Abstract
Background
Volumetric modulated arc therapy (VMAT) for locally advanced rectal cancer (LARC) has emerged as a promising technique, but the planning process can be time-consuming and dependent on planner expertise. We aimed to develop a fully automated VMAT planning program for LARC and evaluate its feasibility and efficiency.
Methods
A total of 26 LARC patients who received VMAT treatment and the computed tomography (CT) scans were included in this study. Clinical target volumes and organs at risk were contoured by radiation oncologists. The automatic planning program, developed within the Raystation treatment planning system, used scripting capabilities and a Python environment to automate the entire planning process. The automated VMAT plan (auto-VMAT) was created by our automated planning program with the 26 CT scans used in the manual VMAT plan (manual-VMAT) and their regions of interests. Dosimetric parameters and time efficiency were compared between the auto-VMAT and the manual-VMAT created by experienced planners. All results were analyzed using the Wilcoxon signed-rank sum test.
Results
The auto-VMAT achieved comparable coverage of the target volume while demonstrating improved dose conformity and uniformity compared with the manual-VMAT. V30 and V40 in the small bowel were significantly lower in the auto-VMAT compared with those in the manual-VMAT (p < 0.001 and < 0.001, respectively); the mean dose of the bladder was also significantly reduced in the auto-VMAT (p < 0.001). Furthermore, auto-VMAT plans were consistently generated with less variability in quality. In terms of efficiency, the auto-VMAT markedly reduced the time required for planning and expedited plan approval, with 93% of cases approved within one day.
Conclusion
We developed a fully automatic feasible VMAT plan creation program for LARC. The auto-VMAT maintained target coverage while providing organs at risk dose reduction. The developed program dramatically reduced the time to approval.
Similar content being viewed by others
Background
Colorectal cancer causes more than 1.9 million deaths worldwide annually and its mortality rate ranks second among all cancers, and rectal cancer accounts for approximately one-third of all colorectal cancers [1]. The treatment outcome of patients with locally advanced rectal cancer (LARC) has been unsatisfactory because of the lack of locoregional control achieved with only surgical resection [2]. The introduction of total mesorectal excision and neoadjuvant chemoradiation for LARC has improved locoregional control [3, 4]. Three-dimensional conformal radiotherapy is the most common radiotherapy technique [5]. However, it is difficult to spare organs at risk (OAR) such as the small bowel and the bladder while keeping the dose to the target in three-dimensional conformal radiotherapy for LARC [6]. Recently, volumetric modulated arc therapy (VMAT) has been widespread and plays an essential role in radiation therapy for LARC. While VMAT provides better dose distribution compared with three-dimensional conformal radiotherapy, VMAT planning and validation require additional effort [7,8,9]. Furthermore, the quality of the VMAT plan depends on the experience and skill of the individual planner [10,11,12,13].
Several studies have reported on automatic planning aimed at reducing planner labor and standardizing plan quality [14]. Currently, the mainstream methods of automatic planning are knowledge-based planning, protocol-based automatic iterative optimization, and multicriteria optimization [15]. Knowledge-based planning predicts dose-volume histograms by learning a large number of clinically accepted plans. While this approach allows for efficient and standardized treatment planning, the quality of the dose distribution depends on the input data [16, 17]. The protocol-based automated iterative optimization automatically updates constraints toward clinical goals but requires experience in setting up calculations [18,19,20]. Multicriteria optimization is a method of simultaneously optimizing multiple scenarios with different trade-off relationships. This method can provide a desired plan from multiple completed plans, but the calculation time increases as the number of scenarios increases [21, 22]. In contrast, script-based auto planning does not require a pre-learning step or initial input. It has the potential to achieve higher accuracy and speed than commercial auto-planning systems. However, previous reports of script-based auto plans using Raystation are still scarce [23, 24].
In this study, we developed a fully automatic VMAT planning program for LARC using the development environment of Raystation with a high degree of freedom. We evaluated the feasibility of our automated planning through dose indices and time efficiency in comparison with manual planning.
Methods
Patient and image dataset
The VMAT plans for 26 patients with LARC treated at our institution from April 2020 to March 2022 and the computed tomography (CT) scans were included in this study (Table 1). The automated VMAT plan (auto-VMAT) was created by our planning program using the 26 CT scans used in the manual VMAT plan (manual-VMAT) and their regions of interest (ROIs). Simulation CT images were acquired using Aquilion One (Canon Medical Systems, Tochigi, Japan), and the image slice thickness was 2 mm. A 400 ml water load and a waiting time of 30–50 min were used for patient pretreatment to fill the bladder before the CT scan. The contents of the study, including the investigation procedure and the handling of patient information, were approved by the institutional review board of the National Cancer Center Hospital East (IRB No. 2018-076).
Contouring
The clinical target volume (CTV), the planning target volume (PTV), and OARs including bladder, small intestine, and femoral heads were delineated by radiation oncologists. The CTV of the initial plans included the primary tumor, metastatic lymph nodes, and relevant regional lymph nodes (mesorectum, internal iliac, obturator, presacral, and external iliac depending on T-stage). The CTV of the boost plans was created based on the primary tumor. A 0.5 cm margin in anterior-posterior and right-left directions and 2 cm margin in craniocaudal directions were given to make the CTV of both initial and boost plans. No margin was given in all directions for metastatic lymph nodes to create CTV. An expansion with a margin of 0.5 cm was given in all directions for CTV to make the PTV.
Treatment Planning and clinical goals
The prescribed doses were 45 Gy and 5.4 Gy for initial and boost plans, respectively. All treatment plans were generated with 10-MV photon beams; the collimator angle was arbitrary for the manual-VMAT and 355° for the auto-VMAT. A single full 360° coplanar arc was adopted for both initial and boost plans. The isocenter was set at the center of PTV for the initial plan (PTV initial) and PTV for the boost plan (PTV boost). Treatment planning was performed using Raystation (RaySearch Laboratories AB, Stockholm, Sweden). The manual-VMAT was created by four experienced planners. Both the auto-VMAT and manual-VMAT were calculated using the Collapsed Cone V5.3 algorithm. Table 2 shows the clinical goals for dose indices. The goals of the PTV coverage were to reach D93 = 98% in both the initial and boost plans. Dose constraints for PTVs and OARs were set with reference to RTOG 0822 [25]. Exceeding the maximum dose clearance to the small bowel and bladder was clinically allowed considering the overlap with the PTV. Adherence to OAR dose was prioritized in determining clinical acceptability.
Script-based Auto Planning
Figure 1 shows the overview of the automatic planning system. The automatic planning system was built with scripting capabilities within Raystation and a Python (version 3.6) environment. The consistency of the defined ROI name and physician contouring was checked before starting the automatic program. After starting the program, the CT electron density conversion table was automatically selected, and a virtual couch was inserted. ROIs for optimization are automatically generated. The program automatically created the ROIs necessary to generate the plan by referencing the names of the ROIs imported from the contour data. Additional ROIs for OARs were created by removing the overlap with the PTV from the bladder and small bowel. Additional ROIs to control the dose distribution shape were created by expanding from the PTV in the abdominal direction. The additional ROIs generated by this method can easily form a bowel bag-like shape without depending on the PTV shape and size to reduce the OAR dose. Prescription dose and geometry were automatically input. The isocenter was set at the center of PTV initial and PTV boost. We adopted a dose fall off constraint to each OAR to achieve short-term optimization. Objects for dose constraint were automatically input, and optimization was performed two times for initial and boost plans. The first dose distribution was created; next, hot-spots were extracted from the distribution, and optimization was performed three times with additional dose objects for hot-spot correction. Constrained objects were added to achieve the clinical goal for PTV and optimization was performed. The ROI display and dose distribution color bar were then automatically set, and physicians checked the dose distribution.
Evaluation
Dose indices for PTVs and OARs were compared in the manual-VMAT and auto-VMAT. OAR doses were evaluated for the sum of the initial and boost plans. In dose indices for OARs, the mean dose and maximum dose were evaluated for the bladder; V30, V40, and maximum dose for small bowel; and mean dose and maximum dose for femoral heads. D93, D2, conformity index (CI), and homogeneity index (HI) were evaluated for initial and boost PTVs. CI and HI are defined in formulas 1 and 2.
Where TV_PIV is the volume of the target covered by the prescription isodose volume (PIV), TV is the target volume, and PIV is the volume covered by the prescribed isodose.
Where D2 is the dose received by 2% of the target volume, D98 is the dose received by 98% of the target volume, and D_ prescription is the prescribed dose.
To evaluate work efficiency, we measured the time required for planning by our program and the number of days required until plan approval. Days to plan approval was defined as the number of days from the time the physician finished contouring to the time the plan was approved by physicians. The time and days at which contouring, plans, and approvals were completed were measured using the in-house developed system.
Plan specific quality assurance
Patient-specific quality assurance (QA) was conducted for all auto-VMATs and manual-VMATs using Delta4® (ScandiDos, Uppsala, Sweden). Criteria of the gamma passing rate were a dose difference of 3% and a distance to agreement of 2 mm (3%/2mm). The gamma pass rate of greater than 98% was the threshold to indicate robust delivery in this study. Modulation Complexity Score (MCS) was calculated to assess the clinical feasibility of the auto-VMAT using Simple MU Analysis® (Triangle Products Co.Ltd, Chiba, Japan). MCS range 0.0–1.0 and the closer to 0 means the more complex the plan [26].
Statistics
All results were analyzed using the Wilcoxon signed-rank sum test. p < 0.05 indicated statistical significance. The analyses were two-sided and performed using the exactRankTests packages in R v.4.2.2.
Results
Dosimetry Comparison
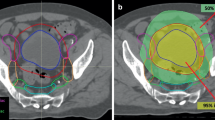
A comparison of the representative dose distributions and dose-volume histograms between auto-VMAT and manual-VMAT is shown in Fig. 2A–D. Table 3 shows the comparison of the manual-VMAT and auto-VMAT for PTV coverage and dose to OARs. The D93 of the auto-VMAT was significantly higher in both the initial and boost plans (p = 0.019 and < 0.001, respectively). The CI value of the manual-VMAT was significantly higher in the initial plan (p = 0.002). The HI value of the auto-VMAT was significantly higher for both initial and boost plans (p = 0.003 and < 0.001, respectively). The V30 and V40 of the small bowel of the auto-VMAT were significantly lower compared with the manual-VMAT (p < 0.001 and < 0.001, respectively). No significant difference in the maximum dose of the small bowel was observed between the manual-VMAT and auto-VMAT. The mean dose of the bladder of the auto-VMAT was significantly lower than the manual-VMAT (p = < 0.001). No significant difference was observed for the maximum dose of the bladder between the manual-VMAT and auto-VMAT (p = 0.829). The mean and maximum dose of femoral heads of the manual-VMAT was significantly lower for both the right (p < 0.001 and < 0.001, respectively) and left (p < 0.001 and = 0.043, respectively) sides.
Work efficiency
Figure 3 shows the days required from the end of the physician’s contouring to the created VMAT plan approval. In the manual-VMAT, over 40% of cases required more than 5 days to approve the plan, and in only 19% of cases, approval was obtained on the same day that the physician’s contouring was completed. In the auto-VMAT, 93% of cases were approved within one day, and 72% of cases were approved on the same day that the physician’s contouring was completed.
Treatment delivery parameters
Table 4 shows the gamma pass ratio, total MU and MCS in the auto-VMAT and manual-VMAT. All plans passed the patient-specific QA. And in both the initial and boost plans, there was no significant difference in the gamma pass ratio between the auto-VMAT and manual-VMAT (p = 0.675 and = 0.610, respectively). In both the initial and boost plans, total MU in the auto-VMAT was significantly higher than in the manual-VMAT (p < 0.001 and p = 0.012, respectively). In initial plans, MCS in the auto-VMAT were significantly lower than in the manual-VMAT (p = 0.031). In boost plans, MCS in the manual-VMAT were significantly lower than in the auto-VMAT (p = 0.012).
Discussion
In this study, we developed a fully automatic feasible VMAT plan creation program that works within a commercial treatment planning machine for LARC. The developed program automatically sets the prescription dose, determines the irradiation geometry, inputs the dose constraint for optimization, creates the dose distribution, and dramatically reduced the time to approval. Because the additional ROIs for dose distribution adjustment in the development program change the shape in accordance with the shape of the PTV, it is possible to create a dose distribution with constant quality for each patient.
The auto-VMAT maintained high uniformity, reduced D2, and kept PTV coverage comparable to the manual-VMAT. All auto-VMATs passed the patient-specific QA. Of particular interest were the reductions in the mean dose, V30, and V40 in the bladder and small bowel with the auto-VMAT. This may be the effect of the additional ROIs and dose fall-off technique used in the auto-VMAT. In contrast, planners may have prioritized achieving PTV coverage constraints or may not have performed sufficient trial-and-error efforts to reduce OAR doses. Differences in planner experience and skills may have affected the results. Song et al. developed an automatic VMAT generation program for LARC using Pinnacle3 with the model for the Elekta Synergy accelerator and compared it with the manual-VMAT [18]. The authors reported that the auto-VMAT achieved quality equal to or better than the manual-VMAT and was particularly effective in reducing the small bowel dose. In our study, small bowel and bladder V30, V40, and mean dose were significantly lower in the auto-VMAT and similar to the previously reported results. CI in the auto-VMAT was lower than the manual-VMAT in our initial plan. This was probably because the restrictions for achieving PTV coverage set in the auto-VMAT were too strong. This may also be because of the steeper concave shape of the PTV compared with those reported in previous studies.
In comparing the time from completion of physician contouring to plan approval, the manual-VMAT required more than 2 days for plan approval in 72% of cases. In the auto-VMAT, 93% of cases obtained plan approval within 1 day. The auto-VMAT may have provided a drastic reduction of hands-on time for planning and the reduction of the review burden on physicians because of the standardization of plan quality. This made it possible to approve plans in a short period of time. In the future, vendor improvements to dose calculation algorithms and systems may yield further speedups. In addition, combining auto-contouring technology may reduce the overall planning times [27, 28].
The setting of the isocenter position, prescription dose, and the insertion of the virtual couch are manually performed by planners in the manual-VMAT. However, in radiotherapy, manual input and recognition errors can cause accidents. According to the TG100, it is difficult to detect errors in treatment planning, and these errors have a significant impact on patients [29]. In our development system, these setting inputs are performed fully automatically in the plan creation process, eliminating manual operation by humans and realizing a high level of medical safety. Unlike dose-volume histogram prediction–type automatic planning systems, our program is robust against differences in definitions of PTV shapes among facilities because the VMAT create section is dependent on the planner’s process. Furthermore, if the facility has a Raystation installed, our program can be implemented with a single text file.
This study has several limitations. First, the number of cases examined was small. Second, the manual-VMAT depended on the experience and skill of each planner. Third, in the manual-VMAT, when planners have multiple plan tasks, they may not start on low-priority plans immediately. This may be a bias that extends the time required for approval in the manual-VMAT.
Conclusions
We developed a fully automatic feasible VMAT plan creation program for LARC. The auto-VMAT maintained target coverage while providing organs at risk dose reduction. The developed program dramatically reduced the time to approval.
Data Availability
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- auto-VMAT:
-
Automated VMAT plan
- CI:
-
Conformity index
- CTV:
-
Clinical target volume
- DVH:
-
Dose-volume histograms
- HI:
-
homogeneity index
- LARC:
-
Locally advanced rectal cancer
- manual-VMAT:
-
Manual VMAT plan
- MCS:
-
Modulation complexity score
- MU:
-
Monitor unit
- OAR:
-
organs at risk
- PTV:
-
Planning target volume
- QA:
-
Quality assurance
- VMAT:
-
volumetric modulated arc therapy
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and Mortality Worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49.
Affleck A, Koprowski MA, Nabavizadeh N, Tsikitis VL. The evolution of rectal cancer treatment: the journey to total neoadjuvant therapy and organ preservation. Ann Gastroenterol. 2022;35:226–33.
Heald RJ, Ryall RD. Recurrence and survival after total mesorectal excision for rectal cancer. Lancet (London, England). England; 1986;1:1479–82.
Papaccio F, Roselló S, Huerta M, Gambardella V, Tarazona N, Fleitas T, et al. Neoadjuvant chemotherapy in locally advanced rectal cancer. Cancers (Basel). 2020;12:1–12.
Wo JY, Anker CJ, Ashman JB, Bhadkamkar NA, Bradfield L, Chang DT et al. Radiation Therapy for Rectal Cancer: Executive Summary of an ASTRO Clinical Practice Guideline. Pract. Radiat. Oncol. United States; 2021. p. 13–25.
Appelt AL, Sebag-Montefiore D. Technological advances in radiotherapy of rectal cancer. Curr Opin Oncol [Internet]. 2016;28:353–8. Available from: http://journals.lww.com/00001622-201607000-00016.
Regnier A, Ulbrich J, Münch S, Oechsner M, Wilhelm D, Combs SE, et al. Comparative analysis of efficacy, toxicity, and patient-reported outcomes in rectal cancer patients undergoing preoperative 3D conformal radiotherapy or VMAT. Front Oncol. 2017;7:1–8.
Stuyck C, Wegge M, Bulens P, Joye I, Haustermans K. Moderate dose escalation with volumetric modulated arc therapy improves outcome in rectal cancer. Acta Oncol (Madr) [Internet]. Informa UK Limited, trading as Taylor & Francis Group; 2017;56:1501–6. https://doi.org/10.1080/0284186X.2017.1350286.
Dröge LH, Weber HE, Guhlich M, Leu M, Conradi LC, Gaedcke J et al. Reduced toxicity in the treatment of locally advanced rectal cancer: A comparison of volumetric modulated arc therapy and 3D conformal radiotherapy. BMC Cancer [Internet]. BMC Cancer; 2015;15:1–8. https://doi.org/10.1186/s12885-015-1812-x.
Chung HT, Lee B, Park E, Lu JJ, Xia P. Can all Centers Plan intensity-modulated Radiotherapy (IMRT) effectively? An external audit of dosimetric comparisons between Three-Dimensional Conformal Radiotherapy and IMRT for Adjuvant Chemoradiation for gastric Cancer. Int J Radiat Oncol Biol Phys. 2008;71:1167–74.
Chen J, Dai J, Nobah A, Bai S, Bi N, Lai Y, et al. A special report on 2019 International Planning Competition and a comprehensive analysis of its results. Front Oncol. 2020;10:1–11.
Nelms BE, Robinson G, Markham J, Velasco K, Boyd S, Narayan S, et al. Variation in external beam treatment plan quality: an inter-institutional study of planners and planning systems. Pract Radiat Oncol United States. 2012;2:296–305.
Peters LJ, O’Sullivan B, Giralt J, Fitzgerald TJ, Trotti A, Bernier J, et al. Critical impact of radiotherapy protocol compliance and quality in the treatment of advanced head and neck cancer: results from TROG 02.02. J Clin Oncol off J Am Soc Clin Oncol United States. 2010;28:2996–3001.
Hussein M, Heijmen BJM, Verellen D, Nisbet A. Automation in intensity modulated radiotherapy treatment planning-a review of recent innovations. Br J Radiol. 2018;91.
Lu L, Sheng Y, Donaghue J, Liu Shen Z, Kolar M, Wu QJ, et al. Three IMRT advanced planning tools: a multi-institutional side-by-side comparison. J Appl Clin Med Phys. 2019;20:65–77.
Wu H, Jiang F, Yue H, Zhang H, Wang K, Zhang Y. Applying a RapidPlan model trained on a technique and orientation to another: A feasibility and dosimetric evaluation. Radiat Oncol [Internet]. Radiation Oncology; 2016;11:1–7. https://doi.org/10.1186/s13014-016-0684-9.
Wu H, Jiang F, Yue H, Li S, Zhang Y. A dosimetric evaluation of knowledge-based VMAT planning with simultaneous integrated boosting for rectal cancer patients. J Appl Clin Med Phys. 2016;17:78–85.
Song Y, Wang Q, Jiang X, Liu S, Zhang Y, Bai S. Fully automatic volumetric modulated arc therapy plan generation for rectal cancerAutomatic rectal VMAT planning. Radiother Oncol [Internet]. Elsevier Ireland Ltd; 2016;119:531–6. https://doi.org/10.1016/j.radonc.2016.04.010.
Hazell I, Bzdusek K, Kumar P, Hansen CR, Bertelsen A, Eriksen JG, et al. Automatic planning of head and neck treatment plans. J Appl Clin Med Phys. 2016;17:272–82.
Speer S, Klein A, Kober L, Weiss A, Yohannes I, Bert C. Automatisierte Bestrahlungsplanung: Auswertung von mit Pinnacle3 via Scripting und Auto-Planning erzeugten Bestrahlungsplänen von Patienten mit Kopf-Hals-Tumor. Strahlentherapie und Onkol. 2017;193:656–65.
Rønde HS, Wee L, Pløen J, Appelt AL. Feasibility of preference-driven radiotherapy dose treatment planning to support shared decision making in anal cancer. Acta Oncol (Madr) [Internet]. Informa UK Limited, trading as Taylor & Francis Group; 2017;56:1277–85. https://doi.org/10.1080/0284186X.2017.1315174.
Ghandour S, Matzinger O, Pachoud M. Volumetric-modulated arc therapy planning using multicriteria optimization for localized prostate cancer. J Appl Clin Med Phys. 2015;16:258–69.
Gleeson IAN, Bolger N, Chun H, Hutchinson K. Implementation of automated personalised breast radiotherapy planning techniques with scripting in Raystation. 2023.
Lou Z, Cheng C, Mao R, Li D, Tian L, Li B et al. Physica Medica A novel automated planning approach for multi-anatomical sites cancer in Raystation treatment planning system. Phys Medica [Internet]. Elsevier Ltd; 2023;109:102586. https://doi.org/10.1016/j.ejmp.2023.102586.
Garofalo MC, Hong T, Hospital MG, Bendell J, North A, Krishnan S, et al. Radiation Therapy Oncology Group Rtog 0822 a phase ii evaluation of Preoperative Chemoradiotherapy utilizing intensity modulated Radiation Therapy (Imrt) in Combination with Capecitabine and Oxaliplatin. for Patients With Locally Advanced; 2011.
McNiven AL, Sharpe MB, Purdie TG. A new metric for assessing IMRT modulation complexity and plan deliverability. Med Phys. 2010;37:505–15.
Udupa JK, Liu T, Jin C, Zhao L, Odhner D, Tong Y, et al. Combining natural and artificial intelligence for robust automatic anatomy segmentation: application in neck and thorax auto-contouring. Med Phys. 2022;49:7118–49.
Mackay K, Bernstein D, Glocker B, Kamnitsas K, Taylor A. A Review of the Metrics Used to Assess Auto-Contouring Systems in Radiotherapy. Clin Oncol [Internet]. The Authors; 2023;35:354–69. https://doi.org/10.1016/j.clon.2023.01.016.
Huq MS, Fraass BA, Dunscombe PB, Gibbons JP, Ibbott GS, Mundt AJ, et al. The report of Task Group 100 of the AAPM: application of risk analysis methods to radiation therapy quality management. Med Phys. 2016;43:4209–62.
Acknowledgements
The authors wish to express sincere gratitude to Yuichi Nagai General Manager of Radiation Technology Department for supporting the research facilities and the environment for conducting this research. We would like to express our deep gratitude to the Takaki Ariji Chief of Radiation Technology for his guidance regarding the knowledge and skills of Python programming. We thank Gabrielle White Wolf, PhD, from Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.
Funding
This study was supported by the University of Tsukuba.
Author information
Authors and Affiliations
Contributions
Concept and design: KH, KT. Automatic planning program creation: KH. Data analysis and interpretation: KH, KT. Important advice and critical discussion: HO, VR. Research management and supervision: MI, TS. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The contents of the study, including the investigation procedure and the handling of patient information, were approved by the institutional review board of the National Cancer Center Hospital East (IRB No. 2018-076). Informed consent was not required for this planning study on anonymized patient data.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Hirotaki, K., Tomizawa, K., Moriya, S. et al. Fully automated volumetric modulated arc therapy planning for locally advanced rectal cancer: feasibility and efficiency. Radiat Oncol 18, 147 (2023). https://doi.org/10.1186/s13014-023-02334-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13014-023-02334-0