Abstract
Background
The potential impact of menthol versus non-menthol cigarette use on smoking behaviors is an intensely scrutinized topic in the public health arena. To date, several general literature reviews have been conducted, but findings and conclusions have been discordant. This systematic review followed PRISMA guidelines to examine the Key Question, “Does menthol cigarette use have a differential impact on smoking cessation compared with non-menthol cigarette use?”
Methods
Six databases—Cochrane Central Register of Controlled Trials, Cochrane Database of Systematic Reviews, Database of Abstracts of Reviews of Effects, MEDLINE, Embase and PsycInfo—were queried from inception to June 12, 2020. Articles comparing menthol versus non-menthol cigarette smokers in terms of at least one predefined smoking cessation outcome were included. Risk of bias was assessed using the Agency for Healthcare Research and Quality Evidence-Based Practice Center approach. A random-effects model utilizing the DerSimonian and Laird method to pool adjusted odds ratio was applied. Variations among pooled studies were assessed using Cochran’s Q statistic, and heterogeneity was quantified using the inconsistency index (I2).
Results
Forty-three demographically adjusted studies (22 rated “good”, 20 rated “fair”, and one study rated “poor” individual study quality) comparing menthol and non-menthol smokers were qualitatively synthesized across the following measures (study count; strength of evidence): duration of abstinence (2; low); quit attempts (15; insufficient); rate of abstinence/quitting (29; moderate); change in smoking quantity/frequency (5; insufficient); and, return to smoking/relapse (2; insufficient). Overall, the qualitative synthesis failed to show a consistent trend for an association between menthol cigarette use and smoking cessation across outcomes. Meta-analyses found no difference between menthol and non-menthol cigarette use and either quit attempts or abstinence.
Conclusions
Given the lack of consistency or statistical significance in the findings—combined with a “low” overall strength of evidence grade, based on deficiencies of indirectness and inconsistency—no consistent or significant associations between menthol cigarette use and smoking cessation were identified. Recommendations for future studies include increased focus on providing longitudinal, adjusted data collected from standardized outcome measures of cessation to better inform long-term smoking cessation and menthol cigarette use. Such improvements should also be further considered in more methodologically rigorous systematic reviews characterized by objectivity, comprehensiveness, and transparency with the ultimate objective of better informing public health and policy decision making.
Similar content being viewed by others
Background
Currently, the proportion of smokers who use menthol cigarettes is higher among youth than among adults, with about three out of ten adult cigarette smokers choosing to smoke menthol cigarette brands [1]. Based on data from the U.S. Centers for Disease Control [2], rates of adult cigarette smoking have steadily declined over the last half century, from 42% in 1965 to 17% in 2014. Despite this overall decline in smoking, the Substance Abuse and Mental Health Services Administration [3] has noted that menthol cigarette use seems to be characterized by a contradictory upward trend among younger adults, females, males, Hispanics, and Asians. Thus, trends in smoking are inconsistent between menthol and non-menthol cigarette smokers.
In recent years, the potential impact of menthol versus non-menthol cigarette use on smoking behaviors has been an intensely scrutinized topic in the public health arena. More recently, the issue has been brought to the forefront of tobacco policy and decision making, as evidenced by the Food and Drug Administration’s (FDA) recently-declared intent to explore a ban on mentholated tobacco products. Given the FDA’s own commitment to evidenced-based actions [4], there is a clear need for the potential associations between menthol cigarettes and smoking behaviors to be explored scientifically. To date, several narrative reviews have been conducted. However, study methods and the included individual publications have varied, and conclusions have been discordant [5,6,7]. Some of the discord may reflect the complicated constructs related to smoking behaviors and the varying measurements across studies [8, 9].
A recent meta-analysis by Smith and colleagues [10] concluded that, among Blacks/African Americans in the U.S. (one sample including respondents from Canada), menthol smokers had approximately 12% lower odds of smoking cessation compared to non-menthol smokers. However, the meta-analysis was not based on a full, PRISMA-guided systematic review of the available evidence. A second systematic review by Smith et al. [9] found that both men and women exhibit minimal switching between menthol and non-menthol cigarettes, suggesting that preference is established early in an individual’s smoking trajectory. However, these findings were based on a single included study in the review of smoking initiation, and therefore conclusions are limited in generalizability. Similarly, a systematic review by Villanti et al. [7] reported an association between menthol cigarette smoking and increased initiation among youth, increased dependence especially among youth, and reduced cessation among non-Hispanic Whites and racial and ethnic subgroups. However, the validity of these findings are undermined by the failure to apply an adequate appraisal tool—such AMSTAR 2 [8] which would have identified significant methodological insufficiencies.
Given the methodological deficiencies in the current evidence base, the purpose of our review was to systematically assess the potential association between menthol cigarette use and smoking cessation, with a strict methodological focus to the measures and methods used by the included studies.
Further, given that smoking behaviors can vary across different population subgroups—suggesting that both individual and environmental factors influence smoking [11, 12]—it is essential that factors that influence smoking behaviors be considered to the extent possible based on available data. To this end, this review applied the Socio-Ecological Model created by McLeroy et al. [13] to guide consideration of the interrelationships between individuals and their social (micro-), physical (meso-), and policy (macro-) environments. The socio-ecological model includes three main levels of factors that influence an individual’s smoking behaviors: characteristics of the individual (“micro”); characteristics of the individual’s social environment (“meso”); and characteristics of the systems-level environment in which the individual exists (“macro”). Our review also attempted to quantitatively synthesize the evidence with meta-analyses; to the best of the authors’ knowledge, quantitative synthesis of data from a systematic review has not been previously conducted for this evidence base.
Methods
Overview
The methods used for this systematic review followed PRISMA guidelines and were applied to a larger literature search strategy of the association between menthol cigarette use and three smoking behaviors—initiation, cessation, and dependence—of which cessation is the focus of this analysis. Specifically, current results assess the Key Question (KQ), “Does menthol cigarette use have a differential impact on smoking cessation compared to non-menthol cigarette use?” The protocol for this systematic review was registered with the PROSPERO international prospective register of systematic reviews on March 22, 2016 and updated on January 10, 2019. The record is available at: https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=119301.
Literature search strategy
The literature searches were conducted by an Information Specialist. Search terms were developed using text words related to the associations between menthol cigarette use and cessation of cigarette smoking. The search strategy included using synonyms of search terms, truncation, wild card symbols, Boolean logic, proximity operators, and limits to focus the search towards the most relevant clinical literature (see SUPPLEMENTAL SECTION 1: Literature Search Strategy).
The following online databases were searched for relevant articles published from inception to 14 December 2018 (for the initial literature search) and from 01 January 2018 to 12 June 2020 (for the updated literature search): Cochrane Central Register of Controlled Trials, Cochrane Database of Systematic Reviews, Database of Abstracts of Reviews of Effects, MEDLINE, Embase and PsycInfo.
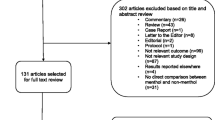
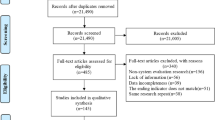
The initial literature search (from inception to 14 December 2018) identified 853 potentially relevant articles, with 838 articles from online databases and 15 additional articles through other sources. An updated literature search (from 01 January 2018 to 12 June 2020) identified an additional 358 potentially relevant articles; however, 149 of the articles were duplicate articles across the two searches, due to a required overlap in the two search timeframes (searches are best conducted from the first of the year). Thus, 209 unique articles were identified in the update literature searching, bringing the total of potentially relevant articles to 1062. After independent review of titles and abstracts by two members of the research team, 603 references were excluded, resulting in 459 articles being screened at the full-text level. An additional 324 articles were excluded at the full-text level (provided in SUPPLEMENTAL SECTION 2: Studies excluded at full-text level screening (with reason for exclusion)), resulting in 135 relevant articles eligible for inclusion; 73 studies (eight of which were reported in paired studies) evaluated the association between menthol cigarette smoking and smoking cessation or cessation-related outcomes (Fig. 1). The weighted overall kappa for inter-rated reliability at full-text screening was 0.96 for the initial literature search, and 0.95 for the updated literature search.
Eligibility criteria
Eligibility criteria were developed according to the PICO framework and are presented in Table 1. Studies of solely non-U.S. residents were excluded on the basis of variations in national tobacco legislation limiting the generalizability of such studies to the U.S. population.
Data extraction
Data were extracted and managed through DistillerSR (Evidence Partners, Ottawa, Canada). Articles were initially screened at the title/abstract level; full-text articles were obtained for studies not excluded based on the title/abstract alone. Two reviewers independently screened articles based on the inclusion/exclusion criteria. Any discrepancies between the two were resolved in a joint-reviewer decision. Any unresolved disagreements were adjudicated by a third clinical reviewer; reasons for exclusions were documented.
Data were independently extracted by one research associate and checked by a second research associate. Discrepancies were resolved through discussion and included a third team member when necessary. Data extraction forms were created in DistillerSR.
Study quality assessment
Study quality rating
A random and sufficient sample of included studies was assessed independently by two members of the review team. The level of agreement between those researchers was evaluated based on the mean difference in scores between the two reviewers. The mean difference was 0.25 points (95% CI, − 0.53 to 1.03), indicating that, on average, reviewers had a high level of agreement that the true mean difference was no greater than one point on the scale. The difference in score across studies was distributed normally, suggesting no systematic bias. Based on the high level of agreement, the ratings were not found to be subject to individual reviewer bias, and a single reviewer reviewed the remaining included studies.
Downs and Black checklist
The quality of the studies included in this systematic review was assessed at the study level using the Downs and Black checklist [14]. The instrument was used as reported in the original publication, with only one adaptation of the power question as to whether the study was adequately powered (yes/no). The maximum achievable score for a study was 28, and score ranges were grouped into the following four quality levels: “excellent” (26–28); “good “(20-25); “fair” (15–19); and “poor” (≤14). When data from a single study were reported in multiple references, all references were considered to determine an overall rating for the study.
Assessment of confounding
A list of potential confounding factors was identified a priori based on evidence and expert opinion from members of the research team and external advisors. Variables that individual study authors considered were recorded for additional post hoc consideration.
This review assessed evidence that adequately controlled for confounding bias according to the predetermined confounders of age, race/ethnicity, and gender. Studies that also adjusted for additional potential meso- (e.g., living with a smoker) or macro-level factors (e.g., cigarette taxes) were flagged for inclusion in sensitivity analyses. Studies with potential overadjustment or adjustment for factors in the causal pathway were also flagged for further examination in sensitivity analyses.
Conceptual framework
This review applied the Socio-Ecological Model [13] to guide consideration of the interrelationships between individuals and their social (micro-), physical (meso-), and policy (macro-) environments.
Outcomes and related psychometrics
Included studies reported on at least one of the following cessation—or cessation-related—outcomes: duration of abstinence, quit attempts (any quit attempts; number of quit attempts per person), rate of abstinence/quitting, change in smoking quantity/frequency, and return to smoking/relapse. Recognizing that not all the outcome measures are likely to be equally valid and reliable, this review examined the following Contextual Question (CQ) to provide additional information and context for the results, “Have measures used to examine cigarette smoking cessation been psychometrically assessed as valid and reliable?” The applied scoring approach was informed by the IARC Handbook of Cancer Prevention [15].
Data analysis
The strongest evidence to assess whether menthol cigarette use has a differential impact on smoking cessation compared to non-menthol cigarette use would be expected to be provided by longitudinal analyses that adjusted or controlled for key confounding factors – age, race/ethnicity, and gender – by inclusion criteria, modeling, or stratification. Consequently, all studies that controlled for, at minimum, age, gender, and race/ethnicity were qualitatively synthesized.
Longitudinal analytic results were considered the highest available evidence and, as such, were weighed more heavily in the strength of evidence analysis and qualitative synthesis below. In the absence of longitudinal analytic results, the highest level of available evidence was synthesized according to studies that controlled for the predefined demographic factors.
Statistical significance
Estimates of the difference between menthol and non-menthol smokers are presented with the best measure of precision (i.e., 95% confidence intervals) or statistical significance (i.e., p-value) reported in the included studies. The words “significant” and “significantly” are used herein to indicate statistical significance (i.e., p < 0.05 and/or confidence interval excludes 1.0).
Meta-analysis
For the meta-analyses, all included studies were controlled, at minimum, for age, gender, race/ethnicity. Menthol cigarette use was defined as either self-reported menthol use, current use, usual cigarette/brand used, or remaining with menthol cigarettes through the length of the study. Subgroup analysis was conducted to compare differences between study designs (prospective cohort and cross-sectional designs in abstinence [no duration]) and differences in measures (past year and ever quit attempt [ever quit attempts, any quit attempts between 2001 and 2005, and any quit attempts in the past 2, 3, or 5 years]). Further, sensitivity analyses were also completed according to race/ethnicity and abstinence verification (eCO verified), when possible. Pooled adjusted odds ratios (AORs) and 95% confidence intervals (CIs) with two-sided P values are reported from random-effects models utilizing the DerSimonian and Laird method [16] to measure the likelihood of reporting having made a quit attempt and abstaining among menthol compared to non-menthol smokers. Variations among pooled studies were assessed using Cochran’s Q statistic and heterogeneity was quantified using the inconsistency index (I2). A p value less than 0.10 was considered significant. I2 expresses the percent of variability in point estimates due to heterogeneity and results here follow the categories of low (I2 = 25%), moderate (I2 = 50%), and high (I2 = 75%) [17]. All data were analyzed through Review Manager version 5.3 [18].
Strength of evidence evaluation
Recognizing the inherent limitations when assessing confidence in empirical conclusions based on observational data [19,20,21,22], the Agency for Healthcare Research and Quality (AHRQ) Evidence-Based Practice Center (EPC) approach – based largely on the methods developed by the Grading of Recommendations Assessment, Development and Evaluation (GRADE) Working Group [23] – was deemed acceptable for this review. Strength of evidence for this review was evaluated based on the four required domains:
-
1)
Study limitations (previously called risk of bias) – The degree to which included studies for a given outcome have a high likelihood of adequate protection against bias (ie, good internal validity), assessed through two main elements, study design and study conduct.
-
2)
Directness – Whether evidence links interventions directly to a health outcome of specific importance for the review and, for comparative studies, whether the results are based on head-to-head comparisons.
-
3)
Consistency – The degree to which included studies find either the same direction or similar magnitude of effect, as assessed by direction of effect and/or magnitude of effect.
-
4)
Precision – The degree of certainty surrounding an effect estimate with respect to a given outcome, based on the sufficiency of sample size and number of events.
Reporting bias is one of the strength of evidence (SOE) domains typically assessed for systematic reviews, but the methods used to detect such bias are designed for use with controlled trials. Although observational studies may be susceptible to reporting bias, no comparable methods exist for assessing reporting bias for these study designs. As a result, reporting bias was not assessed for the purposes of this systematic review, which comprised of only observational studies, in accordance with methodological recommendations [24].
For this review, the SOE was assessed in two ways for each outcome measure. First, SOE was assessed for the studies that adjusted for the key confounders of age, race/ethnicity, and gender (through multivariable modeling, sample stratifications, or predefined study inclusion criteria). These results minimized the potential for confounding bias, represented the “best evidence,” and thus may be more likely to represent the “true” association between menthol cigarette use and smoking behaviors.
Next, a sensitivity analysis was conducted to include the results from analyses that did not control for the key confounders. The unadjusted results reflected the effect of menthol cigarette use but allow all other variables—measured and unmeasured—to vary, potentially obscuring the actual effect of menthol smoking.
In both SOE assessments, measures with “acceptable” reliability and/or validity were weighed more heavily than the “inconclusive” measures (to minimize the impact of misclassification bias).
The final SOE judgment was necessarily qualitative but reflected a sound, reasoned weighing of domain ratings.
The overall strength of the body of evidence was graded as “high,” “moderate,” “low,” or “insufficient” using the Evidence-Based Practice Center (EPC) approach (Table 2).
Sensitivity analysis
Additionally, three sensitivity analyses were conducted in order to evaluate the SOE, to include: limitation of the study pool to those that also adjusted for meso- and/or macro-level variables; exclusions of “poor” quality studies (according to the Downs and Black study quality assessment); and exclusion of studies with potential overadjustment and/or inappropriate adjustment.
Results
A total of 73 studies, reported in 81 unique references, evaluated the potential associations between menthol cigarette use and smoking cessation. Adjusted studies were considered a higher level of evidence and, therefore, all subsequent analyses were restricted to studies that adjusted for key demographic characteristics. A total of 43 studies, reported in 47 unique references, provided adjusted data for relevant smoking cessation outcomes; complete study characteristics are shown in Table 3. The definitions of the specific outcome measures for smoking cessation applied across the adjusted studies are presented in SUPPLEMENTAL SECTION 3: Outcome Measures for Smoking Cessation across Adjusted Studies.
Table 4 contains a summary of the identified published assessments of the psychometric foundations for the smoking cessation measures. Empirical data regarding reliability or validity qualified four of the five smoking cessation measures (duration of abstinence, quit attempts, rate of abstinence/quitting, change in smoking quantity/frequency) as “acceptable”.
Synthesis of the best available evidence
Summaries of the best available evidence — controlling for age, race/ethnicity, and gender — are presented by outcome measure below. Outcome measures are presented with a corresponding overview table for each measure in the following order: duration of abstinence; quit attempts; rate of abstinence/quitting; change in smoking quantity/frequency; and return to smoking/relapse. Where two references reported the same data, the most recent publication was used as the data source. The complete data extraction for all included adjusted studies can be found in SUPPLEMENTAL SECTION 4: Evidence Table, Modeled / Adjusted Results.
Duration of abstinence
Two studies, presented in Table 5, reported duration of abstinence.
Levy at al [46]. reported significantly lower odds of being a “recent” and “long-term” quitter for menthol compared with non-menthol smoking, across all models (AORs ranged from 0.92 to 0.97 across models). Cubbin et al. [29] reported duration of abstinence for six gender-race/ethnicity interactions, yielding only one significant finding that suggested White female menthol smokers had been abstinent significantly longer than White female non-menthol smokers (14.8 years vs. 12.5 years; p < 0.01). Given the limited number of studies and the inconsistent findings reported for this measure, an association between menthol cigarette use and duration of abstinence is unclear and undefined in the evidence base.
Quit attempts (any quit attempts; number of quit attempts per person)
Fifteen studies (from 16 references), as presented in Table 6, reported measures of quit attempts.
Kahende at al [42]. reported White menthol smokers had significantly lower odds than White non-menthol smokers of having made a past-year quit attempt (AOR = 0.91, 95% CI: 0.84 to 0.99; p < 0.05).
Ten studies (from 11 references) found no difference between menthol and non-menthol smokers in terms of having made at least one quit attempt (within various timeframes), across all models and subgroup analyses/stratifications performed [25, 29, 33, 41, 43, 53,54,55, 63, 64, 70]. In addition, Stahre et al. [65] found no significant difference in the odds of using any type of quit aid between menthol and non-menthol current smokers, nor menthol and non-menthol former smokers.
Three studies reported mixed findings. Levy et al. [46] reported that menthol cigarette smokers had significantly higher odds of past-year quit attempts compared to non-menthol users (AOR = 1.03, 95% CI: 1.02 to 1.03; p < 0.001); this result remained unchanged when adding nicotine dependence to the model. However, a third model (adjusting for additional, unspecified covariates) reported significantly lower odds of past year quit attempts among menthol cigarette smokers (AOR = 0.98, 95% CI: 0.98 to 0.98). In Keeler et al. [44], the overall odds of past-year quit attempts between menthol and non-menthol smokers were no different. Both the 2017 and 2018 studies by Keeler at al [44, 45]. found that, among black smokers, menthol users were significantly more likely to report past-year quit attempts (2018: AOR = 1.39, 95% CI: 1.16 to 1.67; p < 0.001; 2017: AOR = 1.37, 95% CI: 1.16 to 1.61; p = 0.0002); no such differences were reported for other racial/ethnic subgroups. The majority of the results from these 14 studies reported no differences between menthol and non-menthol smoking in terms of quit attempts.
Rate of abstinence/quitting
Twenty-nine studies (from 33 references), presented below in Table 7, reported on rate of abstinence/quitting outcomes.
Four studies found that menthol smokers had significantly lower odds of quitting than non-menthol smokers; two studies reported 7-day PPA (between weeks 14 and 26 [61]; and at the previous 7 days and at week 7 [34]), while two studies examined cessation at different time points (1 year abstinence from purchasing a pack of cigarettes [47]; and abstinence at 3 to 6 week follow-up [68]).
Sixteen studies (from 18 references) found no difference in the rate of abstinence between menthol and non-menthol smokers, both overall and within subgroup analyses, in terms of: 7-day PPA in six studies [28, 35, 36, 52, 66, 71]; 30-day PPA in one study [30]; quit rates from baseline to follow-up in three studies from four references [40, 41, 50, 54]; cessation of greater than 3 months in two studies [44, 45]; PA in two studies [56, 57]; successful cessation between two survey waves in one study from two references [63, 64]; and past-year abstinence in one study [49].
Nine studies (from 11 references), reported mixed significance [27, 31, 32, 37, 39, 51, 58,59,60, 67, 69]. Using NHIS data, Sulsky et al. [67] found that White menthol and non-menthol regular and daily smokers were no different in odds of past-year abstinence; similar results were observed in Black menthol and non-menthol daily smokers. Using TUS-CPS data, the authors found no significant difference in one- to three-year abstinence between White menthol and non-menthol smokers (both regular and daily). For other race/ethnicities, no difference was detected between menthol and non-menthol use in terms of abstinence among regular and daily smokers. However, for Black daily (AOR = 0.89, 95% CI: 0.81 to 0.98) and regular (AOR = 0.87, 95% CI: 0.80 to 0.95) smokers, menthol use was significantly associated with lower odds of abstinence.
Reitzel et al. [60] found that menthol and non-menthol smokers were no different in terms of short-term abstinence for the overall sample. However, among White participants, menthol use predicted a significant decrease in short-term abstinence (β = − 1.56, SE = 0.79; χ2 = 3.96; p = 0.05) as well as 7-day PPA (β = − 1.60, SE = 0.79; χ2(1) = 4.06; p = .04; n = 132). No such differences were reported for either outcome among Black participants (short-term abstinence: β = 0.54, SE = 0.55; p = 0.33; and 7-day PPA: β = 1.00, SE = 0.67; p = 0.11).
Blot et al. [27] found that White menthol smokers had significantly greater odds of having quit compared with non-menthol smokers (AOR = 1.55, 95% CI: 1.41 to 1.70); however, Black menthol and non-menthol smokers were no different.
Trinidad et al. [69] reported that, among White, Black, Asian-American/Pacific Islander, and Hispanic participants, menthol smoking was associated with significantly lower odds of abstinence greater than 6 months (AORs ranged from 0.28 to 0.48). However, among Native American/Alaskan native participants, menthol and non-menthol smokers were no different in terms of the odds of abstinence greater than 6 months.
Delnevo et al. [31, 32] reported on the odds of being a former smoker across five racial/ethnic subgroups and the following five sample restrictions (according to past and current smoking status): former smokers who quit within the past 5 years and all current smokers (regardless of quit attempt history); former smokers who quit within the past 5 years and all current smokers (regardless of quit attempt history), both of whom currently do not use other tobacco products; former smokers who quit within the past 5 years and current smokers who reported ever having made a quit attempt; former smokers who quit within the past 5 years and current smokers who reported ever having made a quit attempt, both of whom currently do not use other tobacco products; and, past 12-month cigarette smokers who made a quit attempt or quit (i.e., former smokers). Among the overall sample, across four of the five restrictions, menthol cigarette smokers were significantly less likely than non-menthol smokers to be former smokers with AORs ranging from 0.90 to 0.92.
Black menthol smokers were significantly less likely to be former smokers compared to Black non-menthol smokers in all five restrictions with AORs ranging from 0.68 to 0.81. White menthol, versus non-menthol, smokers were significantly less likely to be a former smoker across three restrictions. However, Hispanic menthol and non-menthol smokers were no different across four of the five restrictions; and, were significantly less likely to be a former smoker in one restriction.
In Reitzel’s ‘Project Mom’ [58, 59], menthol cigarette use did not predict continuous abstinence from smoking. However, among White women, menthol smokers were significantly less likely to maintain continuous abstinence compared to non-menthol smokers (AOR = 0.19, 95% CI: 0.04 to 0.89).
Gandhi et al. [37] found no difference between White menthol and non-menthol smokers in odds of abstinence at both 4 weeks and 6 months. Black menthol smokers had significantly lower odds of abstinence compared to Black non-menthol smokers at both time points, 4 weeks (measured by 7-day PPA) (AOR = 0.32, 95% CI: 0.16 to 0.62) and at 6 months post-quit (AOR = 0.48, 95% CI: 0.25 to 0.90). Hispanic menthol smokers had significantly lower odds of abstinence at 4 weeks compared to Hispanic non-menthol smokers (AOR = 0.43, 95% CI: 0.1 to 0.9); at 6 months, Hispanic menthol and non-menthol smokers were no different in odds of abstinence.
Gundersen et al. [39] suggested no significant difference in being a former smoker between menthol and non-menthol smokers in the overall sample, and among Black smokers. However, odds of being a former smoker were significantly higher for White menthol compared to White non-menthol smokers (AOR = 1.17, 95% CI: 1.00 to 1.36; p < 0.05). Odds of being a former smoker were significantly lower for Hispanic menthol compared to Hispanic non-menthol smokers (AOR = 0.61, 95% CI: 0.39 to 0.97; p = 0.04), and for non-White menthol compared to non-White non-menthol smokers (AOR = 0.55, 95% CI: 0.43 to 0.71; p < 0.01).
Okuyemi et al. [51] reported no significant difference in odds of quitting between menthol and non-menthol smokers among adults ≥50 years of age; however, in adults < 50 years of age, the odds of quitting for menthol smokers were significantly lower for menthol smokers (AOR = 2.02, 95% CI: 1.03 to 3.95).
Across the 28 studies, the majority of studies (15 studies) found no difference between menthol and non-menthol smokers in the rate of abstinence. Four studies reported that menthol smokers were significantly less likely to quit smoking and nine studies reported results of mixed significance based on various stratifications. Overall, the evidence for this outcome was inconsistent for the association between menthol cigarette use and the rate of abstinence/quitting.
Change in smoking quantity/frequency
Five studies (from six references), presented in Table 8, provided adjusted analysis of change in smoking quantity/frequency.
Azagba et al. [26] found that menthol cigarette smokers had significantly higher odds of using cigarettes at least 10 days (versus (1–9 days) in the past 30 days compared with non-menthol cigarette smokers, in the full sample (AOR = 1.48, 95% CI, 1.14 to 1.94; p < 0.05) and among both middle (AOR = 2.36, 95% CI, 1.01 to 5.49; p < 0.05) and high school students (AOR = 1.41, 95% CI, 1.09 to 1.82; p < 0.05). Similarly, menthol cigarette smokers had significantly higher odds of using at least 20 days (versus (1–19 days) in the past 30 days compared with non-menthol cigarette smokers, in the full sample AOR = 1.62, 95% CI, 1.15 to 2.28; p < 0.05) and among both middle (AOR = 3.76, 95% CI, 1.21 to 11.71; p < 0.05) and high school students (AOR = 1.49, 95% CI, 1.07 to 2.07; p < 0.05).
One study, from two references [40, 41], reported no difference between menthol and non-menthol cigarette smokers for changes in smoking frequency; similarly, one study reported that cigarettes per day (CPD) was not significantly associated with menthol cigarette use [38].
Two studies reported mixed significance. Reitzel [58] found that Black female menthol smokers reported substantially less cigarette reduction (measured by CPD) over the course of 26 weeks (β = 3.82, SE = 3.77; p = 0.02; n = 71), but no difference was found in changes in smoking frequency for the overall sample. Sawdey et al. [62] found no significant difference in the odds of moderate smokers (on 6 to 19 days in the past 30 days) being menthol versus non-menthol smokers (AOR = 1.17, 95% CI: 0.86–1.59); however, the odds of frequent smokers (on ≥20 days in the past 30 days) being menthol smokers was significantly higher than being non-menthol smokers (AOR = 1.57, 95% CI: 1.08–2.29). The overall p = value across both groups was non-significant (p = 0.064).
The overall evidence base for this outcome was limited by the small number of included studies, and the mixed significance of findings across studies precludes clear conclusions from the available evidence.
Return to smoking/relapse
Two studies, presented in Table 9, provided analyses of return to smoking/relapse.
In Muench and Juliano [48], menthol smokers were at a significantly greater risk of lapsing compared with non-menthol smokers, in both the univariate regression (AOR = 3.474, p < 0.05) and lapse survival curve analyses (HR = 2.798, Wald statistic = 2.79; p = 0.048). Pletcher et al. [54] reported that young adult menthol smokers had a significantly higher likelihood of returning to smoking, compared to non-menthol smokers (AOR = 1.89, 95% CI: 1.17 to 3.05; p = 0.009).
These results suggest a higher likelihood of menthol smokers relapsing. However, the small number of studies—neither based on nationally representative samples—limit the generalizability of the findings.
Sensitivity analyses
Three sensitivity analyses were conducted in order to test whether the results differed after more stringent inclusion and exclusion criteria were applied. Overall, results from the sensitivity analyses suggested little to no change. Full details on the sub-group analysis and sensitivity analyses are provided in SUPPLEMENTAL SECTION 5: Sensitivity Analyses.
Results of meta-analyses
After screening all included adjusted studies, pooled data were included and extracted for two outcome measures: nine studies in the meta-analyses for quit attempts [25, 33, 41, 42, 44, 46, 53, 54, 70] and 12 studies for abstinence [27, 32, 34,35,36,37, 39, 51, 52, 54, 61, 66]. Full details are provided in SUPPLEMENTAL SECTION 6: Characteristics, Definitions, and Covariates of Studies Included in the Meta-Analysis.
Adjusted odds of reporting a quit attempt (past year or ever)
Results from five studies were pooled to measure the association of menthol use and past year quit attempts. Pooled results from five studies (Fig. 2) showed a significant association between menthol, versus non-menthol, cigarette use and the increasing odds for past year quit attempts (OR = 1.02, 95% CI: 1.01 to 1.03, p-value = 0.003, I2 = 1%). However, pooled result was different for a group of studies measuring ever quit attempts (Alexander et al. [2010]: AOR = 0.98, 95% CI: 0.83 to 1.15), any quit attempts between 2001 to 2005 (Hyland and Rivard, [2010]: AOR = 0.91, 95% CI: 0.72 to 1.15), and any quit attempt in the past 2, 3, or 5 years (Pletcher et al. [2006]: 0.77, 95% CI: 0.57 to 1.06), finding no significant difference in the odds of making a quit attempt among menthol users compared to non-menthol cigarette smokers (OR = 0.93, 95% CI, 0.82 to 1.05, p = 0.23, I2 = 0%; Fig. 3) [25, 41, 54]. In a subgroup analysis of the five studies with past year quit attempts as one group, and the group of three studies measuring ever quit attempts, any quit attempts between 2001 to 2005, and any quit attempt in the past 2, 3, or 5 years (Fig. 4), results remained non-significant (OR = 1.01, 95% CI: 0.98 to 1.04, p = 0.49, I2 = 14%). Test for subgroup difference showed moderate heterogeneity (I2 = 55.4%).
Results from two studies were pooled to measure for the association of menthol cigarette use and quit attempts (past year and quit attempts between 2001 and 2005) among Black participants (Fig. 5) [41, 44]. Pooled results showed a significant increase in the odds of Black menthol, versus non-menthol, smokers reporting quit attempts (OR = 1.37, 95% CI: 1.17 to 1.61, p = 0.00001, I2 = 14%). In contrast, among White menthol respondents in three studies (Fig. 6), the odds of making a quit attempt were significantly lower for menthol compared to non-menthol smokers (OR = 0.95, 95% CI: 0.91 to 0.99, I2 = 0%) [41, 42, 44].
Adjusted odds of abstinence (no definition and 7-day PPA)
Four studies presented results for the association of menthol use and abstinence (self-reported) with no specified duration of abstinence. Two of the studies were cross-sectional in design [32, 39], and two were prospective cohort [27, 54]. Pooled results of cross-sectional studies showed that odds of abstinence with no defined duration among menthol smokers compared to non-menthol smokers was not significant (OR = 0.96, 95% CI: 0.84 to 1.10, p = 0.58, I2 = 71%). A non-significant result was likewise found in synthesis of prospective cohorts (OR = 0.88, 95% CI: 0.62 to 1.27, p = 0.50, I2 = 70%). Synthesizing the results of the four studies showed that the association of abstinence with no defined duration among menthol smokers compared to non-menthol smokers was not significant (OR = 0.96, 95% CI: 0.86 to 1.06, p = 0.41, I2 = 60%; Fig. 7). Test of subgroup differences between both groups (cross-sectional and longitudinal) manifested low heterogeneity (I2 = 0%).
Three studies presented results for the association of menthol use and abstinence with no specified duration of abstinence for Black participants [27, 32, 39]. Pooled results (Fig. 8) showed that the association between abstinence with no defined duration among menthol smokers compared to non-menthol smokers was not significant (OR = 0.90, 95% CI: 0.73 to 1.10, p = 0.29, I2 = 73%). Studies likewise allowed for analysis of association of menthol use and abstinence from smoking with no specified duration of abstinence for White participants [27, 32, 39]. Similar to Black participants, among White participants, results showed that the association of abstinence with no defined duration among menthol smokers compared to non-menthol smokers was not significant (OR = 1.19, 95% CI: 0.83 to 1.69, p = 0.34, =98%; Fig. 9). The heterogeneity was noted to be high for this analysis.
Four cohort studies presented results for the association of menthol use and abstinence from smoking measured by 7-day PPA. For purposes of the following analysis, the studies were grouped by their specific research design. Two of the studies were analyses of RCT by design [36, 61], and two were cohort in nature [35, 66]. Seven-day PPA was self-reported at 4 weeks follow-up for Foulds et al. [35], self-reported at 2 years for Fu et al. [36], and self-reported and eCO verified for Steinberg et al. [66] and Rojewski et al. [61] at 26 weeks follow-up. All published AORs in the study used in the meta-analysis were standardized to have non-menthol use as the reference group [35, 61]. Pooled results of analyses from all four studies (Fig. 10) showed that odds of 7-day PPA among menthol smokers compared to non-menthol smokers was not significant (OR = 0.88, 95% CI: 0.59 to 1.30, p = 0.52, I2 = 70%). Similarly, results of longitudinal studies alone and RCT studies alone (Fig. 10) showed that odds of 7-day PPA among menthol smokers compared to non-menthol smokers was not significant (cohort: OR = 0.83, 95% CI: 0.61 to 1.14, p = 0.25, I2 = 30%; RCT: OR = 0.78, 95% CI: 0.25 to 2.45, p = 0.67, I2 = 84%). Test for subgroup difference showed low heterogeneity (I2 = 0%).
Four studies presented results for the association of menthol use and 7-day PPA among Black participants [34, 37, 51, 52]. For the four studies, 7-day PPA was self-reported at 4 weeks follow-up for Gandhi et al. [37], self-reported at 6 weeks for Okuyemi et al. [51], cotinine verified (cut-off< 15 ng/ml) for Faseru et al. [34] at 7 weeks follow-up, and cotinine verified (cut-off< 20 ng/ml) and eCO verified (< 10 ppm) for Okuyemi et al. [52] at 26 weeks follow-up. All published AORs in the study used in the meta-analysis were standardized to have non-menthol use as the reference group [34, 51, 52]. Results showed that the odds for Black menthol smokers exhibiting 7-day PPA were significantly lower when compared to Black non-menthol smokers (OR = 0.52, 95% CI: 0.38 to 0.70, p < 0.0001, I2 = 0; Fig. 11).
A sensitivity analysis was conducted for eCO verified 7-day PPA (≤10 ppm) with two studies (Fig. 12) [61, 66]. Rojewski et al. [61] was standardized to have non-menthol use as the reference group. Meta-analysis results showed that the odds for eCO verified 7-Day PPA among menthol smokers compared to non-menthol smokers was not significant (OR = 0.70, 95% CI: 0.28 to 1.70, p = 0.42, I2 = 71%).
Stength of evidence
Table 10 provides the SOE for the outcome measures used in the current review to examine the association between menthol cigarette use and cessation outcomes. Most measures were “indirect” and limited by the varying and/or undefined measures of abstinence. As presented in Table 11, the overall strength of evidence for an association between menthol cigarette use and smoking cessation was graded as “low” based on deficiencies in the available evidence base.
Discussion
The findings in this systematic review differ from several existing literature reviews on this topic. The 2013/2015 FDA Report/Addendum [6, 7] concluded that menthol in cigarettes was “likely associated with reduced success in smoking cessation, especially among Black menthol smokers.” That finding was not supported by this newer, more comprehensive review. Similarly, the evidence that contributed to this review does not support the conclusion in the 2011 Report by the FDA’s Tobacco Products Scientific Advisory Committee [5] that “[e] vidence is sufficient to conclude that a relationship is more likely than not that the availability of menthol cigarettes results in lower likelihood of smoking cessation in Blacks.”
Studies in the qualitative synthesis of this review were considered to provide the best available evidence on any differential impact of menthol versus non-menthol cigarette use on smoking cessation. Across studies, a variety of sampling and recruitment methods were used with varying definitions of current smoking and abstinence, and a range of study designs that, in many instances, did not directly address the current research question. Further, the available studies provided evidence that was inconsistent and imprecise—both across studies and within the same study.
Analyses of large cross-sectional studies yielded inconsistent findings. Among studies that used data from nationally representative samples, TUS-CPS and NHIS, population and sub-population results were mixed, based on modeling variation or definitions used; specifically, significantly positive and negative associations between menthol cigarette use and smoking cessation were reported, as well as numerous non-significant findings.
Clinical trials are designed to assess associations between interventions and outcomes, providing the temporal component that cross-sectional data lack. No clinical trials included in this review were designed with menthol cigarette use as the “intervention” to which participants were assigned. Therefore, these studies were re-classified as short-term prospective cohort studies. There was no consistent pattern of a differential impact of menthol versus non-menthol cigarette use on smoking cessation, even when data were stratified by type of cessation intervention, duration of intervention and follow up, or definition of outcome measure (including biochemical validation of self-reported abstinence). Both the shortest (6 weeks) and the longest (12 months) clinical studies found mixed or equivalent results. In addition, trials of cessation inherently include self-selected participants at least interested or motivated to quit smoking. Relying solely—or mainly—on clinical trial data to draw conclusions about the association between menthol cigarette use and smoking cessation will yield a result with limited generalizability to the overall smoking population.
The included prospective studies varied in follow-up duration — a critical factor in assessing the durability of cessation. Of the 11 prospective cohort studies that reported cessation, nine reported outcomes at 6 months or longer post-baseline. Specifically, three reported outcomes at 6 to 12 months, one followed participants for 1 to 2 years, one followed participants for 3 to 5 years, and four assessed outcomes beyond 5 years post-baseline. Two of the three 6- to 12-month cohort studies included a cessation intervention of some form — 7-day and 30-day PPA. The third 6- to 12-month cohort study reported continuous abstinence.
In the longer-term cohort studies, results were of mixed significance. COMMIT (a community-based public health intervention conducted in 11 matched pairs of communities) assessed menthol smoking at baseline in 1988; participants were interviewed again in 1993, 1998, 2001, and 2005. Investigators found no difference between menthol, versus non-menthol, smokers and smoking cessation during 17 years of follow up. The CARDIA study, a cohort of young adults at baseline, found no association between menthol cigarette use and cessation at 15-year follow up. However, a significantly positive association between menthol cigarette use and the risk of smoking relapse was identified. Finally, a study that investigated the association between menthol smoking and quit rate found that menthol smokers had a significantly lower likelihood of quitting compared with non-menthol smokers.
Return to smoking/relapse and change in smoking quantity/frequency were each reported by only two studies. Data were too limited to draw a reliable conclusion about the association between menthol cigarette use and either measure. Quit attempts — making at least one attempt and the number of quit attempts per person — were reported by several studies, but the measure does not reflect actual cessation. Given the lack of a significant difference between menthol and non-menthol smokers on either measure of quit attempts and the empirical uncertainty of the association between making a quit attempt or the number of quit attempts and actual cessation, there is no confident conclusion that can be drawn regarding an association with menthol smoking.
Pooled data for the meta-analyses were extracted for two outcome measures, quit attempts and abstinence. Pooled results from five studies suggested a significant association between menthol cigarette use and increased odds for past year quit attempts. However, pooled data from three studies measuring ever quit attempts found no difference between menthol and non-menthol smokers in the odds of making a quit attempt. Pooling data from all eight studies revealed no consistent differences.
Additional analysis of pooled data from two studies presenting results on quit attempts among Black participants showed that Black menthol, versus non-menthol, smokers were significantly more likely to make a quit attempt. Further, pooled data from three studies suggested that White menthol, versus non-menthol, smokers were significantly less likely of making a quit attempt.
Four cohort studies presented results for examining the association between menthol use and abstinence, with no specified duration. Pooled results showed no difference between menthol and non-menthol smokers in terms of abstinence, even in sub-analyses of Black and White participants, using data from three of the four studies.
Across all four cohort studies, pooled results on the association between menthol use and abstinence, again with no specified duration, showed no difference between menthol and non-menthol smokers, overall, in the odds of abstinence. However, when measuring abstinence by 7-day PPA, pooled data suggest that Black menthol smokers were significantly less likely than Black non-menthol smokers to be abstinent. Recognizing inconsistent results were reported across studies in the qualitative synthesis, meta-analytic results, generally, showed no difference between menthol cigarette use and quit attempts (pooled results from ever, past year quit attempts, any quit attempts between 2001 to 2005, and any quit attempt in the past 2, 3, or 5 years), abstinence with no defined duration, and 7-day PPA.
Limitations
This systematic review was conducted according to established methodological standards and with inherent limitations. For example, the variation in the definitions of several outcome measures made it difficult to summarize results, which limited the reviewers’ ability to draw confident conclusions. Most of the smoking behavior data were self-reported. However, any differential impact of reliance on self-reported data was expected to be minimal. The Downs and Black checklist has some limitations when applied across a variety of study designs. Furthermore, a study’s quality score on the Downs and Black checklist may reflect the quality of reporting rather than the quality of the study as conducted. Finally, the conclusions in this review are based on studies conducted in the U.S. and may or may not be generalizable to other countries due to the potential impact of important influences, such as cultural norms, smoking policies, and taxes on smoking behaviors outside of the U.S.
Conclusions
In summary, the findings of this systematic review suggest that the current evidence base is not strong or consistent enough to support a clear association—positive or negative—between menthol cigarette use and smoking cessation. Having comprehensively reviewed the available literature, this review—which included nearly three times the number of studies as the 2013 FDA Report and 2015 Addendum, including 16 studies that analyzed data among Black smokers only—recommends that future studies assessing the association between menthol cigarette smoking and smoking behaviors can be strengthened in several ways. Specifically, longitudinal data that measures cessation for 12 months or longer to reflect more sustained measures of cessation and adjusting for key demographic variables, at a minimum, will provide more insight into the potential association of menthol cigarette smoking and smoking cessation. Further, given the transparent, comprehensive, and objective approach taken in this review, it is the authors’ hope that these findings—as well as findings from their continued monitoring of the literature—will inform future policy decision-making, as well as influence the methodological approach of future systematic reviews towards an equivalent degree of strict methodological rigor.
Availability of data and materials
All data and materials considered in this review are publicly available.
Abbreviations
- AOR:
-
Adjusted odds ratio
- BRFSS:
-
Behavioral Risk Factor Surveillance System
- CARDIA:
-
Coronary Artery Risk Development in Young Adults
- CI:
-
Confidence interval
- COMMIT:
-
Community Intervention Trial for Smoking Cessation
- CPD:
-
Cigarettes per day
- CQ:
-
Contextual Question
- EPC:
-
Evidence-Based Practice Center
- HR:
-
Hazard ratio
- ITC-4:
-
International Tobacco Control Four Country Survey
- KIS:
-
Kick it at Swope
- KQ:
-
Key Question
- LYAC:
-
Legacy Young Adult Cohort Study
- NA:
-
Not Applicable
- NHIS:
-
National Health Interview Survey
- NHIS-CCS:
-
National Health Interview Survey Cancer Control Supplement
- PA:
-
Prolonged abstinence
- PATH:
-
Population Assessment of Tobacco and Health
- POR :
-
Prevalence odds ratio
- PPA:
-
Point (or period) prevalence abstinence
- PR:
-
Prevalence ratio
- SE:
-
Standard error
- SOE :
-
Strength of Evidence
- TUS-CPS:
-
The Tobacco Use Supplement to the Current Population Survey
- U.S.:
-
United States
- WSHS:
-
Wisconsin Smokers Health Study
References
Caraballo RS, Asman K. Epidemiology of menthol cigarette use in the United States. Tob Induc Dis. 2011;9(Suppl 1):S1.
Centers for Disease Control and Prevention. Current Cigarette Smoking Among Adults — United States, 2011 Nov 9, 2012 [Available from: https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6144a2.htm].
Substance Abuse and Mental Health Services Administration. The NSDUH Report: Recent Trends in Menthol Cigarette Use. Rockville: Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality; 2011.
Administration UFaD. FDA commits to evidence-based actions aimed at saving lives and preventing future generations of smokers. Washington D.C.: US Food and Drug Administration; 2021. [updated 29 April. Available from: https://www.fda.gov/news-events/press-announcements/fda-commits-evidence-based-actions-aimed-saving-lives-and-preventing-future-generations-smokers]
Tobacco Products Scientific Advisory Committee. Menthol Report and Recommendation 2011 [Available from: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/TobaccoProductsScientificAdvisoryCommittee/UCM269697.pdf].
U.S. Food and Drug Administration. Preliminary scientific evaluation of the possible public health effects of menthol versus nonmenthol cigarettes 2013 [Available from: http://www.fda.gov/downloads/ScienceResearch/SpecialTopics/PeerReviewofScientificInformationandAssessments/UCM361598.pdf].
U.S. Food and Drug Administration. Reference addendum: preliminary scientific evaluation of the possible public health effects of menthol versus non-menthol cigarettes 2015 [Available from: http://www.fda.gov/downloads/ScienceResearch/SpecialTopics/PeerReviewofScientificInformationandAssessments/UCM362600.pdf].
Stanton CA, Papandonatos G, Lloyd-Richardson EE, Niaura R. Consistency of self-reported smoking over a 6-year interval from adolescence to young adulthood. Addiction. 2007;102(11):1831–9. https://doi.org/10.1111/j.1360-0443.2007.01974.x.
Soulakova JN, Hartman AM, Liu B, Willis GB, Augustine S. Reliability of adult self-reported smoking history: data from the tobacco use supplement to the current population survey 2002-2003 cohort. Nicotine Tob Res. 2012;14(8):952–60. https://doi.org/10.1093/ntr/ntr313.
Smith PH, Assefa B, Kainth S, Salas-Ramirez KY, McKee SA, Giovino GA. Use of mentholated cigarettes and likelihood of smoking cessation in the United States: a meta-analysis. Nicotine Tob Res. 2020;22(3):307-16.
Kandel DB, Kiros G-E, Schaffran C, Hu M-C. Racial/ethnic differences in cigarette smoking initiation and progression to daily smoking: a multilevel analysis. Am J Public Health. 2004;94(1):128–35. https://doi.org/10.2105/AJPH.94.1.128.
Yi Z, Mayorga ME, Hassmiller Lich K, Pearson JL. Changes in cigarette smoking initiation, cessation, and relapse among U.S. adults: a comparison of two longitudinal samples. Tobacco Induced Dis. 2017;15:17.
McLeroy KR, Bibeau D, Steckler A, Glanz K. An ecological perspective on health promotion programs. Health Educ Q. 1988;15(4):351–77. https://doi.org/10.1177/109019818801500401.
Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52(6):377–84. https://doi.org/10.1136/jech.52.6.377.
International Agency for Research on Cancer. Section 3.3 Measurement of nicotine dependence. Methods for Evaluating Tobacco Control Policies IARC Handbook of Cancer Prevention, vol. 12. Lyon: World Health Organization; 2008.
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88. https://doi.org/10.1016/0197-2456(86)90046-2.
Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–58. https://doi.org/10.1002/sim.1186.
The Nordic Cochrane Centre. Review manager (RevMan). The Cochrane collaboration. 5.3 ed. Copenhagen: The Cochrane Collaboration; 2014.
Wells GA, Shea B, Higgins JP, Sterne J, Tugwell P, Reeves BC. Checklists of methodological issues for review authors to consider when including non-randomized studies in systematic reviews. Res Synth Methods. 2013;4(1):63–77. https://doi.org/10.1002/jrsm.1077.
Schunemann HJ, Tugwell P, Reeves BC, Akl EA, Santesso N, Spencer FA, et al. Non-randomized studies as a source of complementary, sequential or replacement evidence for randomized controlled trials in systematic reviews on the effects of interventions. Res Synth Methods. 2013;4(1):49–62. https://doi.org/10.1002/jrsm.1078.
Norris SL, Moher D, Reeves BC, Shea B, Loke Y, Garner S, et al. Issues relating to selective reporting when including non-randomized studies in systematic reviews on the effects of healthcare interventions. Res Synth Methods. 2013;4(1):36–47. https://doi.org/10.1002/jrsm.1062.
Higgins JP, Ramsay C, Reeves BC, Deeks JJ, Shea B, Valentine JC, et al. Issues relating to study design and risk of bias when including non-randomized studies in systematic reviews on the effects of interventions. Res Synth Methods. 2013;4(1):12–25. https://doi.org/10.1002/jrsm.1056.
Group GW. Grading quality of evidence and strength of recommendations. BMJ. 2004;328(7454):1490.
Berkman ND, Lohr KN, Ansari MT, Balk EM, Kane R, McDonagh M, et al. Grading the strength of a body of evidence when assessing health care interventions: an EPC update. J Clin Epidemiol. 2015;68(11):1312–24. https://doi.org/10.1016/j.jclinepi.2014.11.023.
Alexander LA, Crawford T, Mendiondo MS. Occupational status, work-site cessation programs and policies and menthol smoking on quitting behaviors of US smokers. Addiction. 2010;105(Suppl 1):95–104. https://doi.org/10.1111/j.1360-0443.2010.03227.x.
Azagba S, King J, Shan L, Manzione L. Cigarette smoking behavior among menthol and nonmenthol adolescent smokers. J Adolesc Health. 2020;66(5):545–50. https://doi.org/10.1016/j.jadohealth.2019.11.307.
Blot WJ, Cohen SS, Aldrich M, McLaughlin JK, Hargreaves MK, Signorello LB. Lung cancer risk among smokers of menthol cigarettes. J Natl Cancer Inst. 2011;103(10):810–6. https://doi.org/10.1093/jnci/djr102.
Cropsey KL, Weaver MF, Eldridge GD, Villalobos GC, Best AM, Stitzer ML. Differential success rates in racial groups: results of a clinical trial of smoking cessation among female prisoners. Nicotine Tob Res. 2009;11(6):690–7. https://doi.org/10.1093/ntr/ntp051.
Cubbin C, Soobader MJ, LeClere FB. The intersection of gender and race/ethnicity in smoking behaviors among menthol and non-menthol smokers in the United States. Addiction. 2010;105(Suppl 1):32–8. https://doi.org/10.1111/j.1360-0443.2010.03191.x.
D'Silva J, Boyle RG, Lien R, Rode P, Okuyemi KS. Cessation outcomes among treatment-seeking menthol and nonmenthol smokers. Am J Prev Med. 2012;43(5 Suppl 3):S242–8. https://doi.org/10.1016/j.amepre.2012.07.033.
Delnevo CD, Gundersen DA, Hrywna M. Examining the relationship between menthol smoking and cessation using data from the 2003 and 2006/7 Tobacco Use Supplement: Center for Tobacco Surveillance and Evaluation Researc. New Jersey: University of Medicine & Dentistry of New Jersey - School of Public Health; 2010. p. 2011.
Delnevo CD, Gundersen DA, Hrywna M, Echeverria SE, Steinberg MB. Smoking-cessation prevalence among U.S. smokers of menthol versus non-menthol cigarettes. Am J Prev Med. 2011;41(4):357–65. https://doi.org/10.1016/j.amepre.2011.06.039.
Fagan P, Augustson E, Backinger CL, O'Connell ME, Vollinger RE Jr, Kaufman A, et al. Quit attempts and intention to quit cigarette smoking among young adults in the United States. Am J Public Health. 2007;97(8):1412–20. https://doi.org/10.2105/AJPH.2006.103697.
Faseru B, Nollen NL, Mayo MS, Krebill R, Choi WS, Benowitz NL, et al. Predictors of cessation in African American light smokers enrolled in a bupropion clinical trial. Addict Behav. 2013;38(3):1796–803. https://doi.org/10.1016/j.addbeh.2012.11.010.
Foulds J, Gandhi KK, Steinberg MB, Richardson DL, Williams JM, Burke MV, et al. Factors associated with quitting smoking at a tobacco dependence treatment clinic. Am J Health Behav. 2006;30(4):400–12. https://doi.org/10.5993/AJHB.30.4.6.
Fu SS, Okuyemi KS, Partin MR, Ahluwalia JS, Nelson DB, Clothier BA, et al. Menthol cigarettes and smoking cessation during an aided quit attempt. Nicotine Tob Res. 2008;10(3):457–62. https://doi.org/10.1080/14622200801901914.
Gandhi KK, Foulds J, Steinberg MB, Lu SE, Williams JM. Lower quit rates among African American and Latino menthol cigarette smokers at a tobacco treatment clinic. Int J Clin Pract. 2009;63(3):360–7. https://doi.org/10.1111/j.1742-1241.2008.01969.x.
Gubner NR, Williams DD, Pagano A, Campbell BK, Guydish J. Menthol cigarette smoking among individuals in treatment for substance use disorders. Addict Behav. 2018;80:135–41. https://doi.org/10.1016/j.addbeh.2018.01.015.
Gundersen DA, Delnevo CD, Wackowski O. Exploring the relationship between race/ethnicity, menthol smoking, and cessation, in a nationally representative sample of adults. Prev Med. 2009;49(6):553–7. https://doi.org/10.1016/j.ypmed.2009.10.003.
Hyland A, Garten S, Giovino GA, Cummings KM. Mentholated cigarettes and smoking cessation: findings from COMMIT. Tob Control. 2002;11(2):135–9. https://doi.org/10.1136/tc.11.2.135.
Hyland A, Rivard C. Analysis of mentholated cigarettes using the COMMIT data -- summary. New York: Department of Health Behavior, Roswell Park Cancer Institute; 2010.
Kahende JW, Malarcher AM, Teplinskaya A, Asman KJ. Quit attempt correlates among smokers by race/ethnicity. Int J Environ Res Public Health. 2011;8(10):3871–88. https://doi.org/10.3390/ijerph8103871.
Kasza KA, Hyland AJ, Bansal-Travers M, Vogl LM, Chen J, Evans SE, et al. Switching between menthol and nonmenthol cigarettes: findings from the U.S. cohort of the international tobacco control four country survey. Nicotine Tob Res. 2014;16(9):1255–65. https://doi.org/10.1093/ntr/ntu098.
Keeler C, Max W, Yerger V, Yao T, Ong MK, Sung H-Y. The Association of menthol cigarette use with quit attempts, successful cessation, and intention to quit across racial/ethnic groups in the United States. Nicotine Tob Res. 2017;19(12):1450–64. https://doi.org/10.1093/ntr/ntw215.
Keeler C, Max W, Yerger VB, Yao T, Wang Y, Ong MK, et al. Effects of cigarette prices on intention to quit, quit attempts, and successful cessation among African American smokers. Nicotine Tob Res. 2020;22(4):522-31.
Levy DT, Blackman K, Tauras J, Chaloupka FJ, Villanti AC, Niaura RS, et al. Quit attempts and quit rates among menthol and nonmenthol smokers in the United States. Am J Public Health. 2011;101(7):1241–7. https://doi.org/10.2105/AJPH.2011.300178.
Lewis M, Wang Y, Berg CJ. Tobacco control environment in the United States and individual consumer characteristics in relation to continued smoking: differential responses among menthol smokers? Prev Med. 2014;65:47–51. https://doi.org/10.1016/j.ypmed.2014.04.019.
Muench C, Juliano LM. Predictors of smoking lapse during a 48-hour laboratory analogue smoking cessation attempt. Psychol Addict Behav. 2017;31(4):415–22. https://doi.org/10.1037/adb0000246.
Muscat JE, Richie JP Jr, Stellman SD. Mentholated cigaettes and smoking habits in whites and blacks. Tob Control. 2002;11(4):368–71. https://doi.org/10.1136/tc.11.4.368.
Nonnemaker J, Hersey J, Homsi G, Busey A, Allen J, Vallone D. Initiation with menthol cigarettes and youth smoking uptake. Addiction. 2012;108(1):171–8. https://doi.org/10.1111/j.1360-0443.2012.04045.x.
Okuyemi KS, Ahluwalia JS, Ebersole-Robinson M, Catley D, Mayo MS, Resnicow K. Does menthol attenuate the effect of bupropion among African American smokers? Addiction. 2003;98(10):1387–93. https://doi.org/10.1046/j.1360-0443.2003.00443.x.
Okuyemi KS, Faseru B, Sanderson Cox L, Bronars CA, Ahluwalia JS. Relationship between menthol cigarettes and smoking cessation among African American light smokers. Addiction. 2007;102(12):1979–86. https://doi.org/10.1111/j.1360-0443.2007.02010.x.
Park J-Y. Tobacco use behaviors among vulnerable populations. Dissertation Abstracts Int: Section B: Sci Eng. 2017;77(12-B(E)):No-Specified.
Pletcher MJ, Hulley BJ, Houston T, Kiefe CI, Benowitz N, Sidney S. Menthol cigarettes, smoking cessation, atherosclerosis, and pulmonary function. Arch Intern Med. 2006;166(17):1915–22. https://doi.org/10.1001/archinte.166.17.1915.
Rath JM, Villanti AC, Williams VF, Richardson A, Pearson JL, Vallone DM. Patterns of longitudinal transitions in menthol use among US young adult smokers. Nicotine Tob Res. 2015;17(7):839–46. https://doi.org/10.1093/ntr/ntu247.
Reitzel LR. Menthol cigarettes, tobacco dependence, and smoking cessation: project BREAK FREE final report; 2011.
Reitzel LR. Menthol cigarettes, tobacco dependence, and smoking cessation: project CARE final report; 2011.
Reitzel LR. Menthol cigarettes, tobacco dependence, and smoking cessation: project MOM final report; 2011.
Reitzel LR, Nguyen N, Cao Y, Vidrine JI, Daza P, Mullen PD, et al. Race/ethnicity moderates the effect of prepartum menthol cigarette use on postpartum smoking abstinence. Nicotine Tob Res. 2011;13(12):1305–10. https://doi.org/10.1093/ntr/ntr095.
Reitzel LR, Li Y, Stewart DW, Cao Y, Wetter DW, Waters AJ, et al. Race moderates the effect of menthol cigarette use on short-term smoking abstinence. Nicotine Tob Res. 2013;15(5):883–9. https://doi.org/10.1093/ntr/nts335.
Rojewski AM, Toll BA, O'Malley SS. Menthol cigarette use predicts treatment outcomes of weight-concerned smokers. Nicotine Tob Res. 2014;16(1):115–9. https://doi.org/10.1093/ntr/ntt137.
Sawdey MD, Chang JT, Cullen KA, Rass O, Jackson KJ, Ali FRM, et al. Trends and associations of menthol cigarette smoking among US middle and high school students-National Youth Tobacco Survey, 2011-2018. Nicotine Tob Res. 2020;22(10):1726–35. https://doi.org/10.1093/ntr/ntaa054.
Schneller LM. Assessment of various delivery methods of menthol in cigarettes sold in the US. Cancer Science, Roswell Park Graduate Division; 2019.
Schneller LM, Bansal-Travers M, Mahoney MC, McCann SE, O'Connor RJ. Menthol cigarettes and smoking cessation among adult smokers in the US. Am J Health Behav. 2020;44(2):252–6. https://doi.org/10.5993/AJHB.44.2.12.
Stahre M, Okuyemi KS, Joseph AM, Fu SS. Racial/ethnic differences in menthol cigarette smoking, population quit ratios and utilization of evidence-based tobacco cessation treatments. Addiction. 2010;105(Suppl 1):75–83. https://doi.org/10.1111/j.1360-0443.2010.03200.x.
Steinberg MB, Bover MT, Richardson DL, Schmelzer AC, Williams JM, Foulds J. Abstinence and psychological distress in co-morbid smokers using various pharmacotherapies. Drug Alcohol Depend. 2011;114(1):77–81. https://doi.org/10.1016/j.drugalcdep.2010.06.022.
Sulsky SI, Fuller WG, Van Landingham C, Ogden MW, Swauger JE, Curtin GM. Evaluating the association between menthol cigarette use and the likelihood of being a former versus current smoker. Regul Toxicol Pharmacol. 2014;70(1):231–41. https://doi.org/10.1016/j.yrtph.2014.07.009.
Thihalolipavan S, Jung M, Jasek J, Chamany S. Menthol smokers in large-scale nicotine replacement therapy program. Am J Public Health. 2014;104(11):e3–4. https://doi.org/10.2105/AJPH.2014.302168.
Trinidad DR, Perez-Stable EJ, Messer K, White MM, Pierce JP. Menthol cigarettes and smoking cessation among racial/ethnic groups in the United States. Addiction. 2010;105(Suppl 1):84–94. https://doi.org/10.1111/j.1360-0443.2010.03187.x.
Webb Hooper M, Zhao W, Byrne MM, Davila E, Caban-Martinez A, Dietz NA, et al. Menthol cigarette smoking and health, Florida 2007 BRFSS. Am J Health Behav. 2011;31(1):3–14.
Winhusen TM, Adinoff B, Lewis DF, Brigham GS, Gardin JG 2nd, Sonne SC, et al. A tale of two stimulants: mentholated cigarettes may play a role in cocaine, but not methamphetamine, dependence. Drug Alcohol Depend. 2013;133(3):845–51. https://doi.org/10.1016/j.drugalcdep.2013.09.002.
Brigham J, Lessov-Schlaggar CN, Javitz HS, Krasnow RE, McElroy M, Swan GE. Test-retest reliability of web-based retrospective self-report of tobacco exposure and risk. J Med Internet Res. 2009;11(3):e35. https://doi.org/10.2196/jmir.1248.
Colby SM, Clark MA, Rogers ML, Ramsey S, Graham AL, Boergers J, et al. Development and reliability of the lifetime interview on smoking trajectories. Nicotine Tob Res. 2012;14(3):290–8. https://doi.org/10.1093/ntr/ntr212.
Borland R, Yong HH, O'Connor RJ, Hyland A, Thompson ME. The reliability and predictive validity of the heaviness of smoking index and its two components: findings from the international tobacco control four country study. Nicotine Tob Res. 2010;12(Suppl):S45–50. https://doi.org/10.1093/ntr/ntq038.
Brener ND, Kann L, McManus T, Kinchen SA, Sundberg EC, Ross JG. Reliability of the 1999 youth risk behavior survey questionnaire. J Adolesc Health. 2002;31(4):336–42. https://doi.org/10.1016/S1054-139X(02)00339-7.
Brigham J, Lessov-Schlaggar CN, Javitz HS, Krasnow RE, Tildesley E, Andrews J, et al. Validity of recall of tobacco use in two prospective cohorts. Am J Epidemiol. 2010;172(7):828–35. https://doi.org/10.1093/aje/kwq179.
Hughes JR, Carpenter MJ, Naud S. Do point prevalence and prolonged abstinence measures produce similar results in smoking cessation studies? A systematic review. Nicotine Tob Res. 2010;12(7):756–62. https://doi.org/10.1093/ntr/ntq078.
Velicer WF, Prochaska JO. A comparison of four self-report smoking cessation outcome measures. Addict Behav. 2004;29(1):51–60. https://doi.org/10.1016/S0306-4603(03)00084-4.
Rahman A, Mohamad MHN, Jamshed S. Evaluating effectiveness and safety toward electronic cigarette among Malaysian vapers: one-month observational study. Arch Pharma Pract. 2016;7(2):43. https://doi.org/10.4103/2045-080X.181038.
Abrams DB, Follick MJ, Biener L, Carey KB, Hitti J. Saliva cotinine as a measure of smoking status in field settings. Am J Public Health. 1987;77(7):846–8. https://doi.org/10.2105/AJPH.77.7.846.
From Attebring M, Herlitz J, Berndt AK, Karlsson T, Hjalmarson A. Are patients truthful about their smoking habits? A validation of self-report about smoking cessation with biochemical markers of smoking activity amongst patients with ischaemic heart disease. J Intern Med. 2001;249(2):145–51. https://doi.org/10.1046/j.1365-2796.2001.00770.x.
Bender D, Haubruck P, Boxriker S, Korff S, Schmidmaier G, Moghaddam A. Validity of subjective smoking status in orthopedic patients. Ther Clin Risk Manag. 2015;11:1297–303. https://doi.org/10.2147/TCRM.S86212.
Gerritsen M, Berndt N, Lechner L, de Vries H, Mudde A, Bolman C. Self-reporting of smoking cessation in cardiac patients: how reliable is it and is reliability associated with patient characteristics? J Addict Med. 2015;9(4):308–16. https://doi.org/10.1097/ADM.0000000000000137.
Hilberink SR, Jacobs JE, Breteler MH, de Vries H, Grol RP. General practice counseling for patients with chronic obstructive pulmonary disease to quit smoking: impact after 1 year of two complex interventions. Patient Educ Couns. 2011;83(1):120–4. https://doi.org/10.1016/j.pec.2010.04.009.
Studts JL, Ghate SR, Gill JL, Studts CR, Barnes CN, LaJoie AS, et al. Validity of self-reported smoking status among participants in a lung cancer screening trial. Cancer Epidemiol Biomark Prev. 2006;15(10):1825–8. https://doi.org/10.1158/1055-9965.EPI-06-0393.
Tourangeau R, Yan T, Sun H, Hyland A, Stanton CA. Population Assessment of Tobacco and Health (PATH) reliability and validity study: selected reliability and validity estimates. Tob Control. 2019;28(6):663-8.
van der Aalst CM, de Koning HJ. Biochemical verification of the self-reported smoking status of screened male smokers of the Dutch-Belgian randomized controlled lung cancer screening trial. Lung Cancer. 2016;94:96–101. https://doi.org/10.1016/j.lungcan.2016.02.001.
Hymowitz N, Cummings KM, Hyland A, Lynn WR, Pechacek TF, Hartwell TD. Predictors of smoking cessation in a cohort of adult smokers followed for five years. Tob Control. 1997;6(Suppl 2):S57–62. https://doi.org/10.1136/tc.6.suppl_2.S57.
Rohsenow DJ, Martin RA, Tidey JW, Monti PM, Colby SM. Comparison of the cigarette dependence scale with four other measures of nicotine involvement: correlations with smoking history and smoking treatment outcome in smokers with substance use disorders. Addict Behav. 2013;38(8):2409–13. https://doi.org/10.1016/j.addbeh.2013.03.019.
Brozek G, Jankowski M, Zejda J, Jarosinska A, Idzik A, Banka P. E-smoking among students of medicine - frequency, pattern and motivations. Adv Respir Med. 2017;85(1):8–14. https://doi.org/10.5603/ARM.2017.0003.
Grant BF, Dawson DA, Stinson FS, Chou PS, Kay W, Pickering R. The alcohol use disorder and associated disabilities interview schedule-IV (AUDADIS-IV): reliability of alcohol consumption, tobacco use, family history of depression and psychiatric diagnostic modules in a general population sample. Drug Alcohol Depend. 2003;71(1):7–16. https://doi.org/10.1016/S0376-8716(03)00070-X.
Ramo DE, Hall SM, Prochaska JJ. Reliability and validity of self-reported smoking in an anonymous online survey with young adults. Health Psychol. 2011;30(6):693–701. https://doi.org/10.1037/a0023443.
Stanton WR, McClelland M, Elwood C, Ferry D, Silva PA. Prevalence, reliability and bias of adolescents’ reports of smoking and quitting. Addiction. 1996;91(11):1705–14. https://doi.org/10.1111/j.1360-0443.1996.tb02273.x.
Blank MD, Breland AB, Enlow PT, Duncan C, Metzger A, Cobb CO. Measurement of smoking behavior: Comparison of self-reports, returned cigarette butts, and toxicant levels. Exp Clin Psychopharmacol. 2016;24(5):348-55.
Weinberger AH, Reutenauer EL, Allen TM, Termine A, Vessicchio JC, Sacco KA, et al. Reliability of the Fagerstrom test for nicotine dependence, Minnesota nicotine withdrawal scale, and Tiffany questionnaire for smoking urges in smokers with and without schizophrenia. Drug Alcohol Depend. 2007;86(2–3):278–82. https://doi.org/10.1016/j.drugalcdep.2006.06.005.
Rostron BL, Corey CG, Chang JT, van Bemmel DM, Miller ME, Chang CM. Associations of cigarettes smoked per day with biomarkers of exposure among U.S. Adult cigarette smokers in the Population Assessment of Tobacco and Health (PATH) Study Wave 1 (2013–2014). Cancer Epidemiol Biomark Prev. 2019;28(9):1443.
Acknowledgements
The authors would like to acknowledge the Venebio Group, LLC (Richmond, VA) for providing their systematic review expertise to execute the original synthesis of the evidence base and all study activities across all levels of the review process. The authors also acknowledge Thera-Business (Ontario CANADA) for providing their systematic review expertise to all study activities across all levels of the updated review process.
Funding
All study activities were executed by providers external to RAI Services Company (Venebio and Thera-Business). These providers were financially compensated for services according to contractual terms with RAI Services Company. RAI Services Company is a wholly owned subsidiary of Reynolds American Inc., whose operating companies manufacture and market tobacco products. Reynolds American Inc. was acquired by British American Tobacco in July 2017.
Author information
Authors and Affiliations
Contributions
M.K. and G.C. co-lead the conceptualization of this review and served as subject matter experts throughout the review process. M.K. led the preparation and finalization of the manuscript. G.C. served as the second author for all aspects of the manuscript preparation process including critically revising the manuscript and reviewing accuracy of all technical details. The corresponding author attests that the listed authors meet authorship criteria and that no others meeting the criteria have been omitted. The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Consent for publications
Not applicable.
Ethics approval and consent to participate
Not applicable.
Competing interests
Both Drs. Kim and Curtin serve as full-time employees of RAI Services Company, a wholly owned subsidiary of Reynolds American Inc., a manufacturer and marketer of tobacco products.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Literature Search Strategy.
Additional file 2.
Studies excluded at full-text level screening (with reason for exclusion).
Additional file 3.
Outcome Measures for Smoking Cessation across Adjusted Studies.
Additional file 4
Evidence Table, Modeled / Adjusted Results (Duration of Abstinence, Any Quit Attempt, Number of Quit Attempts per Person, Rate of Abstinence/Quitting, Change in Smoking Quantity/Frequency, and Return to Smoking/Relapse) (n = 43 studies; n = 47 references).
Additional file 5.
Sensitivity Analyses.
Additional file 6.
Characteristics, Definitions, and Covariates of Studies Included in the Meta-Analysis.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Kim, M.M., Curtin, G.M. Assessing the evidence on the differential impact of menthol versus non-menthol cigarette use on smoking cessation in the U.S. population: a systematic review and meta-analysis. Subst Abuse Treat Prev Policy 16, 61 (2021). https://doi.org/10.1186/s13011-021-00397-4
Accepted:
Published:
DOI: https://doi.org/10.1186/s13011-021-00397-4