Abstract
Background
Tumor-infiltrating lymphocytes (TILs), investigated using routine hematoxylin and eosin (H&E)-stained section slides (H&E-sTILs), provide a robust prognostic biomarker in various types of solid cancer. The purpose of the present study was to investigate the prognostic significance of H&E-sTILs in patients with small cell lung cancer (SCLC).
Methods
The clinical data of patients with SCLC who had been treated in our cancer center between January 2013 and October 2019 were collected and retrospectively reviewed. The H&E-sTILs were re-assessed by two experienced pathologists independently. H&E-sTILs that affected the overall survival (OS), progression free survival (PFS) and brain-metastasis free survival (BMFS) rates were explored using the Kaplan–Meier method, and the log-rank test was used to assess the differences. Multivariate analysis was subsequently performed using the Cox proportion hazards model.
Results
A total of 159 patients with SCLC who fulfilled the inclusion criteria were enrolled in the current study. The OS rates at 1, 2 and 3 years were 59.8, 28.6 and 19.8%, respectively, for the whole group. The 3-year OS, PFS and BMFS rates for the H&E-sTILs(+) and H&E-sTILs(−) groups were 25.1% cf. 5.1% (P = 0.030), 14.0% cf. 4.0% (P = 0.013), and 66.0% cf. 11.4% (P = 0.023), respectively. Multivariate analyses subsequently revealed that H&E-sTILs, clinical M stage, the cycles of chemotherapy and short-term response to thoracic radiotherapy were independent factors affecting OS, whereas H&E-sTILs, clinical N stage, clinical M stage and short-term response to chemotherapy were factors affecting PFS. The H&E-sTILs affected OS, PFS and BMFS simultaneously.
Conclusions
The results of this retrospective study have shown that H&E-sTILs may be considered as a prognostic biomarker affecting the short-term response to treatment, and they are the one and only risk factor for BMFS. However, due to the limitations of the nature of the retrospective design and shortcomings in visually assessing the TILs based on the H&E-stained slides, further prospective studies are required to confirm these conclusions.
Similar content being viewed by others
Background
Tumor-infiltrating lymphocytes (TILs), comprising T cells, B cells, and natural killer (NK) cells, have recently been shown to serve as an effective prognostic biomarker in various types of solid cancer [1,2,3]. Although immunohistochemistry (IHC) has been applied to differentiate the various TIL subsets, to assess their density, distribution and localization, and to evaluate their function, this method cannot be used to assess entirely the role of TILs in cancer. Using routine hematoxylin and eosin (H&E) staining to assess TILs in tissue H&E-stained section slides (H&E-sTILs) is an easy procedure that may be integrated into the workflow of pathology laboratories without extra staining protocols, and which has been shown to be a valuable prognostic biomarker of patients [2, 4,5,6,7,8,9]. However, to date, no consensus has been reached on a standard method of quantification for H&E-sTILs in small cell lung cancer (SCLC), and neither has the manner in which H&E-sTILs affect SCLC been identified, especially with respect to predicting brain metastasis (BM) [10,11,12]. The aim of the present study was therefore to assess the value of H&E-sTILs in patients with SCLC.
Methods
Patient selection and data collection
The present retrospective study was approved by the Fujian Province Cancer Hospital Institutional Review Board. The eligibility and exclusion criteria employed in the current study were similar to those of a previously published study [13]. In brief, the eligibility criteria were as follows: primary histologically proven SCLC; a sufficiently good performance status to enable treatment; efficient pretreatment workup for tumor staging and treatment response evaluation; complete follow-up data; good quality of H&E slides and/or adequate tissue in paraffin-embedded formalin-fixed blocks; and treatment with at least one cycle of chemotherapy of a dual-agent. Patients who survived for < 1 month following treatment were considered as adverse event fatalities, and were excluded from the present study.
Treatment strategy
All patients in the current study were administered chemotherapy with at least one cycle of a dual-agent combination of etoposide or irinotecan with cisplatin (EP, IP), carboplatin (EC), lobaplatin (EL) or nidaplatin (EN). Thoracic radiotherapy (TRT) with a ≥ 45 Gy radiation dose was performed using the intensity-modulated radiotherapy (IMRT) technique when necessary, depending on the clinician and the patient’s condition. The details of TRT, including the gross tumor volume (GTV), clinical tumor volume (CTV) and organ at risk (OAR), have been reported previously [13].
Criteria for treatment toxicity and the short-term response
The toxicities of chemotherapy or TRT were evaluated according to the National Cancer Institute Common Toxicity Criteria (NCI CTC) v.4.0 [14] or the Radiation Therapy Oncology Group (RTOG) criteria [15], respectively.
The short-term response of chemotherapy or TRT was evaluated at 3–4 weeks after the most recent cycle of chemotherapy or the completion of TRT, and subsequently confirmed 4 weeks later. The short-term responses were categorized as a clinically complete response (CR), a partial response (PR), stable disease (SD) or progressive disease (PD) according to the guidelines of RECIST1.1 [16]. The CR and PR categories were considered as the sensitive-to-treatment group, whereas the SD and PD were considered as the resistance-to-treatment group in the current study.
TIL assessment
Histopathological assessment of TILs was performed on H&E slides by two experienced pathologists (Dan Hu and Gang Chen) according to the International Immuno-Oncology Biomarkers Working Group [17]. Briefly, every patient was assessed with regard to TILs in at least two section (4–5 µm) by microscopy (magnification of ×200–400; ocular magnification, ×10, objective magnification, ×20–×40). The tissue was primarily obtained from primary biopsies, although some were from lymph node biopsies. The primary method for evaluating sTIL in lymph nodes was based on partial metastatic tumor deposits, which can be determined under a microscope, and the boundary between pre-existing lymphatic tissue and tumor sTIL was clearly discernible. The pre-existing lymphoid stroma was excluded from the evaluation. To minimize selection bias, at least four standard-compliant vision fields per section were randomly selected, and the mean infiltrative areas were considered as the last results enrolled for analysis.
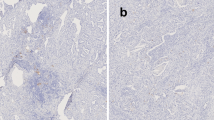
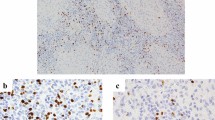
Considering that our pre-study TIL assessment indicated that intratumoral TILs were rarely found, we used the stromal TIL assessment of H&E staining [18] in the current study (Fig. 1). The denominator used to determine the percentage of stromal TILs was the area of stromal tissue (i.e. the area occupied by mononuclear inflammatory cells over the total intratumoral stromal area), not the number of stromal cells (i.e. the fraction of total stromal nuclei that represents mononuclear inflammatory cell nuclei) [17]. However, due to the limitation of standard values and the lower numbers of H&E-sTILs identified in SCLC to date, the H&E-sTILs status was defined simply as either positive(+) or negative (−) for subsequent analysis as follows: ≤ 0%, negative (−), > 1%, positive (+); similar to the method of analysis used by Rao et al. [19].
Surveillance and statistical analysis
The survival outcomes were evaluated in June 2020. The outcomes of interest were the overall survival (OS), progression-free survival (PFS), BM-free survival (BMFS) and BM rates. The survival time was calculated in a similar manner to that described in our previous study [13]. In brief, OS was defined from the date of diagnosis to either the date of death or the date of the last follow-up. The PFS was calculated from the date of diagnosis to the date of disease progression, whereas the BMFS was determined as the duration between the date of diagnosis and BM. Patients who were censored at the last follow-up date or who had died without evidence of BM were censored for BM incidence [13].
Data were analyzed using SPSS version 24.0 (IBM Corp., Armonk, NY, USA). The survival curves were constructed using the Kaplan–Meier method, and comparisons were performed using log-rank tests. Univariate and multivariate analyses of the associations of clinical baseline characteristics [including sex, age, H&E-sTILs, clinical TNM (cTNM) stage including clinical T stage (cT), clinical N stage (cN) and clinical M stage (cM), prophylactic cerebral irradiation (PCI), regimens and cycles of chemotherapy, TRT dose and short-term response to chemotherapy or TRT] with OS, PFS and BMFS rates were performed using the Cox proportional hazards model. Confidence intervals (CIs) represented 95% lower and upper limits. The variables that were statistically significantly correlated with OS, PFS or BMFS were entered in the multivariate analysis using logistic regression.
Receiver operating characteristic (ROC) curve analysis was applied to establish the cut-off values of continuous variables using the area under the curve (AUC). Finally, propensity score matching (PSM) analyses were used to minimize the differences in characteristics between the compared groups, similar to the procedure followed in our previous study [20]. In brief, all the imbalances in tumor variables that may have affected the OS were compared using a χ2 test first. Then, a propensity score was calculated using the variables that were statistically significantly correlated with OS in the logistic regression analysis in the multivariate analysis. Finally, all analyses regarding OS were adjusted based on the generated propensity score. A Pearson’s χ2 test was subsequently performed to compare the differences between the H&E-sTILs (−) and H&E-sTILs (+) groups after matching.
Results
Patient characteristics
Between February 2012 and August 2018, 173 patients were reviewed. A total of 159 patients who fulfilled the inclusion criteria were enrolled in the current study, of whom 77 (48.4%) patients were determined to be H&E-sTILs(+) and 82 (51.6%) were H&E-sTILs(−). The differences in clinical characteristics, including sex, age, cTNM stage, regimens and cycles of chemotherapy, TRT, dose of TRT and PCI between the H&E-sTILs(+) and H&E-sTILs(−) patient groups were not significant (Table 1).
Short-term response to treatment and H&E-sTILs
The short-term response to chemotherapy and TRT values are presented in Table 2. Irrespective of whether the chemotherapy was administered alone or as TRT combined with chemotherapy, patients who were H&E-sTILs(+) exhibited sensitivity in terms of the short-term response to the treatment, whereas patients who were H&E-sTILs(−) displayed resistance in terms of their short-term responses to treatment.
OS, PFS and TILs
The median follow-up time in the entire cohort and in the surviving patients was 16 (range: 2–62) and 19 (range: 6–62) months, respectively. At the last follow-up, 48 patients remained alive and 111 patients had died, of whom 48 patients (48/111; 43.2%) had succumbed to extracranial progression (including locoregional or distant recurrence) alone, 20 to BM, 8 to both, and 35 to unascertainable intracranial or/and extracranial progression; those 35 patients were considered to have died from unknown causes when conducting the survival analysis.
The OS and PFS rates at the 1-, 2- and 3-year stages for the entire group, the H&E-sTILs(+) group and the H&E-sTILs(−) group are summarized in Table 2. The mOS of patients with H&E-sTILs(+) was markedly superior compared with patients with H&E-sTILs(−) (18 months cf. 12 months; P = 0.030) (Fig. 2A). The difference in mPFS between the two groups was also found to be statistically significant (10 months cf. 8 months, P = 0.013) (Fig. 2B).
Univariate and multivariate analyses for the entire group revealed that H&E-sTILs, cM stage, the cycles of chemotherapy and short-term response to TRT were independent factors affecting OS, whereas H&E-sTILs, cN stage, cM stage and short-term response to chemotherapy were considered as factors affecting PFS (Table 3).
As far as the entire cohort of patients was concerned, patients who received ≥ 6 cycles of chemotherapy achieved markedly improved OS rates compared with patients with < 6 cycles chemotherapy. However, following PSM analysis, the difference was found to be not significant. Furthermore, in the PSM subgroups, the difference between the OS rates of the H&E-sTILs(−) and H&E-sTILs(+) groups was significant in patients who received < 6 cycles chemotherapy, but not with patients who received ≥ 6 cycles chemotherapy (Figs. 3A, B, 4).
BM, BMFS and TILs analysis
The correlation between H&E-sTILs and BM was subsequently explored in the ‘non-PCI and non-BM at initial diagnosis’ subgroup (Table 2). A total of 133 patients were enrolled in the subgroup analysis, of whom 38 (38/133; 28.6%) patients in the subgroup experienced BM post-treatment. The 1-, 2- and 3-year BMFS rates of patients in the H&E-sTILs(−) and H&E-sTILs(+) groups are summarized in Table 2 (Fig. 2C). Univariate and multivariate analyses indicated that the H&E-sTILs were the unique factor affecting the BMFS (Table 3).
TILs and cTNM stage analysis
The cM stage is considered as one independent significant risk factor according to the univariate and multivariate analyses explored in the entire group. For the entire group, patients with M0 stage cancer were shown to have increased prospects of survival compared with patients with M1 stage cancer. Comparing the survival analyses of the M0 and H&E-sTILs(+), M0 and H&E-sTILs(−), M1 and H&E-sTILs(+) and M1 and H&E-sTILs(−) subgroups revealed a sequentially decreasing survival rate. The M0 and H&E-sTILs(+) group was associated with the best prospect of survival, whereas the M1 and H&E-sTILs(−) subgroup had the worst survival outlook; in addition, the difference in survival rate between the M0 and H&E-sTILs(−) and M1 and H&E-sTILs(+) subgroups was found to be not significant (Fig. 5).
Discussion
TILs have been demonstrated to serve as a prognostic factor in several types of solid cancer [2, 4,5,6,7,8,9], although studies on TILs in SCLC have only been rarely reported. A couple of decades ago, Eerola et al. [10] performed a study to analyze the associations of T cells using IHC in the prognosis of patients with operated SCLC. This group found that higher levels of TILs were associated with significantly more favorable survival times. However, it is not clear whether all mononuclear immune cells are T cells, and the relative proportions of macrophages, dendritic cells, myeloid-derived suppressor cells and plasma cells in the immune infiltrate may also serve important immune roles in cancer [21]. Furthermore, due to inaccurate measurement of the test variable without controlled calibration, the digital quantification of IHC-stained sections may yield different results and conclusions [9]. In more recent times, the histological evaluation of TILs is emerging as a more promising biomarker in various types of solid tumors [22], and this method has been proposed as a biomarker for inclusion in routine histopathological reporting and in the implementation of TNM staging for predicting patients’ prognosis [23, 24]. H&E-stained sections are easy to distinguish; thus, the evaluation of TILs using H&E-stained sections may be useful for predicting the prognosis in SCLC patients.
However, to the best of our knowledge, no similar studies have been performed to evaluate the relationships of TILs based on H&E-stained slides in SCLC. Therefore, we consider that the present study is the first to have explored H&E-sTILs in SCLC. The current study demonstrated that, for the entire cohort of enrolled patients, even in the absence of any significant differences in clinical characteristics comparing between the H&E-sTILs(+) and H&E-sTILs(−) patient groups, patients in the H&E-sTILs(+) group exhibited superior survival rates in terms of the OS, PFS and BMFS rates compared with patients in the H&E-sTILs(−) group. These results suggested that H&E-sTILs may serve as a potential biomarker in predicting prognosis of SCLC.
The short-term response to treatment, including chemotherapy or radiotherapy, is a factor that has been confirmed to be associated with survival. Liu et al.[25] reported that there is a correlation between TILs and the chemotherapy response in non-small cell lung cancer. Similarly, in the current study, irrespective of whether the patients were treated with chemotherapy alone or chemotherapy combined with TRT, those in the H&E-sTILs(+) group exhibited a greater sensitivity in terms of the short-term response to treatment compared with patients in the H&E-sTILs(−) group, which suggested that, for patients in the H&E-sTILs(−) group, more intensive treatment strategies should be implemented in order to improve their treatment response and survival prospects.
Chemotherapy is a cornerstone in the treatment of patients with SCLC [26]. In the current study, ROC analysis indicated that patients who received ≥ 6 cycles of chemotherapy achieved significantly improved OS rates compared with patients who received < 6 cycles of chemotherapy. Further analysis of the H&E-sTILs in the different subgroups of patients treated with differing numbers of chemotherapy cycles indicated that the difference in the OS rate between the H&E-sTILs(−) and the H&E-sTILs(+) subgroups was significant in patients who received < 6 cycles of chemotherapy, but for those patients who received ≥ 6 cycles of chemotherapy, the difference was not significant. These findings suggested that, for the H&E-sTILs(+) patients, a rational decision may be taken to administer fewer cycles of chemotherapy, but for the H&E-sTILs(−) patients, who were experiencing a poorer immune tumor environment and more dismal prospects of survival, more intensive treatment strategies [for example, with a greater number of cycles of chemotherapy or with the inclusion of added immune checkpoint inhibitors (ICIs)] should be considered in the clinic.
It has been shown in numerous studies that TRT is able to improve patients’ survival across the board, whether the patients have localized or extensive stage disease [26]. Similarly, the present study indicated that, compared with the non-TRT treatment group, patients who received TRT successfully achieved improved survival rates, regardless of the cTNM stage. In addition, the differences in the OS rate comparing between H&E-sTILs(−) and H&E-sTILs(+) patients in the TRT subgroup almost reached the level of statistical significance, although the failure to do so may have been influenced by the limited number of cases of enrolled patients. These results suggested that, for patients with H&E-sTILs(+), it may be possible to take a rational decision to use a lower radiation dose or a restricted field of TRT, especially when considering the lung toxicity that is associated with radiotherapy combined with ICIs.
The TNM staging system is the system accepted worldwide in terms of guiding cancer treatment and predicting prognosis, of which the M stage is associated with the most dismal prospects of survival. In the current study, neither the cT stage nor the cN stage, but only the cM stage was considered as a risk factor in the TNM staging system influencing the OS, PFS and BMFS rates. The different cM stage subgroups comprising the different H&E-sTILs statuses were subsequently explored to identify the best method for predicting patients` survival. The survival rates were found to decrease sequentially and significantly comparing among the M0 and H&E-sTILs(+), M0 and H&E-sTILs(−), M1 and H&E-sTILs(+) and M1 and H&E-sTILs(−) subgroups, although the difference between the M0 and H&E-sTILs(−) and M1 and H&E-sTILs(+) subgroups was not found to be statistically significant on account of the limited number of enrolled patients. These results demonstrated that H&E-sTILs should be considered in the TNM staging system as a prognostic factor to predict survival more accurately [23, 27].
With the development of systematic treatment strategies, especially in the case of ICIs applied in the clinic, BM has become the crucial failure model of treatment in SCLC. A study published previously by our research group [13] revealed that approximately one-third of patients encountered BM post-treatment, and poor short-term response to TRT and larger Dmax-T values were identified as risk factors for BM. However, in the current study, univariate and multivariate analyses indicated that H&E-sTILs were the unique factor affecting the BM and BMFS parameters. Although the BM rate between the H&E-sTILs(−) and H&E-sTILs(+) groups was not statistically different due to the limited number of enrolled cases and surveillance time, the difference in BMFS values exhibited a marked significance, which indicated that, for non-BM patients who were H&E-sTILs(+) at the initial diagnosis, PCI may not be necessary.
The global familiar studies Impower133 [28] and CASPIAN [29] have confirmed that ICIs achieve sustained improvements in the OS rates of patients with SCLC at the advanced and extensive stages of the disease. However, an effective biomarker of ICIs in SCLC had not yet been established, in spite of the fact that several other studies have reported that TILs may serve as a biomarker of ICI treatment [30]. Regrettably, given the fact that none of the patients enrolled in the current study received ICIs, it was not possible for us to investigate this further in terms of assessing the value of H&E-sTILs in SCLC for patients treated with ICIs, although will be an important focus of our future work.
Conclusions
In conclusion, the present study has shown that H&E-sTILs are the one and only significant prognostic factor affecting OS, PFS and BMFS simultaneously. Compared with patients in the H&E-sTILs(+) subgroup, patients in the H&E-sTILs(−) group had shorter survival times, more frequently occurring BM, and therefore these patients should be administered more intensive treatments in the clinic.
However, the present study did have several limitations, including its retrospective design from a single institution, the variability associated with visual TIL assessments using small biopsy tissue, and both the insufficient follow-up duration of the patients and the limited number of patients enrolled. The results of our investigation must therefore be interpreted with caution, and further prospective clinical trials are required to confirm the conclusions.
Availability of data and materials
All datasets presented in this study are included in the article.
Abbreviations
- BM:
-
Brain metastasis
- BMFS:
-
Brain-metastasis free survival
- CR:
-
Complete response
- CTV:
-
Clinical tumor volume
- GTV:
-
Gross tumor volume
- H&E:
-
Hematoxylin and eosin
- H&E-sTILs:
-
Assess TILs in tissue H&E-stained section slides
- ICIs:
-
Immune checkpoint inhibitors
- IMRT:
-
Intensity-modulated radiotherapy
- OAR:
-
Organ at risk
- OS:
-
Overall survival
- PCI:
-
Prophylactic cerebral irradiation
- PD:
-
Progressive disease
- PFS:
-
Progression free survival
- PR:
-
Partial response
- PSM:
-
Propensity score matching
- ROC:
-
Receiver operating characteristic
- SCLC:
-
Small cell lung cancer
- SD:
-
Stable disease
- TRT:
-
Thoracic radiotherapy
- TILs:
-
Tumor-infiltrating lymphocytes
References
Saito T, Nishikawa H, Wada H, Nagano Y, Sugiyama D, Atarashi K, Maeda Y, Hamaguchi M, Ohkura N, Sato E, Nagase H, Nishimura J, Yamamoto H, Takiguchi S, Tanoue T, Suda W, Morita H, Hattori M, Honda K, Mori M, Doki Y, Sakaguchi S. Two FOXP3(+)CD4(+) T cell subpopulations distinctly control the prognosis of colorectal cancers. Nat Med. 2016;22:679–84.
Brambilla E, Le Teuff G, Marguet S, Lantuejoul S, Dunant A, Graziano S, Pirker R, Douillard JY, Le Chevalier T, Filipits M, Rosell R, Kratzke R, Popper H, Soria JC, Shepherd FA, Seymour L, Tsao MS. Prognostic effect of tumor lymphocytic infiltration in resectable non-small-cell lung cancer. J Clin Oncol Off J Am Soc Clin Oncol. 2016;34:1223–30.
Perez EA, Ballman KV, Tenner KS, Thompson EA, Badve SS, Bailey H, Baehner FL. Association of stromal tumor-infiltrating lymphocytes with recurrence-free survival in the N9831 adjuvant trial in patients with early-stage HER2-positive breast cancer. JAMA Oncol. 2016;2:56–64.
Rakaee M, Kilvaer TK, Dalen SM, Richardsen E, Paulsen E-E, Hald SM, Al-Saad S, Andersen S, Donnem T, Bremnes RM. Evaluation of tumor-infiltrating lymphocytes using routine H&E slides predicts patient survival in resected non-small cell lung cancer. Hum Pathol. 2018;79:188–98.
Ruffini E, Asioli S, Filosso PL, Lyberis P, Bruna MC, Macrì L, Daniele L, Oliaro A. Clinical significance of tumor-infiltrating lymphocytes in lung neoplasms. Ann Thorac Surg. 2009;87:365–72.
Horne ZD, Jack R, Gray ZT, Siegfried JM, Wilson DO, Yousem SA, Nason KS, Landreneau RJ, Luketich JD, Schuchert MJ. Increased levels of tumor-infiltrating lymphocytes are associated with improved recurrence-free survival in stage 1A non-small-cell lung cancer. J Surg Res. 2011;171:1–5.
Kilic A, Landreneau RJ, Luketich JD, Pennathur A, Schuchert MJ. Density of tumor-infiltrating lymphocytes correlates with disease recurrence and survival in patients with large non-small-cell lung cancer tumors. J Surg Res. 2011;167:207–10.
Feng W, Li Y, Shen L, Cai X-W, Zhu Z-F, Chang J-H, Xiang J-Q, Zhang Y-W, Chen H-Q, Fu X-L. Prognostic value of tumor-infiltrating lymphocytes for patients with completely resected stage IIIA (N2) non-small cell lung cancer. Oncotarget. 2016;7:7227.
Hendry S, Salgado R, Gevaert T, Russell PA, John T, Thapa B, Christie M, van de Vijver K, Estrada MV, Gonzalez-Ericsson PI, Sanders M, Solomon B, Solinas C, Van den Eynden G, Allory Y, Preusser M, Hainfellner J, Pruneri G, Vingiani A, Demaria S, Symmans F, Nuciforo P, Comerma L, Thompson EA, Lakhani S, Kim SR, Schnitt S, Colpaert C, Sotiriou C, Scherer SJ, Ignatiadis M, Badve S, Pierce RH, Viale G, Sirtaine N, Penault-Llorca F, Sugie T, Fineberg S, Paik S, Srinivasan A, Richardson A, Wang Y, Chmielik E, Brock J, Johnson DB, Balko J, Wienert S, Bossuyt V, Michiels S, Ternes N, Burchardi N, Luen SJ, Savas P, Klauschen F, Watson PH, Nelson BH, Criscitiello C, O’Toole S, Larsimont D, de Wind R, Curigliano G, Andre F, Lacroix-Triki M, van de Vijver M, Rojo F, Floris G, Bedri S, Sparano J, Rimm D, Nielsen T, Kos Z, Hewitt S, Singh B, Farshid G, Loibl S, Allison KH, Tung N, Adams S, Willard-Gallo K, Horlings HM, Gandhi L, Moreira A, Hirsch F, Dieci MV, Urbanowicz M, Brcic I, Korski K, Gaire F, Koeppen H, Lo A, Giltnane J, Rebelatto MC, Steele KE, Zha J, Emancipator K, Juco JW, Denkert C, Reis-Filho J, Loi S, Fox SB. Assessing tumor-infiltrating lymphocytes in solid tumors: a practical review for pathologists and proposal for a standardized method from the international immunooncology biomarkers working group: part 1: assessing the host immune response, TILs in invasive breast carcinoma and ductal carcinoma in situ, metastatic tumor deposits and areas for further research. Adv Anat Pathol. 2017;24:235–51.
Eerola A, Soini Y, Pääkkö P. A high number of tumor-infiltrating lymphocytes are associated with a small tumor size, low tumor stage, and a favorable prognosis in operated small cell lung carcinoma. Clin Cancer Res Off J Am Assoc Cancer Res. 2000;6:1875–81.
Wang W, Hodkinson P, McLaren F, Mackean M, Williams L, Howie S, Wallace W, Sethi T. Histologic assessment of tumor-associated CD45(+) cell numbers is an independent predictor of prognosis in small cell lung cancer. Chest. 2013;143:146–51.
Bonanno L, Pavan A, Dieci M, Di Liso E, Schiavon M, Comacchio G, Attili I, Pasello G, Calabrese F, Rea F, Favaretto A, Rugge M, Guarneri V, Fassan M, Conte P. The role of immune microenvironment in small-cell lung cancer: distribution of PD-L1 expression and prognostic role of FOXP3-positive tumour infiltrating lymphocytes. Eur J Cancer (Oxford, England: 1990). 2018;101:191–200.
Wu S, Wang J, Zhang W, Li J, Wu H, Huang Z, Zhou G, Pan J, Chen M. Analysis of factors affecting brain metastasis in limited-stage small-cell lung cancer treated with definitive thoracic irradiation. Front Oncol. 2020;10:556634.
U.D.o. Health, N.C.I. Human Services %J National Institutes of Health, Common terminology criteria for adverse events (CTCAE) version 4.0. 4 (2009).
Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys. 1995;31:1341–6.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47.
Hendry S, Salgado R, Gevaert T, Russell PA, John T, Thapa B, Christie M, van de Vijver K, Estrada MV, Gonzalez-Ericsson PI, Sanders M, Solomon B, Solinas C, Van den Eynden G, Allory Y, Preusser M, Hainfellner J, Pruneri G, Vingiani A, Demaria S, Symmans F, Nuciforo P, Comerma L, Thompson EA, Lakhani S, Kim SR, Schnitt S, Colpaert C, Sotiriou C, Scherer SJ, Ignatiadis M, Badve S, Pierce RH, Viale G, Sirtaine N, Penault-Llorca F, Sugie T, Fineberg S, Paik S, Srinivasan A, Richardson A, Wang Y, Chmielik E, Brock J, Johnson DB, Balko J, Wienert S, Bossuyt V, Michiels S, Ternes N, Burchardi N, Luen SJ, Savas P, Klauschen F, Watson PH, Nelson BH, Criscitiello C, O’Toole S, Larsimont D, de Wind R, Curigliano G, Andre F, Lacroix-Triki M, van de Vijver M, Rojo F, Floris G, Bedri S, Sparano J, Rimm D, Nielsen T, Kos Z, Hewitt S, Singh B, Farshid G, Loibl S, Allison KH, Tung N, Adams S, Willard-Gallo K, Horlings HM, Gandhi L, Moreira A, Hirsch F, Dieci MV, Urbanowicz M, Brcic I, Korski K, Gaire F, Koeppen H, Lo A, Giltnane J, Rebelatto MC, Steele KE, Zha J, Emancipator K, Juco JW, Denkert C, Reis-Filho J, Loi S, Fox SB. Assessing tumor-infiltrating lymphocytes in solid tumors: a practical review for pathologists and proposal for a standardized method from the International Immuno-oncology Biomarkers Working Group: Part 2: TILs in melanoma, gastrointestinal tract carcinomas, non-small cell lung carcinoma and mesothelioma, endometrial and ovarian carcinomas, squamous cell carcinoma of the head and neck, genitourinary carcinomas, and primary brain tumors. Adv Anat Pathol. 2017;24:311–35.
Salgado R, Denkert C, Demaria S, Sirtaine N, Klauschen F, Pruneri G, Wienert S, Van den Eynden G, Baehner F, Penault-Llorca F, Perez E, Thompson E, Symmans W, Richardson A, Brock J, Criscitiello C, Bailey H, Ignatiadis M, Floris G, Sparano J, Kos Z, Nielsen T, Rimm D, Allison K, Reis-Filho J, Loibl S, Sotiriou C, Viale G, Badve S, Adams S, Willard-Gallo K, Loi S. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol Off J Eur Soc Med Oncol. 2015;26:259–71.
Rao U, Lee S, Luo W, Mihm M, Kirkwood J. Presence of tumor-infiltrating lymphocytes and a dominant nodule within primary melanoma are prognostic factors for relapse-free survival of patients with thick (t4) primary melanoma: pathologic analysis of the e1690 and e1694 intergroup trials. Am J Clin Pathol. 2010;133:646–53.
Su L, Chen M, Su H, Dai Y, Chen S, Li J. Postoperative chemoradiotherapy is superior to postoperative chemotherapy alone in squamous cell lung cancer patients with limited N2 lymph node metastasis. BMC Cancer. 2019;19:1023.
Fridman WH, Sautès-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer. 2012;12:298–306.
Liu JY, Yang GF, Chen FF, Peng CW. Evaluating the prognostic significance of tumor-infiltrating lymphocytes in solid tumor: practice of a standardized method from the International Immuno-Oncology Biomarkers Working Group. Cancer Manag Res. 2019;11:6815–27.
Donnem T, Hald SM, Paulsen E-E, Richardsen E, Al-Saad S, Kilvaer TK, Brustugun OT, Helland A, Lund-Iversen M, Poehl M, Olsen KE, Ditzel HJ, Hansen O, Al-Shibli K, Kiselev Y, Sandanger TM, Andersen S, Pezzella F, Bremnes RM, Busund L-T. Stromal CD8+ T-cell density: a promising supplement to TNM staging in non-small cell lung cancer. Clin Cancer Res Off J Am Assoc Cancer Res. 2015;21:2635–43.
Donnem T, Kilvaer T, Andersen S, Richardsen E, Paulsen E, Hald S, Al-Saad S, Brustugun OT, Helland A, Lund-Iversen M. Strategies for clinical implementation of TNM-immunoscore in resected nonsmall-cell lung cancer. Ann Oncol. 2016;27:225–32.
Liu H, Zhang T, Ye J, Li H, Huang J, Li X, Wu B, Huang X, Hou J. Tumor-infiltrating lymphocytes predict response to chemotherapy in patients with advance non-small cell lung cancer. Cancer Immunol Immunother. 2012;61:1849–56.
B.W.L. Apar Kishor P. Ganti, Jr., Michael Bassetti, Collin Blakely, et al., Small Cell Lung Cancer, Vison 2. 2022.
Al-Shibli KI, Donnem T, Al-Saad S, Persson M, Bremnes RM, Busund L-T. Prognostic effect of epithelial and stromal lymphocyte infiltration in non-small cell lung cancer. Clin Cancer Res. 2008;14:5220–7.
Horn L, Mansfield AS, Szczęsna A, Havel L, Krzakowski M, Hochmair MJ, Huemer F, Losonczy G, Johnson ML, Nishio M. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med. 2018;379:2220–9.
Paz-Ares L, Dvorkin M, Chen Y, Reinmuth N, Hotta K, Trukhin D, Statsenko G, Hochmair MJ, Özgüroğlu M, Ji JH. Durvalumab plus platinum–etoposide versus platinum–etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet. 2019;394:1929–39.
Park S, Ock C-Y, Kim H, Pereira S, Park S, Ma M, Choi S, Kim S, Shin S, Aum BJ, Paeng K, Yoo D, Cha H, Park S, Suh KJ, Jung HA, Kim SH, Kim YJ, Sun J-M, Chung J-H, Ahn JS, Ahn M-J, Lee JS, Park K, Song SY, Bang Y-J, Choi Y-L, Mok TS, Lee S-H. Artificial intelligence-powered spatial analysis of tumor-infiltrating lymphocytes as complementary biomarker for immune checkpoint inhibition in non-small-cell lung cancer. J Clin Oncol Off J Am Soc Clin Oncol. 2022;40:1916–28.
Acknowledgements
The authors would like to thank all patients who participated in the present study.
Funding
This work was supported by the National Natural Science Foundation of China (No. 82173051), the Joint Funds for the Innovation of Science and Technology, Fujian province (No. 2019Y9036), Scientific Research Foundation of Fujian Cancer Hospital (No. 2021YN01), Fujian Provincial Clinical Research Center for Cancer Radiotherapy and Immunotherapy (No. 2020Y2012), National Clinical Key Specialty Construction Program (No. 2021), Fujian Clinical Research Center for Radiation and Therapy of Digestive, Respiratory and Genitourinary Malignancies and the National Clinical Key Specialty Construction Program.
Author information
Authors and Affiliations
Contributions
All authors listed have made a substantial, direct, and intellectual contribution to the present study, and have approved it for publication. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This present retrospective study was approved by the Fujian Province Cancer Hospital Institutional Review Board (Approval No. K2022-031-01).
Consent for publication
Consent for scientific usage of clinical data was obtained from all patients included in the study.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhou, G., Zheng, J., Chen, Z. et al. Clinical significance of tumor-infiltrating lymphocytes investigated using routine H&E slides in small cell lung cancer. Radiat Oncol 17, 127 (2022). https://doi.org/10.1186/s13014-022-02098-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13014-022-02098-z