Abstract
Background
The purpose of this study was to compare the treatment efficacy and safety of re-irradiation (re-RT) using stereotactic ablative radiotherapy (SABR) and initial SABR for primary, recurrent lung cancer or metastatic lung tumor.
Methods
A retrospective review of the medical records of 336 patients who underwent lung SABR was performed. Re-RT was defined as the overlap of the 70% isodose line of second-course SABR with that of the initial radiotherapy, and 20 patients were classified as the re-RT group. The median dose of re-RT using SABR was 54 Gy (range 48–60 Gy), and the median fraction number was 4 (range 4–6). One-to-three case-matched analysis with propensity score matching was used, and 60 patients were included in the initial SABR group of the matched cohort.
Results
The 1- and 2-year local control rates for the re-RT group were 73.9% and 63.3% and those for the initial SABR group in the matched cohort were 92.9% and 87.7%, respectively (P = 0.013). There was no difference in distant metastasis-free, progression-free, and overall survival rates. The crude grade ≥ 2 toxicity rates were 40.0% for the re-RT group and 25.0% for the initial SABR group (P = 0.318). Re-RT group had higher acute grade ≥ 2 toxicity rates (25.0% vs 5.0%, P = 0.031). One incident of grade 3 toxicity (pulmonary) was reported in the re-RT group; there was no grade 4‒5 toxicity.
Conclusions
The local control rate of the in-field re-RT SABR was lower than that of the initial SABR without compromising the survival rates. The toxicity of re-RT using SABR was acceptable.
Similar content being viewed by others
Introduction
Stereotactic ablative radiotherapy (SABR) is a precise modality to deliver a high radiation dose to the tumor and is more effective than conventional radiotherapy (RT) in terms of the local control of early stage lung cancer [1]. Furthermore, studies have shown that salvage SABR can achieve acceptable local control in recurrent lung cancer after previous surgery or RT [2, 3]. Patients with oligometastatic lung tumors may have longer progression-free survival and extended time intervals between subsequent treatment interventions with SABR [4, 5]. As SABR was proven to be feasible in various clinical situations, several attempts have been made to apply SABR to previously irradiated lung lesions [6, 7]. Re-irradiation (Re-RT) has a higher risk of toxicity than initial irradiation as irreversible normal tissue damage may occur after the re-RT of the lung tumor [8]. Treatment efficacy of re-RT also needs to be considered as these previously irradiated tumors have a higher probability of developing resistance to radiation. Therefore, although the application of SABR to patients without any previous history of thoracic irradiation is feasible, the safety and efficacy of re-RT using SABR need to be verified.
Various treatment outcomes and toxicity profiles of re-RT with SABR have been reported due to heterogeneous clinical settings. The 2-year local control rates of salvage re-RT using SABR after prior external beam RT ranged from 37 to 90%, and the rates of grade 2‒3 radiation-induced lung toxicity ranged from 0 to 100% [9]. Some studies have shown considerable risk of fatal toxicity, such as pneumonitis and hemoptysis, after re-RT SABR [10]. These reports were mostly derived from retrospective single-arm studies, which made it difficult to assess the feasibility of re-RT with SABR. Therefore, this study aimed to evaluate the benefits and risks of re-RT using SABR with respect to in-field recurrence after previous irradiation of lung nodule(s) in patients with primary, recurrent lung cancer or metastatic lung tumor.
Methods
Study population
A retrospective review was performed of the medical records of 336 patients who underwent lung SABR for primary, recurrent lung cancer or metastatic lung tumor from January 2013 to December 2018 at a single institution. Re-RT was defined as the overlap of the 70% isodose line of second-course SABR with that of the initial RT, and 20 patients were classified as the re-RT SABR group. The remaining 316 patients were classified as the initial SABR group of the unmatched cohort.
Treatment and follow-up
Re-RT SABR was applied when recurrence was histologically confirmed or strongly suspected in positron emission tomography / computerized tomography (PET/CT) scan and serial CT scan in the previously irradiated or adjacent areas. Patients who received re-RT SABR did not have other systemic recurrence which required chemotherapy or targeted therapy. In addition, the patients were not eligible for salvage operation due to poor lung function or refusal to surgery according to the patients’ will. Recurrent lung nodules directly abutting critical mediastinal structures such as trachea, main bronchus, great vessels and esophagus were considered not to be amenable for Re-RT SABR.
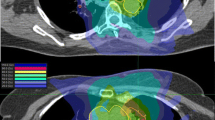
For simulation CT scan, the patients were in the supine position with both arms abducted and immobilized by wing board and vacuum cushions. If the patient had a lung tumor in the apex of the lung, both arms were adducted, and the patient was immobilized by a thermoplastic aquaplast. An abdominal compression device was routinely applied to minimize the respiratory movement of the tumor. A four-dimensional CT (4D-CT) scan was performed for RT simulation to cover all the respiratory phases. Internal target volume (ITV) was delineated for the lung tumor, using each respiratory phase and a maximum-intensity projection image from the 4D-CT. The planning target volume (PTV) was constructed by expanding the ITV by 3‒7 mm. The prescribed dose and PTV margins were defined at the discretion of the radiation oncologists by considering various clinical factors such as tumor histology, location, baseline lung function, and previous irradiation in case of re-RT SABR. The plan was optimized to cover 95% of the PTV by 100% of the prescribed dose. Maximum dose was limited to 110%, but the PTV coverage and normal organ doses had priority over maximum dose, and maximum dose up to 113% was permitted in some cases. The treatment was delivered using the volumetric-modulated arc therapy (VMAT) technique with two 180° arcs of 6 MV photon beam in the most cases. An example of re-RT SABR planning using VMAT is illustrated in Fig. 1. In the re-RT SABR group, 2nd RT course of three (15.0%) patients was delivered by magnetic resonance imaging (MRI)-guided cobalt-60 device with static intensity-modulated radiation therapy (IMRT) technique. A fraction was delivered two or three times a week, without consecutive daily treatment. For every fraction, cone beam CT was applied to verify the patient’s setup and the target location.
An example of re-irradiation SABR planning. A The patient underwent SABR for non-small-cell lung cancer with 54 Gy in 4 fraction. B Local recurrence was suspected one year after initial SABR, and re-irradiation SABR was delivered with 60 Gy in 4 fraction. C Summation of both SABR plan. Red lines are indicating internal target volume, while cyan lines are indicating planning target volume
The patients were followed up with clinical examination and chest CT scans at 6–8 weeks after RT. Thereafter, the patients were advised to follow up with 3-month intervals for 2 years. For the third to fifth years, the recommended follow-up interval was 6 months.
Propensity score matching
The propensity score (PS) was estimated by using the logistic regression model. Factors used in PS calculation were as follows: age (older than 75 versus younger than or equal to 75), tumor histology (lung cancer primary versus others), the existence of underlying pulmonary disease, tumor size (larger than 2 cm vs smaller than or equal to 2 cm), and tumor location (upper and middle lobes versus lower lobe). The PS-matched cohort was constructed using a 1:3 (re-RT SABR group / initial SABR group) ratio, and 60 patients from the unmatched cohort were included in the initial SABR group of the matched cohort. The standardized mean difference (SMD) was calculated to confirm the balance of the matching factors of the matched cohort. In the unmatched cohort, the SMD values of the underlying pulmonary disease and tumor size were larger than 0.2, whereas the SMD values of all variables were equal or smaller than 0.120 in the matched cohort, indicating that the matching factors became fairly balanced after PS matching. The comparisons of the matching factors of the unmatched and matched cohorts are summarized in Table 1.
Clinical outcomes and toxicity profile
The clinical outcomes in this study were local control (LC), distant metastasis-free survival (DMFS), progression-free survival (PFS), and overall survival (OS), which were estimated using the Kaplan–Meier method. These treatment outcomes were measured from the date of the end of RT for each defined event. An LC event was defined as the progression of a lung tumor treated by SABR, while a DMFS event was defined as an occurrence of distant metastasis or death. A new contralateral lung nodule was considered as distant metastasis. For OS, an event was defined as the death of the patient, whereas for PFS, an event was defined as disease progression or death. Survival data were retrieved from the resident registration system of the government of the Republic of Korea. These clinical outcomes of the re-RT and initial SABR groups of the matched cohort were compared using the log-rank test. Univariate analysis was performed for LC, DMFS, PFS, and OS of the re-RT SABR group to identify potential prognostic factors affecting treatment outcomes of re-RT. Multivariate analysis was not performed due to small number of the events in the re-RT SABR group.
For the analysis of RT toxicity, new occurrences or worsening of the symptoms were recorded and graded using the Common Terminology Criteria for Adverse Events (CTCAE) version 5. Toxicity that occurred more than 3 months after the completion of RT was considered late toxicity, while other events were considered acute toxicity. The rate of freedom from grade ≥ 2 toxicity (FFT) was calculated using the Kaplan–Meier method. Univariate analyses were performed for the FFT of the re-RT SABR group using Cox proportional-hazards model.
For dosimetric analysis of organs-at-risk (OARs) for re-RT SABR group, simulation CT scans, structures, and dose distributions of 17 patients transferred from Eclipse (Version 13.6, ARIA Oncology Information System, Varian Medical Systems, Palo Alto, CA) to MIM (Version 6.1.7, MIM Software Inc., Beachwood, OH, USA). Three (15.0%) patients who were treated by MRI-guided cobalt-60 device were excluded, as dose distribution of at least one course of RT could not be retrieved. Dose distributions were converted to dose delivered in 2 Gy fractions (EQD2) using linear-quadratic model with α/β ratio of 3. Two simulation CT scans were fused by rigid assisted alignment offered by MIM, and EQD2 distribution of the first RT course was transferred to simulation CT scan of the second RT course. Summation of the two EQD2 distributions was performed, and dosimetric parameters of OAR were calculated using structures defined at the second RT course. OARs included in the analysis were lung, proximal bronchial tree (from trachea to lobar bronchus), chest wall, aorta, esophagus, and heart. OAR structure for lung was defined as lung subtracting the PTV of re-RT SABR plan. Univariate analyses were performed for pulmonary symptom grade ≥ 2 with dosimetric parameters of lung and proximal bronchial tree, and for chest wall pain grade ≥ 2 with dosimetric parameters of chest wall using logistic regression model. All statistical analyses were performed using R version 4.0.4 (The R Foundation for Statistical Computing, Vienna, Austria).
Results
Patient characteristics and details of re-RT SABR
The characteristics of the patients included in both groups of the matched cohort are summarized in Table 2. The median follow-up of all patients of the matched cohort was 28.0 months (range 3.5–95.8 months). The median ages of the entire matched cohort, re-RT SABR group, and the initial SABR group of the matched cohort were 70 years (range 32–85 years), 73 years (range 51–85 years), and 67.5 years (range 32–85 years), respectively. The patients were predominantly male (80.0%). About one-third of the patients had a history of pulmonary disease: three patients had interstitial lung disease (ILD), five patients had chronic obstructive pulmonary disease (COPD) (two patients were diagnosed both ILD and COPD), one patient had asthma, and one patient had a 30-year history of working at stone quarry. The median age-adjusted Charlson comorbidity index was 7 (range 3–12). Among all the matched patients, 70.0% had a history of smoking, and the median pack-year value of current and previous smokers was 40 (range 3–110 pack-years). Central lung nodules were defined as nodules closer than 2 cm from the proximal bronchial tree [11]; 14 (17.5%) patients had central nodules. 80% of the patients underwent SABR due to primary lung cancer, including both non-small-cell lung cancer and small-cell lung cancer. Among non-small-cell lung cancer patients in the re-RT SABR group, 9 patients had squamous cell carcinoma, and 5 patients had adenocarcinoma. One patient had non-small-cell lung cancer whose subtype could not be determined due to insufficient tumor tissue.
The details of re-RT SABR are summarized in Table 3. The median prescribed dose of re-RT SABR was 54 Gy (range 48–60 Gy), and all but one patient had 4 fractionations. For the initial SABR group of the matched cohort, the median prescribed dose was 60 Gy (range 45–60 Gy), and the median fractionation number was 4 (range 4–8). Most (88.3%) of the patients in the initial SABR group underwent SABR with 4 fractionations. There was no significant difference in the prescribed doses for both groups (P = 0.342). The median biologically equivalent dose with an α/β ratio of 10 (BED10) was 126.9 Gy (range 105.6–150.0 Gy) for the re-RT SABR group and 126.9 Gy (range 85.5–150.0 Gy) for the initial SABR group. There was no significant difference in the BED10 values of both the groups (P = 0.461). Only one patients from the initial SABR group received a BED10 lower than 100 Gy. For the re-RT SABR group, the median cumulative BED10 of two courses of irradiation was 262.6 Gy (range 184.8–300.0 Gy).
For re-RT SABR, the prescribed dose was significantly different from the centrality of the lung nodule (P = 0.039). The median doses for peripheral and central nodules were 57 Gy (range 48–60 Gy) and 52 Gy (range 48–54 Gy), respectively. The BED10 value was not significantly different (P = 0.063) for both the groups: the median BED10 values were 126.9 Gy (range 105.6–150.0 Gy) for peripheral nodules and 119.6 Gy (range 105.6–126.9 Gy) for central nodules. There was no significant difference in the prescribed dose (P = 0.804) and BED10 values (P = 0.658) between primary lung cancer and metastatic lung tumors.
Treatment outcomes
The 1-, 2-, and 3-year LC rates were 73.9%, 63.3%, and 63.3% in the re-RT SABR group and 92.9%, 87.7%, and 87.7% in the initial SABR group of the matched cohort, respectively. There was a significant difference in the LC rates between the two groups (P = 0.013). The 1-, 2-, and 3-year DMFS rates were 75.0%, 37.5%, and 30.0% in the re-RT SABR group and 69.7%, 57.1%, and 52.8% in the initial SABR group, respectively, with no significant difference (P = 0.410) between both the groups. The PFS rates at 1, 2, and 3 years were 55.0%, 25.0%, and 25.0% for the re-RT SABR group and 58.0%, 45.4%, and 41.4% for the initial SABR group, respectively. No significant difference in PFS rates was found between the two groups (P = 0.460). The OS rates at 1, 2, and 3 years were 95.0%, 69.1% and 38.3% for the re-RT SABR group and 90.0%, 74.8%%, and 67.1% for the initial SABR group, respectively. There was no significant difference in the OS rates between the two groups (P = 0.270). These treatment outcomes of the matched cohort are illustrated in Fig. 2.
The results of the univariate analysis of LC, DMFS, PFS, and OS for the re-RT SABR group are summarized in Table 4. There was no significant association between these variables and LC in the univariate analysis. Also, we could not find significant factors for DMFS, although cumulative BED10 was marginally associated with DMFS (hazard ratio [HR] 0.315, 95% confidence interval [CI] 0.099–1.001, P = 0.050). The RT technique of the first course was the only factor that associated with PFS in the univariate analysis (HR 4.847, 95% CI 1.073–21.91, P = 0.040). In terms of OS, the RT technique of the first course (HR 9.167, 95% CI 1.062–79.08, P = 0.044) and any treatment between the RT courses (HR 5.686, 95% CI 1.265–25.55, P = 0.023) were associated.
Toxicity profile and dosimetric analysis
Toxicity profiles of the matched cohort are summarized in Table 5. The reported complications were cough, dyspnea, and chest wall pain. In the patients of the re-RT SABR group, 40.0% experienced grade ≥ 2 toxicity, while 25.0% of the patients in the initial SABR group showed grade ≥ 2 toxicity. There were no significant differences in the crude rates of grade ≥ 2 toxicity between both the groups (P = 0.318). More grade ≥ 2 acute toxicity was reported in the re-RT SABR group (25.0% vs 5.0%, P = 0.031), whereas there was no difference in the occurrence rates of grade ≥ 2 late toxicity (20.0% vs 21.7%, P = 1.000). The 1-, 2-, and 3-year rates of FFT were 60.0%, 60.0%, and 60.0% for the re-RT SABR group and 80.6%, 74.2%, and 74.2% for the initial SABR group, respectively. The log-rank test showed no significant difference in the FFT rates between the two groups (P = 0.160). FFT rates are shown in Fig. 3. The Cox univariate analysis for the re-RT SABR group revealed age-adjusted Charlson comorbidity index ≥ 10 (HR 8.302, 95% CI 2.361–50.66, P = 0.022), and any other treatment between the two courses of RT (HR 8.091, 95% CI 1.609–40.69 P = 0.011) were associated with the FFT rate.
Dosimetric analysis of OARs for 17 patients is summarized in Table 6. Cumulative EQD2 for chest wall was relatively high, while cumulative EQD2 for critical mediastinal structures including proximal bronchial tree, aorta, esophagus, and heart were low. Dosimetric parameters of lung and chest wall did not show significant association with occurrence of grade ≥ 2 pulmonary toxicity (cough, dyspnea) and chest wall pain, respectively, in the univariate analysis.
Discussion
The current study demonstrates that the LC rate of the salvage lung SABR after in-field recurrence is acceptable with tolerable toxicity. In-field locoregional relapse after definitive chemoradiation for locally advanced lung cancer is frequent [12]. Even with high LC rates, the local recurrence after lung SABR is not uncommon and has an actuarial rate of nearly 20% [13]. Several reports have shown that salvage surgical resection can be an alternative [14, 15]; however, surgery is not always an option as many lung cancer patients are morbid and have poor lung function. Therefore, salvage SABR is still a promising treatment option even with in-field recurrence after thoracic irradiation. Nevertheless, it is important to pay attention to potentially worse treatment outcomes of salvage SABR than those of initial irradiation, as the current study found lower LC rates for re-RT SABR by PS matching than for initial lung SABR without any previous irradiation history.
Although there have been several studies on thoracic re-RT, the definition of re-RT varies considerably. Some reports included both in-field and out-field recurrence as targets for re-RT [16], hindering the interpretation of treatment outcomes for patients with high cumulative dose using re-RT. Even in studies on only in-field recurrence, the definition of in-field recurrence is diverse. Some studies used a certain percentage or absolute isodose line [6, 17, 18], while others used criteria directly derived from the target volume [19]. Several studies used overlapping of 50% isodose line as the inclusion criteria [6, 18], but it did not guarantee the inclusion of the patients with higher cumulative dose than commonly prescribed dose when two dose distributions marginally overlap. Our definition of re-RT SABR is the overlap of the 70% isodose line to ensure that the recruited patients had a heavily irradiated area of at least 70% of the cumulative dose.
A few reports of salvage SABR for in-field lung recurrence after previous RT have been published, although the comparison between re-RT SABR and initial SABR is scarce. Wide ranges of LC rates were reported due to various clinical situations of utilization of re-RT SABR [9], making it even more difficult to compare the treatment outcomes among retrospective series. The current study applied PS matching to mitigate these clinical differences and found worse LC rates in the re-RT SABR group. The clinical outcomes reported by the current study would be helpful to assess the actual clinical benefits of re-RT using SABR in settings of in-field local recurrence. Moreover, in contrast to some previous re-RT SABR series, all second-course SABR in the current study comprised irradiation with high doses of more than 100 Gy of BED10, which was considered sufficient to achieve LC of lung nodules [10, 17, 20, 21]. This indicated that even with a sufficient dose, the LC rate of re-RT SABR for in-field recurrent lung nodules may be lower than that of initial SABR.
There are several reasons for low LC of locally relapsed nodules treated with re-RT SABR. One plausible explanation is that surviving cell fractions that cause local recurrence after RT are radioresistant, and underlying mechanisms have been postulated for this phenomenon [22]. Additionally, there is a chance that locally recurrent lung nodules could initially have radioresistant clones. Radiation lung fibrosis may impact tissue oxygenation, and impaired oxygenation could reduce radiosensitivity [23]. Delineating target volume in re-RT SABR may be inaccurate due to radiation pneumonitis after initial RT. The exact reasons of low LC need to be addressed by further study.
Toxicity is an important concern of re-RT, as cumulative radiation damage would result in a greater risk of damage to normal tissue. Over 40% of the patients from the re-RT group of the current study experienced grade ≥ 2 toxicity. However, only one grade 3 toxicity was reported, and there was no grade 4‒5 toxicity. The low rate of severe toxicity of re-RT SABR in the current study might be influenced by the utilization of advanced RT technology such as VMAT. Most (85.0%) re-RT SABR courses in this study were delivered by using the VMAT technique in contrast to other studies in which the patients underwent re-RT SABR by three-dimensional conformal RT or static IMRT. Nevertheless, this result should be interpreted cautiously, as the possibility of severe and fatal toxicity of re-RT SABR cannot be excluded due to the small sample size and the retrospective nature of this study. Some studies have demonstrated high rates of severe toxicity. For instance, a re-RT SABR study by Peulen et al. [19] showed that 37.9% of the patients experienced grade ≥ 3 toxicity with 3 fatal cases. The centrality of the recurrent lung nodules was not a significant risk factor of occurrence of grade ≥ 2 toxicity in the current study. However, additional caution would be needed for central tumors, as several fatal pneumonitis and hemoptysis were reported in previous series [6, 10]. It should be noted that although there were lesions classified as central by the present criteria, no central lung nodules in re-RT SABR group of this study had direct abutment to the trachea, esophagus, or the main bronchus. Central nodules were re-irradiated only when a safe SABR plan could be executed.
There are several different methods for reflecting dose distribution of previous RT course when planning re-RT SABR. Cumulative EQD2 based on linear quadratic equation was applied in a recently published article [24]. As we previously mentioned, dosimetric analysis in the current study showed relatively low cumulative EQD2 for critical mediastinal structures when compared with that of chest wall, reflecting patient selection factor. No significant association between dosimetric parameters and occurrence of grade ≥ 2 toxicity was observed, presumably due to the small number of the event. Since some dosimetric parameters of the current series showed marginal significance, it is expected that a significant dose-response relationship can be confirmed through a larger number of patient studies.
The current study has some limitations. As this series included both primary lung cancer and metastatic lung tumors, various histologic types were included in the analysis, which might have influenced the treatment outcomes due to differences in radioresistance based on tumor histology. Second, the definition of re-RT used in this study was arbitrary, and both conventional fractionation RT and SABR were allowed as first-course RT in the re-RT SABR group, creating a lack of uniformity in the clinical situations. Third, the patients might not be representative due to the small numbers of the recruited cases. It should be considered that it would be impractical to design a prospective trial for re-RT SABR to recurrent lung nodules, as recurrence after initial SABR to lung is not common due to high local control rate of SABR. Therefore, we conducted propensity-score matching analysis using retrospective data. Finally, toxicity could be under-reported due to the retrospective nature of this study. Nevertheless, the results of the present study are clinically significant because it included only those patients who underwent re-RT for in-field recurrence in a strict sense. In terms of the heterogeneous inclusion of histologic types and techniques of the first course of RT, despite the inherent bias in a retrospective study, this study captured complex real-world situations of re-RT by dealing with various cases. Further, the patients in the re-RT SABR group underwent SABR with a more advanced RT technique and had higher BED10 values than those in previous reports. Furthermore, as reports comparing re-RT SABR to initial SABR are rare, this study can contribute to clinical decisions for the treatment of recurrent lung nodules.
Conclusions
Re-RT SABR for in-field local recurrence after thoracic irradiation is effective and feasible, although the LC rate for in-field re-RT SABR was lower than for initial SABR. Albeit only one grade 3 toxicity and no grade 4‒5 toxicity was reported in the re-RT SABR group, it should be noted that patients without direct abutment to critical mediastinal structures underwent re-RT SABR in the current study. The biological background of the worse outcome needs to be further explored in future studies. Even though the toxicity of re-RT SABR was acceptable, more clinical data would be needed to specify the criteria for safe re-RT.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- RT:
-
Radiotherapy
- Re-RT:
-
Re-irradiation
- SABR:
-
Stereotactic ablative radiotherapy
- PET/CT:
-
Positron emission tomography/computerized tomography
- 4D-CT:
-
Four-dimensional computerized tomography
- ITV:
-
Internal target volume
- PTV:
-
Planning target volume
- VMAT:
-
Volumetric-modulated arc therapy
- MRI:
-
Magnetic resonance imaging
- IMRT:
-
Intensity-modulated radiation therapy
- PS:
-
Propensity score
- SMD:
-
Standardized mean difference
- LC:
-
Local control
- DMFS:
-
Distant metastasis-free survival
- PFS:
-
Progression-free survival
- OS:
-
Overall survival
- CTCAE:
-
Common Terminology Criteria for Adverse Events
- FFT:
-
Freedom from grade ≥ 2 toxicity
- OARs:
-
Organs-at-risk
- EQD2:
-
Dose delivered in 2 Gy fractions
- ILD:
-
Interstitial lung disease
- COPD:
-
Chronic obstructive pulmonary disease
- BED10 :
-
Biologically equivalent dose with an α/β ratio of 10
- HR:
-
Hazard ratio
- CI:
-
Confidence interval
References
Ball D, Mai GT, Vinod S, Babington S, Ruben J, Kron T, et al. Stereotactic ablative radiotherapy versus standard radiotherapy in stage 1 non-small-cell lung cancer (TROG 09.02 CHISEL): a phase 3, open-label, randomised controlled trial. Lancet Oncol. 2019;20:494–503.
Sun B, Brooks ED, Komaki R, Liao Z, Jeter M, McAleer M, et al. Long-Term outcomes of salvage stereotactic ablative radiotherapy for isolated lung recurrence of non-small cell lung cancer: a phase II clinical trial. J Thorac Oncol. 2017;12(6):983–92.
Sumodhee S, Bondiau PY, Poudenx M, Cohen C, Naghavi AO, Padovani B, et al. Long term efficacy and toxicity after stereotactic ablative reirradiation in locally relapsed stage III non-small cell lung cancer. BMC Cancer. 2019;19(1):1–14.
Suh YG, Cho J. Local ablative radiotherapy for oligometastatic non-small cell lung cancer. Radiat Oncol J. 2019;37(3):149–55.
Palma DA, Olson R, Harrow S, Gaede S, Louie AV, Haasbeek C, et al. Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (SABR-COMET): a randomised, phase 2, open-label trial. Lancet. 2019;393(10185):2051–8.
Kilburn JM, Kuremsky JG, Blackstock AW, Munley MT, Kearns WT, Hinson WH, et al. Thoracic re-irradiation using stereotactic body radiotherapy (SBRT) techniques as first or second course of treatment. Radiother Oncol. 2014;110(3):505–10.
Mazzola R, Fersino S, Ferrera G, Targher G, Figlia V, Triggiani L, et al. Stereotactic body radiotherapy for lung oligometastases impacts on systemic treatment-free survival: a cohort study. Med Oncol. 2018;35(9):1–7.
De Ruysscher D, Faivre-Finn C, Le Pechoux C, Peeters S, Belderbos J. High-dose re-irradiation following radical radiotherapy for non-small-cell lung cancer. Lancet Oncol. 2014;15(13):e620–4.
Milano MT, Kong FMS, Movsas B. Stereotactic body radiotherapy as salvage treatment for recurrence of non-small cell lung cancer after prior surgery or radiotherapy. Transl Lung Cancer Res. 2018;8(1):78–87.
Trovo M, Minatel E, Durofil E, Polesel J, Avanzo M, Baresic T, et al. Stereotactic body radiation therapy for re-irradiation of persistent or recurrent non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2014;88(5):1114–9.
Timmerman R, McGarry R, Yiannoutsos C, Papiez L, Tudor K, DeLuca J, et al. Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. J Clin Oncol. 2006;24(30):4833–9.
Jouglar E, Isnardi V, Goulon D, Ségura-Ferlay C, Ayadi M, Dupuy C, et al. Patterns of locoregional failure in locally advanced non-small cell lung cancer treated with definitive conformal radiotherapy: results from the gating 2006 trial. Radiother Oncol. 2018;126(2):291–9.
Shintani T, Matsuo Y, Iizuka Y, Mitsuyoshi T, Mizowaki T. A retrospective long-term follow-up study of stereotactic body radiation therapy for non-small cell lung cancer from a single institution: incidence of late local recurrence. Int J Radiat Oncol Biol Phys. 2018;100(5):1228–36.
Bograd AJ, Mann C, Gorden JA, Gilbert CR, Farivar AS, Aye RW, et al. Salvage lung resections after definitive chemoradiotherapy: a safe and effective oncologic option. Ann Thorac Surg. 2020;110(4):1123–30.
Chen F, Matsuo Y, Yoshizawa A, Sato T, Sakai H, Bando T, et al. Salvage lung resection for non-small cell lung cancer after stereotactic body radiotherapy in initially operable patients. J Thorac Oncol. 2010;5(12):1999–2002.
Horne ZD, Dohopolski MJ, Clump DA, Burton SA, Heron DE. Thoracic reirradiation with SBRT for residual/recurrent and new primary NSCLC within or immediately adjacent to a prior high-dose radiation field. Pract Radiat Oncol. 2018;8(3):e117–23.
Kennedy WR, Gabani P, Nikitas J, Robinson CG, Bradley JD, Roach MC. Repeat stereotactic body radiation therapy (SBRT) for salvage of isolated local recurrence after definitive lung SBRT. Radiother Oncol. 2020;2020(142):230–5.
Yang WC, Hsu FM, Chen YH, et al. Clinical outcomes and toxicity predictors of thoracic re-irradiation for locoregionally recurrent lung cancer. Clin Transl Radiat Oncol. 2020;22:76–82.
Peulen H, Karlsson K, Lindberg K, Tullgren O, Baumann P, Lax I, et al. Toxicity after reirradiation of pulmonary tumours with stereotactic body radiotherapy. Radiother Oncol. 2011;101(2):260–6.
Onishi H, Shirato H, Nagata Y, Hiraoka M, Fujino M, Gomi K, et al. Hypofractionated stereotactic radiotherapy (HypoFXSRT) for stage I non-small cell lung cancer: updated results of 257 patients in a Japanese multi-institutional study. J Thorac Oncol. 2007;2(7 SUPPL. 3):S94-100.
Parks J, Kloecker G, Woo S, Dunlap NE. Stereotactic body radiation therapy as salvage for intrathoracic recurrence in patients with previously irradiated locally advanced non-small cell lung cancer. Am J Clin Oncol Cancer Clin Trials. 2016;39(2):147–53.
Barker HE, Paget JTE, Khan AA, Harrington KJ. The tumour microenvironment after radiotherapy: mechanisms of resistance and recurrence. Nat Rev Cancer. 2015;15(7):409–25.
Hughes VS, Wiggins JM, Siemann DW. Tumor oxygenation and cancer therapy-then and now. Br J Radiol. 2019;92(1093):20170955.
Schröder C, Stiefel I, Tanadini-Lang S, et al. Re-irradiation in the thorax—an analysis of efficacy and safety based on accumulated EQD2 doses. Radiother Oncol. 2020;152:56–62.
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
THL was responsible for data analysis and manuscript writing. DYK was responsible for data analysis and study designing. HGW was responsible for project administration and data collection. JHL contributed to data collection. HJK contributed to study designing and project administration. All authors contributed to the revision of the manuscript and approval of the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Institutional Review Board of Seoul National University Hospital (IRB No. H-2103-116-1205) before collecting patient information. Informed consent was waived.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Lee, T.H., Kim, DY., Wu, HG. et al. Treatment outcomes of re-irradiation using stereotactic ablative radiotherapy to lung: a propensity score matching analysis. Radiat Oncol 16, 222 (2021). https://doi.org/10.1186/s13014-021-01948-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13014-021-01948-6