTrials.gov Identifier: NCT 02969473, October 2010).
Similar content being viewed by others
Introduction
Esophageal carcinoma (EC) is the ninth most prevalent cancer and the sixth-highest cancer-related mortality in the world [1]. The incidence of EC in China is the highest in the world, accounting for over 50% of mortality and morbidity. About 90% of EC patients in East Asia have esophageal squamous cell carcinoma (ESCC) [2]. As the standard treatment option for patients not eligible for surgery, definitive radiotherapy with concurrent chemotherapy has been established [3,4,5,6,7]. For EC patients, 5-fluorouracil plus cisplatin (CDDP) (PF regimen) has been the most widely used chemotherapy regimen since the 1980s [3,4,5,6,7,8]. However, the toxicity and the survival of definitive concurrent chemoradiotherapy (CCRT) with cisplatin plus fluorouracil regimen have hitherto not been satisfactory. It was associated with 42.5% grade 3 acute toxicity, 25.5% grade 3 late toxicity, and 26.5% 5-year overall survival (OS) [6]. A great deal of effort has been invested into improving the OS and locoregional control through the combination of different chemotherapy medications. Unfortunately, when compared to the PF regimen, many trials have not shown a statistically significant benefit [3, 9, 10].
In some preclinical studies, it has been improved that, as a kind of semi-synthetic taxane, docetaxel was a radiation sensitizer [11,12,13]. Clinical studies have shown that docetaxel has notable therapeutic effect on head and neck squamous cell carcinoma [14, 15]. According to some studies, docetaxel and cisplatin chemotherapy administered concurrently with radiotherapy was highly effective in treating unresectable localized ESCC with tolerable toxicities, which has been hailed as promising [16, 17]. Therefore, in order to provide strong evidence for the efficacy of the DP regimen, it is necessary to obtain high-quality data from prospective randomized controlled trials.
This phase II prospective randomized study compared the efficacy and toxicity of DP and PF regimens with CCRT in ESCC patients. From October 2010 to March 2015, 86 patients from Sun Yat-sen University Cancer Center (SYSUCC) participated in this study. The preliminary outcomes were published in 2017 after 24 months of follow-up (median 25.1 months) [18]. We additionally investigated the consistency of long-term results with our earlier findings and analyzed the 5-year OS and progression free survival (PFS). We also summarized the late adverse events and post-relapse treatments of some patients, which provides a reference for clinicians.
Materials and methods
Patients
Patients’ inclusion criteria and the procedures of this prospective, single-center, randomized phase II trial have previously been reported. Eligible patients were aged between 18 and 70 years; had adequate bone marrow, hepatic, and renal function; a Karnofsky performance status (KPS) score ≥ 70; and had no history of other malignancies. Only patients with locally advanced (clinical stage II to IVA, including metastatic celiac or cervical nodes, according to the 6th edition of the American Joint Committee on Cancer [AJCC] staging system for esophagus cancer), histologically proven, and potentially curable ESCC, were eligible for inclusion. Primary exclusion criteria were the presence of distant metastasis (except for those confined to the celiac or cervical nodes) before treatment, a history of hypersensitivity to CDDP, 5-fluorouracil and docetaxel, and patients who were pregnant or breastfeeding. Our study protocol was reviewed by the SYSUCC Ethics Committee and approved. All patients gave their written informed consent to participate in the study.
Randomization
After confirming the eligibility criteria, using a computerized randomization program, patients were divided into either the PF (41 patients) or DP (45 patients) treatment group. Trial registration was completed with the US National Institute of Health (ClinicalTrials. gov, Identifier NCT 02969473).
Procedures
All patients underwent pretreatment evaluations, including a complete medical history and physical examination; complete blood count and chemistry profile of the serum; urine test; test of pulmonary function; electrocardiogram; examination of the barium swallow; neck, chest, and upper abdomen computed tomography (CT) scan with contrast; and ultrasonography by endoscopy. The patient underwent bronchoscopy if the diagnosis of bronchial invasion was suspected. Radionuclide bone scans and co-registered 18 F-labeled fluoro-2-deoxy-D-glucose positron emission tomography (PET/CT) scans were also performed when clinically indicated.
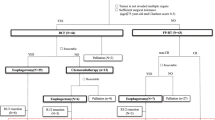
The treatment plan is illustrated in Fig. 1. Two cycles of chemotherapy were administered with CCRT to all patients. For patients assigned to receive CCRT with the DP regimen, docetaxel (60 mg/m2 delivered on day 1) and cisplatin (80 mg/m2 delivered on day 1) were intravenously administered at 3-week intervals. For patients assigned to receive CCRT with the PF regimen, 5-fluorouracil (1000 mg/m2 continuous infusion over 24 h daily on days 1–4) and cisplatin (80 mg/m2 delivered on day 1) were intravenously administered at 3-week intervals. Linear accelerators with a power of 6-MV or 8-MV were used to treat all patients. All patients received conventional radiotherapy at 1.8-2.0 Gy per fraction and five fractions per week. The total concurrent radiation dose was 60–64 Gy, from the first day of the first chemotherapy cycle onwards. Gross tumor volume (GTV) encompassed primary mass and metastatic regional lymph nodes observed on imaging examinations. Emerging evidence has demonstrated that involved field irradiation (IFI) is an acceptable treatment for locally advanced ESCC. In line with this notion, our trial employed IFI as well. The clinical target volume (CTV) included the primary tumour plus a 3.0-cm craniocaudal margin and a 1.0-cm margin in other directions, as well as the metastatic lymph nodes plus a 1.0-cm margin. The planning target volume (PTV1) included the GTV with a 5-mm margin in all directions, and the PTV2 generally included the CTV with a 5-mm margin in all directions. The prescribed dose was 60 to 64 Gy for PTV1 and 50 Gy for PTV2. The patients received either intensity-modulated radiation therapy (IMRT) or three-dimensional conformal radiotherapy (3D-CRT). We use the Monacle planning system (Monacle version 5.11, ELEKTA, US) to calculate the IMRT treatment plans, while 3D-CRT treatment plans were calculated using the Pinnacle planning system (Pinnacle3 version 8.0 m; Philips Medical Systems, Fitchburg, WI, USA). In some cases of severe hematologic or non-hematologic toxicity, dose adjustment was performed in the subsequent chemotherapy cycle. The following section outlines commonly implemented adjustments in chemotherapeutic drug regimens. In the presence of grade 3 thrombocytopenia, febrile grade 3 neutropenia, grade 4 neutropenia, or grade 3 non-hematologic toxicities, drugs are reduced to 75% of the initial dose. In cases of grade 4 thrombocytopenia, febrile grade 4 neutropenia, or grade 4 non-hematologic toxicities, drugs are adjusted to 50% of the original dose. Following dose reduction, if the same grade or higher toxicity recurs, drugs are further adjusted to 50% of the initial dose. In the event of grade 4 toxicity occurring subsequent to dose adjustment for grade 4, treatment is discontinued. The first follow-up took place in the fifth or sixth week after the end of treatment, and every three months thereafter for up to two years. It was planned to follow up every six months for up to five years or when clinically necessary.
Outcomes
The primary endpoint was the OS, which was defined as the time from randomization to death from any cause or the last follow-up date. Secondary endpoints included treatment response rate, PFS, patterns of treatment failure, completion rate of the protocol, and late toxicities associated with the treatment. PFS meant the time from randomization to progression, relapse, death from any cause, or last follow-up date.
Statistical analysis
Intention-to-treat analysis was used to analyze the data. The sample size was calculated using Power and Sample Size Calculations software (Version 3.0, 2009, USA). On the basis of previous study, the current study was estimated to detect an improvement in the median OS of 35 months for the DP group to 20 months for the PF group with a two-sided test with alpha level 0.05 and 80% statistical power. An estimated sample of 182 participants (91 per group) was required. Because recruitment in this trial was slower than expected, we performed an interim analysis in March 2015, after 86 patients had been enrolled. The Kaplan-Meier method was used to estimate survival, and the log-rank test was used to determine significance. It was considered significant only if the P-value was < 0.05 on two sides. Comparing baseline characteristics of the treatment groups was done using the t-test or Mann-Whitney U-test for continuous variables, and the Fisher’s exact test for categorical variables. Statistics were analyzed using SPSS version 19.0.
Results
From 2010 to 2015, we enrolled 86 patients in this trial, PF group had 41 participants and DP group had 45 (Fig. 2). Due to the slow enrollment, we terminate early. There was a good balance between the two groups in terms of demographics and characteristics of tumor (Table 1). The treatment compliance details for the patients are listed in Table 2. All patients in the two groups completed radiotherapy as planned, with 35 (85.4%) receiving IMRT and 6 (14.6%) patients receiving 3D-CRT in the PF group versus 33 (73.3%) receiving IMRT and 12 (26.7%) patients receiving 3D-CRT in the DP group (P = 0.171). As for chemotherapy, PF group had 40 (96.7%) patients complete full doses for two cycles, while the DP group had 32 (71.1%) patients complete full doses for two cycles (P = 0.002). Due to the early treatment-induced toxicity, one (2.4% of the PF patients) required an altered chemotherapy regimen during the second cycle. However, in the DP patients, eight (17.8%) received dose reduction, three (6.7%) received an altered regimen, and two (4.4%) canceled chemotherapy during the second cycle. According to the evaluation criteria for clinical efficacy, in the PF group, 12 patients obtained complete remission (CR), 24 patients achieved partial remission (PR), four patients experienced stable disease (SD), and one suffered progressive disease (PD). In the DP group, there were 14, 24, 5, and 2 patients with CR, PR, SD, and PD, respectively. The overall response rate (ORR: CR + PR) was 87.8% in the PF group and 84.4% in the DP group (P = 0.653) [18].
As of the time of this analysis, the median follow-up period for the surviving patients was 107 months (range 98–116). Of the 86 analyzed patients, 46 deaths (53.5%) were recorded at the final analysis (18 [43.9%] of 41 patients in the PF group and 28 [62.2%] of 45 patients in the DP group). The 5-year OS rates were 62.9% ± 7.6% in the PF group, and 52.7% ± 7.5% in the DP group. There was no significant difference in 5-year OS (62.9% vs. 52.7%, P = 0.131) between the PF and the DP group, respectively (Fig. 3). The 5-year PFS rates were 43.9% ± 7.8% for the PF group, and 40.0% ± 7.3% for the DP group (P = 0.398), respectively (Fig. 4). Twenty-four (27.9%) patients (10 (22.2%) in the DP group, and 14 (34.1%) in the PF group) were alive without disease progression at the time of analysis on March 5, 2022. The first failure patterns are listed in Table 3. Notably, more patients in the DP group had distant metastases at first failure than in the PF group (53.4% vs. 24.2%, P = 0.006), which is consistent with the previous study [18]. For patients who experienced disease progression, we summarized the treatment after progression. Sixteen patients in the DP group, received post-relapse therapy: one patient with simple distant metastasis underwent palliative radiotherapy; two patients with compound metastases received systemic chemotherapy; esophageal surgery was performed in one patient with simple local metastasis; one patient with simple local metastasis received chemoradiotherapy; and eleven patients with local or distant metastasis received chemotherapy alone. In the PF group, thirteen patients received post-relapse therapy: one patient with a simple distant metastasis underwent surgery at the metastatic site; one patient with compound metastasis underwent ablation plus chemotherapy; esophageal surgery was performed in two patients with simple local metastases; one patient with simple local metastasis received chemoradiotherapy; and eight patients with local or distant metastasis received chemotherapy alone. Notably, the salvage treatments and survival of some patients with local residual or local recurrent are summarized in Table 4. The median survival time after salvage treatment was 13.5 months in these patients. The 1, 2, 3-year OS rates were 79.0%, 50.3% and 43.1%. In particular, 3 (20%) of the 15 patients remained disease free after salvage treatment. The Common Terminology Criteria for Adverse Events version 3.0 (CTCAE 3.0) was used to evaluated patients’ adverse events. As for the early adverse events, compared to the PF group, the DP group was significantly more likely to suffer from hematological toxicity. Non-hematological toxicity was comparable between the two groups [18]. After long-term follow-up, late adverse events were also observed in some patients. Thirteen patients had Grade 2 late cardiac events, including heart failure and pericardial effusion (7 in the DP group, 6 in the PF group). One patient had Grade 2 pleural effusion, and required diuretics. Most patients with pneumonia were mild, and only one Grade 2 pneumonia was observed in PF group. One patient in the DP group developed tracheoesophageal fistula. No deaths due to the therapeutic toxicity were observed. In summary, the 1, 2, and 5-year OS rates were 93.7%, 86.2%, and 62.9%, respectively, in the PF group, and 87.3%, 69.1%, and 52.7%, respectively, in the DP group.
Discussion
For clinicians, esophageal carcinoma remains a challenging condition, with a poor prognosis and low survival rate. For fit patients with resectable esophageal carcinoma, the 5-year survival rate was only 25% after surgery [19]. Moreover, approximately 30% of the patients who underwent esophageal surgery, who were clinically considered to have resectable disease, had microscopically irradical resections performed [20,21,22]. Further evidence suggested that neoadjuvant concurrent chemoradiotherapy followed by surgery achieved a survival benefit [23]. Based on the aforementioned, some multi-center prospective trials were initiated to improve the survival benefit of neoadjuvant chemoradiotherapy or postoperative chemotherapy for patients with clinically resectable, locally advanced EC that offers a higher survival benefit over surgery alone, as demonstrated in the CROSS, JCOG9907, and NEOCRTEC5010 trials [24,25,26,27,28].
Nevertheless, at the initial diagnosis, approximately half of the patients are no longer eligible for surgery [29]. Therefore, it is particularly important to improve the survival of these patients. Over the past few decades, many studies have focused on finding surgery alternatives. Based on the results of the Radiation Therapy Oncology Group (RTOG) 8501 study, definitive radiotherapy concurrent with cisplatin plus fluorouracil has become one of the standard treatment regimens for locally advanced EC. Chemoradiotherapy for RTOG 8501 was the cisplatin plus fluorouracil plan, in which cisplatin 75 mg/m2 was administered on the first day of weeks 1, 5, 8, and 11, and a 1000 mg/m2 dose of fluorouracil was administered for the first 4 days of weeks 1, 5, 8, and 11 concurrent with radiation therapy (50 Gy in 25 fractions over 5 weeks). Although combined therapy proved to be superior to radiotherapy alone, the treatment toxicities and the survival were not satisfactory, with 42% grade 3 acute toxicity, 25% grade 3 late toxicity, and 26% 5-year OS [6]. Since then, a growing number of studies have evaluated the clinical outcomes of CCRT in patients with EC. The selected prospective studies and retrospective studies that have been updated are listed in Table 5 [10, 30,31,32,33,34,35,36,37].
To improve the efficacy, some studies have aimed to improve survival by optimizing the radiation dose [5, 31, 38]. Unsurprisingly, none of the results were satisfactory. And recently, an oral presentation from Maarten C.C.M. Hulshof at 2020 ASCO-GI indicated that increasing the radiotherapy dose from 50.4 to 61.6 Gy (primary tumor) did not significantly improve local control rate and OS. In contrast, the number of related side effects increased.
Along with the emergence of other drugs, several active drugs have been used in various clinical studies to obtain a better response. Phase II trial RTOG 0113, which compared non-fluorouracil-based therapy (cisplatin plus paclitaxel) and fluorouracil-based therapy (fluorouracil plus paclitaxel), reported that the median survival time were 14.9 months in the cisplatin group and 28.7 months in the fluorouracil group, respectively [34]. In PRODIGE5/ACCORD17, results showed that definitive chemoradiotherapy with FOLFOX (fluorouracil plus leucovorin and oxaliplatin) did not improve PFS compared to chemoradiotherapy with cisplatin and fluorouracil in patients with unresectable EC [3]. ESO-Shanghai 2, a randomized, multicenter, phase III clinical trial, compared three paclitaxel-based chemoradiotherapy regimens (paclitaxel with fluorouracil, carboplatin, and cisplatin) and suggested that paclitaxel plus fluorouracil was not superior to paclitaxel plus carboplatin or paclitaxel plus cisplatin in terms of OS. CCRT in patients with locally advanced ESCC, the 3-year OS rates were 57.2% in the fluorouracil group, 56.5% in the carboplatin groups, and 60.1% in the cisplatin group, respectively [10].
Docetaxel, a new generation of anti-EC drugs, enhances the radiation response by inducing apoptosis and mitotic arrest in murine tumor cells [16, 17, 39,40,41,42,43]. Given the known activity of cisplatin in EC, we planned to explore the combined effect of docetaxel and cisplatin as an enhanced disease control regimen for locally advanced diseases that are not resectable. Moreover, previous studies have enrolled participants with different histological types (squamous carcinoma and adenocarcinoma) or patients with different disease stages (locally advanced diseases and metastatic diseases) [44, 45]. Therefore, it is time to initiate our study, a phase II prospective randomized trial comparing the efficacy and toxicity of PF and DP regimens with CCRT in patients with ESCC. After the 5-year follow-up, we found that the ORR of the PF group was 87.3% and that of the DP group was 84.4% (P = 0.653). Besides, the 5-year OS did not differ statistically significantly between the PF group and the DP group (62.9% vs. 52.7%, p = 0.131). In the present study, OS was higher than that in previous studies. Several reasons may account for these differences between different studies. Firstly, various studies used different chemotherapy regimens with varying dose intensity. As an example, in Zhang’s study, patients received lower doses of 5-FU and cisplatin. Secondly, in our trial, chemotherapy compliance rate was substantially higher (97.6% in the PF group and 71.1% in the DP group) than that in the RTOG 8501 trial. Thirdly, there have been decades since the RTOG 8501 trial was conducted, without improvements in the techniques of radiotherapy, staging methods, or best supportive care. Finally, there may exist patient selection bias, especially in those retrospective studies, and differences between ethnicities may also play a significant role [46, 47].
At the same time, due to the time of data analysis in this article, more than half of the participants had both local recurrence and distant metastasis, coupled with the fact that a consensus salvage treatment strategy for patients with ESCC who recur after definitive chemoradiotherapy or radiotherapy has not been established. In this study, we made a summarized list of the patients who received different salvage treatments after relapse. Notably, because local progression is more common, the salvage treatments and survival of some patients with local residual or local recurrent are summarized in Table 6. The median survival time after salvage treatment was 13.5 months in these patients. The 1, 2, and 3-year OS rates were 79.0%, 50.3%, and 43.1%, respectively. In particular, three (20%) of the 15 patients remained disease free after salvage treatment. However, the optimal salvage treatment modality remains controversial (Table 6) [48,49,50,51,52,53,54,55,56]. Clinicians should make individualized assessments based on the type, location, and local involvement of the cancer as well as the functional status of each patient to determine the appropriate salvage treatment.
In summary, we were unable to confirm that the docetaxel plus cisplatin regimen performed better in OS than the fluorouracil plus cisplatin regimen in definitive CCRT for patients with ESCC. Based on these findings, our recommendation is to remain the standard role of the fluorouracil plus cisplatin regimen for definitive CCRT in patients with locally advanced ESCC. What’s more, comparing the adverse event (AE) profiles of the two regimens, it was found that the docetaxel plus cisplatin regimen resulted in severe leukocytopenia and neutropenia, and that the treatment had to be interrupted or terminated. As for late adverse effects, there was no significant difference between two groups. One possible reason is the difficulty in collecting late effects data.
In view of the biological characteristics of ESCC, chemotherapy has entered a bottleneck stage; however, it still plays an irreplaceable role in CCRT. Recent studies have indicated that, in combination with immunotherapy, chemoradiotherapy has shown immunomodulatory effects that may result in synergistic treatment responses. Some ongoing studies have explored the combination of chemoradiotherapy regimens with immunotherapies in EC, such as KEYNOTE-975 (study of pembrolizumab in combination with chemoradiotherapy in EC), ESOCORT-CRT (study of camrelizumab (SHR-1210) in combination with concurrent chemoradiotherapy in EC), and Rational-311 (study of Tislelizumab [BGB-A317] versus placebo in combination with chemoradiotherapy in EC), and we expect to hear good news from these studies in the near future.
Conclusions
The 5-year follow-up confirmed that definitive CCRT with the DP regimen did not improve the treatment response, OS, or PFS in patients with ESCC compared to the PF regimen. The PF regimen remains the standard regimen for definitive CCRT for patients with locally advanced ESCC. Long-term follow-up also suggested that appropriate and active salvage treatment has a survival benefit for some patients, and late cardiopulmonary adverse event should be noticed during follow-up.
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- DP:
-
docetaxel and cisplatin
- PF:
-
cisplatin and 5-fluorouracil
- CCRT:
-
concurrent chemoradiotherapy
- ESCC:
-
esophageal squamous cell carcinoma
- OS:
-
overall survival
- PFS:
-
progression free survival
- EC:
-
esophageal carcinoma
- ORR:
-
overall response rate
- CR:
-
complete remission
- PR:
-
partial remission
- SYSUCC:
-
Sun Yat-sen University Cancer Center
- KPS:
-
Karnofsky performance status
- AJCC:
-
American Joint Committee on Cancer
- CT:
-
computed tomography
- PET/CT:
-
positron emission tomography
- GTV:
-
gross tumor volume
- IFI:
-
involved field irradiation
- CTV:
-
clinical target volume
- PTV:
-
planning target volume
- IMRT:
-
intensity-modulated radiation therapy
- 3D-CRT:
-
three-dimensional conformal radiotherapy
- CTCAE 3.0:
-
Common Terminology Criteria for Adverse Events version 3.0
- FOLFOX:
-
fluorouracil plus leucovorin and oxaliplatin
- AE:
-
adverse event
- BMI:
-
body mass index
- CCI:
-
Charlson comorbidity index
- SD:
-
stable disease
- PD:
-
progressive disease
- FOLFIRI:
-
5-fluorouracil + irinotecan + leucovorin
- anti-PD-1:
-
anti-program death-1
- Ad:
-
adenocarcinoma
- MST:
-
median survival time
- QW:
-
every week
- Q3W:
-
every three weeks
- Q4W:
-
every four weeks
- Q5W:
-
every five weeks
- RT:
-
radiotherapy
- Sq:
-
squamous cell carcinoma
- TP:
-
paclitaxel + cisplatin
- TF:
-
paclitaxel + fluorouracil
- TC:
-
paclitaxel + carboplatin
- TPF:
-
fluorouracil + cisplatin + paclitaxel
- sEMR:
-
salvage Endoscopic mucosal resection
- sESD:
-
salvage endoscopic submucosal dissection
- sPDT:
-
salvage photodynamic therapy
- sAPC:
-
salvage Argon plasma coagulation
- NRT:
-
non-radiotherapy
- BED:
-
biological effective dose
- CDDP:
-
cisplatin
- DOC:
-
docetaxel
- Fr:
-
fraction
References
Erratum. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer J Clin. 2020;70(4):313. .21609. PubMed PMID: 32767693.
Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. Cancer J Clin. 2016;66(2):115–32. 21338. PubMed PMID: 26808342.
Conroy T, Galais MP, Raoul JL, Bouché O, Gourgou-Bourgade S, Douillard JY, et al. Definitive chemoradiotherapy with FOLFOX versus fluorouracil and cisplatin in patients with oesophageal cancer (PRODIGE5/ACCORD17): final results of a randomised, phase 2/3 trial. Lancet Oncol. 2014;15(3):305–14. 2045(14)70028-2. PubMed PMID: 24556041.
Ruppert BN, Watkins JM, Shirai K, Wahlquist AE, Garrett-Mayer E, Aguero EG, et al. Cisplatin/Irinotecan versus carboplatin/paclitaxel as definitive chemoradiotherapy for locoregionally advanced esophageal cancer. Am J Clin Oncol. 2010;33(4):346–52. https://doi.org/10.1097/COC.0b013e3181aaca26. Epub 2009/10/21.
Minsky BD, Pajak TF, Ginsberg RJ, Pisansky TM, Martenson J, Komaki R, et al. INT 0123 (Radiation Therapy Oncology Group 94 – 05) phase III trial of combined-modality therapy for esophageal cancer: high-dose versus standard-dose radiation therapy. J Clin Oncology: Official J Am Soc Clin Oncol. 2002;20(5):1167–74. https://doi.org/10.1200/jco.2002.20.5.1167. Epub 2002/03/01.
Cooper JS, Guo MD, Herskovic A, Macdonald JS, Martenson JA Jr, Al-Sarraf M, et al. Chemoradiotherapy of locally advanced esophageal cancer: long-term follow-up of a prospective randomized trial (RTOG 85 – 01). Radiation Therapy Oncology Group Jama. 1999;281(17):1623–7. https://doi.org/10.1001/jama.281.17.1623. Epub 1999/05/11.
Herskovic A, Martz K, al-Sarraf M, Leichman L, Brindle J, Vaitkevicius V, et al. Combined chemotherapy and radiotherapy compared with radiotherapy alone in patients with cancer of the esophagus. N Engl J Med. 1992;326(24):1593–8. https://doi.org/10.1056/nejm199206113262403. Epub 1992/06/11.
al-Sarraf M, Martz K, Herskovic A, Leichman L, Brindle JS, Vaitkevicius VK, et al. Progress report of combined chemoradiotherapy versus radiotherapy alone in patients with esophageal cancer: an intergroup study. J Clin Oncology: Official J Am Soc Clin Oncol. 1997;15(1):277–84. https://doi.org/10.1200/jco.1997.15.1.277. Epub 1997/01/01.
Chen Y, Ye J, Zhu Z, Zhao W, Zhou J, Wu C, et al. Comparing Paclitaxel Plus Fluorouracil Versus Cisplatin Plus Fluorouracil in Chemoradiotherapy for locally advanced esophageal squamous cell Cancer: a Randomized, Multicenter, Phase III Clinical Trial. J Clin Oncology: Official J Am Soc Clin Oncol. 2019;37(20):1695–703. https://doi.org/10.1200/jco.18.02122. Epub 2019/03/29.
Ai D, Ye J, Wei S, Li Y, Luo H, Cao J, et al. Comparison of 3 paclitaxel-based chemoradiotherapy regimens for patients with locally advanced esophageal squamous cell Cancer: a Randomized Clinical Trial. JAMA Netw open. 2022;5(2):e220120. https://doi.org/10.1001/jamanetworkopen.2022.0120. Epub 2022/02/22.
Tishler RB, Schiff PB, Geard CR, Hall EJ. Taxol: a novel radiation sensitizer. Int J Radiat Oncol Biol Phys. 1992;22(3):613–7. https://doi.org/10.1016/0360-3016(92)90888-o. Epub 1992/01/01. PubMed PMID: 1346533.
Ajani JA, Ilson DH, Daugherty K, Pazdur R, Lynch PM, Kelsen DP. Activity of taxol in patients with squamous cell carcinoma and adenocarcinoma of the esophagus. J Natl Cancer Inst. 1994;86(14):1086–91. https://doi.org/10.1093/jnci/86.14.1086. Epub 1994/07/20.
Milas L, Milas MM, Mason KA. Combination of taxanes with radiation: preclinical studies. Semin Radiat Oncol. 1999;9(2 Suppl 1):12–26. Epub 1999/04/21. PubMed PMID: 10210536.
Catimel G, Verweij J, Mattijssen V, Hanauske A, Piccart M, Wanders J, EORTC Early Clinical Trials Group. Docetaxel (Taxotere): an active drug for the treatment of patients with advanced squamous cell carcinoma of the head and neck. Annals of Oncology: Official Journal of the European Society for Medical Oncology. 1994;5(6):533–7. https://doi.org/10.1093/oxfordjournals.annonc.a058908. Epub 1994/07/01.
Dreyfuss AI, Clark JR, Norris CM, Rossi RM, Lucarini JW, Busse PM, et al. Docetaxel: an active drug for squamous cell carcinoma of the head and neck. J Clin Oncology: Official J Am Soc Clin Oncol. 1996;14(5):1672–8. https://doi.org/10.1200/jco.1996.14.5.1672. Epub 1996/05/01.
Zhao T, Chen H, Zhang T. Docetaxel and cisplatin concurrent with radiotherapy versus 5-fluorouracil and cisplatin concurrent with radiotherapy in treatment for locally advanced oesophageal squamous cell carcinoma: a randomized clinical study. Medical oncology (Northwood, London, England). 2012;29(5):3017-23. Epub 2012/04/06. https://doi.org/10.1007/s12032-012-0228-6. PubMed PMID: 22476809.
Li QQ, Liu MZ, Hu YH, Liu H, He ZY, Lin HX. Definitive concomitant chemoradiotherapy with docetaxel and cisplatin in squamous esophageal carcinoma. Dis Esophagus: Official J Int Soc Dis Esophagus. 2010;23(3):253–9. https://doi.org/10.1111/j.1442-2050.2009.01003. Epub 2009/09/08.
Zhu Y, Zhang W, Li Q, Li Q, Qiu B, Liu H, et al. A phase II randomized controlled trial: definitive concurrent chemoradiotherapy with Docetaxel Plus Cisplatin versus 5-Fluorouracil plus cisplatin in patients with oesophageal squamous cell carcinoma. J Cancer. 2017;8(18):3657–66. https://doi.org/10.7150/jca.20053. Epub 2017/11/21.
Herskovic A, Russell W, Liptay M, Fidler MJ, Al-Sarraf M. Esophageal carcinoma advances in treatment results for locally advanced disease: review. Annals of Oncology: Official Journal of the European Society for Medical Oncology. 2012;23(5):1095–103. https://doi.org/10.1093/annonc/mdr433. Epub 2011/10/18.
Hulscher JB, van Sandick JW, de Boer AG, Wijnhoven BP, Tijssen JG, Fockens P, et al. Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the esophagus. N Engl J Med. 2002;347(21):1662–9. Epub 2002/11/22. https://doi.org/10.1056/NEJMoa022343. PubMed PMID: 12444180.
Kelsen DP, Ginsberg R, Pajak TF, Sheahan DG, Gunderson L, Mortimer J, et al. Chemotherapy followed by surgery compared with surgery alone for localized esophageal cancer. N Engl J Med. 1998;339(27):1979–84. https://doi.org/10.1056/nejm199812313392704. Epub 1998/12/31.
Surgical resection with. Or without preoperative chemotherapy in oesophageal cancer: a randomised controlled trial. Lancet (London England). 2002;359(9319):1727–33. https://doi.org/10.1016/s0140-6736. Epub 2002/06/07.
Sjoquist KM, Burmeister BH, Smithers BM, Zalcberg JR, Simes RJ, Barbour A, et al. Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: an updated meta-analysis. Lancet Oncol. 2011;12(7):681–92. PubMed PMID: 21684205.
Shapiro J, van Lanschot JJB, Hulshof M, van Hagen P, van Berge Henegouwen MI, Wijnhoven BPL, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol. 2015;16(9):1090–8. 2045(15)00040-6. PubMed PMID: 26254683.
Eyck BM, van Lanschot JJB, Hulshof M, van der Wilk BJ, Shapiro J, van Hagen P, et al. Ten-year outcome of Neoadjuvant Chemoradiotherapy Plus surgery for esophageal Cancer: the Randomized Controlled CROSS Trial. J Clin Oncology: Official J Am Soc Clin Oncol. 2021;39(18):1995–2004. https://doi.org/10.1200/jco.20.03614. Epub 2021/04/24.
Ando N, Kato H, Igaki H, Shinoda M, Ozawa S, Shimizu H, et al. A randomized trial comparing postoperative adjuvant chemotherapy with cisplatin and 5-fluorouracil versus preoperative chemotherapy for localized advanced squamous cell carcinoma of the thoracic esophagus (JCOG9907). Ann Surg Oncol. 2012;19(1):68–74. https://doi.org/10.1245/s10434-011-2049-9. Epub 2011/09/01.
Yang H, Liu H, Chen Y, Zhu C, Fang W, Yu Z, et al. Neoadjuvant Chemoradiotherapy followed by surgery versus surgery alone for locally advanced squamous cell carcinoma of the Esophagus (NEOCRTEC5010): a phase III Multicenter, Randomized, open-label clinical trial. J Clin Oncology: Official J Am Soc Clin Oncol. 2018;36(27):2796–803. https://doi.org/10.1200/jco.2018.79.1483. Epub 2018/08/09.
Yang H, Liu H, Chen Y, Zhu C, Fang W, Yu Z, et al. Long-term efficacy of Neoadjuvant Chemoradiotherapy Plus surgery for the treatment of locally advanced esophageal squamous cell carcinoma: the NEOCRTEC5010 Randomized Clinical Trial. JAMA Surg. 2021;156(8):721–9. https://doi.org/10.1001/jamasurg.2021.2373. Epub 2021/06/24.
Li R. Cancer burden in China and the role of the cancer registries. Annals of translational medicine. 2014;2(7):69. Epub 2014/10/22. https://doi.org/10.3978/j.issn.2305-5839.2014.06.12. PubMed PMID: 25333044; PubMed Central PMCID: PMCPMC4202461.
Minsky BD, Neuberg D, Kelsen DP, Pisansky TM, Ginsberg R, Benson A 3. Neoadjuvant chemotherapy plus concurrent chemotherapy and high-dose radiation for squamous cell carcinoma of the esophagus: a preliminary analysis of the phase II intergroup trial 0122. J Clin Oncology: Official J Am Soc Clin Oncol. 1996;14(1):149–55. https://doi.org/10.1200/jco.1996.14.1.149. Epub 1996/01/01.
Minsky BD, Neuberg D, Kelsen DP, Pisansky TM, Ginsberg RJ, Pajak T, et al. Final report of Intergroup Trial 0122 (ECOG PE-289, RTOG 90 – 12): phase II trial of neoadjuvant chemotherapy plus concurrent chemotherapy and high-dose radiation for squamous cell carcinoma of the esophagus. Int J Radiat Oncol Biol Phys. 1999;43(3):517–23. https://doi.org/10.1016/s0360-3016(98)00463-5. Epub 1999/03/17.
Ohtsu A, Boku N, Muro K, Chin K, Muto M, Yoshida S, et al. Definitive chemoradiotherapy for T4 and/or M1 lymph node squamous cell carcinoma of the esophagus. J Clin Oncology: Official J Am Soc Clin Oncol. 1999;17(9):2915–21. https://doi.org/10.1200/jco.1999.17.9.2915. Epub 1999/11/24.
Ishida K, Ando N, Yamamoto S, Ide H, Shinoda M. Phase II study of cisplatin and 5-fluorouracil with concurrent radiotherapy in advanced squamous cell carcinoma of the esophagus: a Japan Esophageal Oncology Group (JEOG)/Japan Clinical Oncology Group trial (JCOG9516). Jpn J Clin Oncol. 2004;34(10):615–9. https://doi.org/10.1093/jjco/hyh107. Epub 2004/12/14.
Ajani JA, Winter K, Komaki R, Kelsen DP, Minsky BD, Liao Z, et al. Phase II randomized trial of two nonoperative regimens of induction chemotherapy followed by chemoradiation in patients with localized carcinoma of the esophagus: RTOG 0113. J Clin Oncology: Official J Am Soc Clin Oncol. 2008;26(28):4551–6. https://doi.org/10.1200/jco.2008.16.6918. Epub 2008/06/25.
Kato K, Muro K, Minashi K, Ohtsu A, Ishikura S, Boku N, et al. Phase II study of chemoradiotherapy with 5-fluorouracil and cisplatin for stage II-III esophageal squamous cell carcinoma: JCOG trial (JCOG 9906). Int J Radiat Oncol Biol Phys. 2011;81(3):684–90. PubMed PMID: 20932658.
Nishimura Y, Hiraoka M, Koike R, Nakamatsu K, Itasaka S, Kawamura M, et al. Long-term follow-up of a randomized phase II study of cisplatin/5-FU concurrent chemoradiotherapy for esophageal cancer (KROSG0101/JROSG021). Jpn J Clin Oncol. 2012;42(9):807–12. https://doi.org/10.1093/jjco/hys112. Epub 2012/07/20.
Zhang P, Xi M, Li QQ, Hu YH, Guo X, Zhao L, et al. Concurrent cisplatin and 5-fluorouracil versus concurrent cisplatin and docetaxel with radiotherapy for esophageal squamous cell carcinoma: a propensity score-matched analysis. Oncotarget. 2016;7(28):44686–94. https://doi.org/10.18632/oncotarget.9301. Epub 2016/05/18.
Gaspar LE, Qian C, Kocha WI, Coia LR, Herskovic A, Graham M. A phase I/II study of external beam radiation, brachytherapy and concurrent chemotherapy in localized cancer of the esophagus (RTOG 92 – 07): preliminary toxicity report. Int J Radiat Oncol Biol Phys. 1997;37(3):593–9. https://doi.org/10.1016/s0360-3016(96)00591-3. Epub 1997/02/01.
Mason KA, Hunter NR, Milas M, Abbruzzese JL, Milas L. Docetaxel enhances tumor radioresponse in vivo. Clin cancer Research: Official J Am Association Cancer Res. 1997;3(12 Pt 1):2431–8. Epub 1998/11/17. PubMed PMID: 9815644.
Hennequin C, Giocanti N, Favaudon V. Interaction of ionizing radiation with paclitaxel (taxol) and docetaxel (Taxotere) in HeLa and SQ20B cells. Cancer Res. 1996;56(8):1842–50. Epub 1996/04/15. PubMed PMID: 8620502.
Hihara J, Yoshida K, Hamai Y, Emi M, Yamaguchi Y, Wadasaki K. Phase I study of docetaxel (TXT) and 5-fluorouracil (5-FU) with concurrent radiotherapy in patients with advanced esophageal cancer. Anticancer Res. 2007;27(4c):2597–603. Epub 2007/08/19. PubMed PMID: 17695421.
Choy H. Combining taxanes with radiation for solid tumors. Int J Cancer. 2000;90(3):113–27. Epub 2000/07/20. PubMed PMID: 10900423.
Mauer AM, Masters GA, Haraf DJ, Hoffman PC, Watson SM, Golomb HM, et al. Phase I study of docetaxel with concomitant thoracic radiation therapy. J Clin Oncology: Official J Am Soc Clin Oncol. 1998;16(1):159–64. https://doi.org/10.1200/jco.1998.16.1.159. Epub 1998/01/24.
Ilson DH, Bains M, Ginsberg RJ, Kelsen DP. Neoadjuvant therapy of esophageal cancer. Surg Oncol Clin N Am. 1997;6(4):723–40. Epub 1997/10/06. PubMed PMID: 9309090.
Siewert JR, Stein HJ, Feith M, Bruecher BL, Bartels H, Fink U. Histologic tumor type is an independent prognostic parameter in esophageal cancer: lessons from more than 1,000 consecutive resections at a single center in the western world. Ann Surg. 2001;234(3):360–7. https://doi.org/10.1097/00000658-200109000-00010. discussion 8–9. Epub 2001/08/29.
Allemani C, Matsuda T, Di Carlo V, Harewood R, Matz M, Nikšić M, et al. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet (London England). 2018;391(10125):1023–75. https://doi.org/10.1016/s0140-6736(17)33326-3. Epub 2018/02/06.
Deng J, Chen H, Zhou D, Zhang J, Chen Y, Liu Q, et al. Comparative genomic analysis of esophageal squamous cell carcinoma between asian and caucasian patient populations. Nat Commun. 2017;8(1):1533. https://doi.org/10.1038/s41467-017-01730-x. Epub 2017/11/17.
Makazu M, Kato K, Takisawa H, Yoshinaga S, Oda I, Saito Y, et al. Feasibility of endoscopic mucosal resection as salvage treatment for patients with local failure after definitive chemoradiotherapy for stage IB, II, and III esophageal squamous cell cancer. Dis Esophagus: Official J Int Soc Dis Esophagus. 2014;27(1):42–9. https://doi.org/10.1111/dote.12037. Epub 2013/02/28.
Yano T, Muto M, Hattori S, Minashi K, Onozawa M, Nihei K, et al. Long-term results of salvage endoscopic mucosal resection in patients with local failure after definitive chemoradiotherapy for esophageal squamous cell carcinoma. Endoscopy. 2008;40(9):717–21. https://doi.org/10.1055/s-2008-1077480. Epub 2008/09/06.
Takeuchi M, Kobayashi M, Hashimoto S, Mizuno K, Kawaguchi G, Sasamoto R, et al. Salvage endoscopic submucosal dissection in patients with local failure after chemoradiotherapy for esophageal squamous cell carcinoma. Scand J Gastroenterol. 2013;48(9):1095–101. https://doi.org/10.3109/00365521.2013.822092. Epub 2013/08/03.
Hatogai K, Yano T, Kojima T, Onozawa M, Daiko H, Nomura S, et al. Salvage photodynamic therapy for local failure after chemoradiotherapy for esophageal squamous cell carcinoma. Gastrointest Endosc. 2016;83(6):1130. PubMed PMID: 26608125. 9.e3.
Matsutani T, Nomura T, Hagiwara N, Matsuda A, Uchida E. Salvage endoscopic argon plasma Coagulation after Chemoradiotherapy for Inoperable Esophageal Cancer. Surgical laparoscopy, endoscopy & percutaneous techniques. 2017;27(5):384–90. Epub 2017/07/21. https://doi.org/10.1097/sle.0000000000000454. PubMed PMID: 28727635.
Yoo C, Park JH, Yoon DH, Park SI, Kim HR, Kim JH, et al. Salvage esophagectomy for locoregional failure after chemoradiotherapy in patients with advanced esophageal cancer. Ann Thorac Surg. 2012;94(6):1862–8. https://doi.org/10.1016/j.athoracsur.2012.07.042. Epub 2012/09/11.
Chen Y, Lu Y, Wang Y, Yang H, Xia Y, Chen M, et al. Comparison of salvage chemoradiation versus salvage surgery for recurrent esophageal squamous cell carcinoma after definitive radiochemotherapy or radiotherapy alone. Dis Esophagus: Official J Int Soc Dis Esophagus. 2014;27(2):134–40. https://doi.org/10.1111/j.1442-2050.2012.01440. Epub 2012/10/24.
Zhou ZG, Zhen CJ, Bai WW, Zhang P, Qiao XY, Liang JL, et al. Salvage radiotherapy in patients with local recurrent esophageal cancer after radical radiochemotherapy. Radiation oncology (London. England). 2015. https://doi.org/10.1186/s13014-015-0358-z. 10:54. Epub 2015/04/19.
Li J, Wen Y, Xiang Z, Du H, Geng L, Yang X, et al. Radical radiotherapy for metachronous oligometastasis after initial treatment of esophageal cancer. Radiotherapy and Oncology: Journal of the European Society for Therapeutic Radiology and Oncology. 2021;154:201–6. https://doi.org/10.1016/j.radonc.2020.09.042. Epub 2020/09/28.
Acknowledgements
The authors would like to thank all patients and their families, for their participation in this study.
Funding
This study was funded in part by Medical Scientific Research Foundation of Guangdong Province, China, grant number No. B2014157.
Author information
Authors and Affiliations
Contributions
All authors have made substantial contributions to all of the following: (1) HJ, KJBBMKLK, BQC, MX, QQL, YHH, and YJZ contributed to the conception and design of the study. (2) HJ, KJBBMKLK, and BQC contributed to the acquisition, analysis, and interpretation of data. (3) HJ, KJBBMKLK, and YJZ drafted the article and submitted it. All authors revised and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was reviewed and approved by the SYSUCC Ethics Committee.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Jiang, H., Makelike, K., Chen, B. et al. Definitive concurrent chemoradiotherapy with docetaxel plus cisplatin versus 5-fluorouracil plus cisplatin in patients with esophageal squamous cell carcinoma: long-term follow-up results of a phase II randomized controlled trial. Radiat Oncol 18, 150 (2023). https://doi.org/10.1186/s13014-023-02339-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13014-023-02339-9