Abstract
Background
Aneuploidy, a condition caused by an imbalance between the relative dosages of chromosomes, generally produces a novel phenotype specific to the molecular karyotype. Few techniques are currently available for detecting the molecular karyotypes of aneuploids in plants.
Results
Based on this imbalance in chromosome dosage, a new approach (referred to as ‘SSR-qPCR’) combining simple sequence repeat (SSR) markers and quantitative real-time PCR (qPCR) has been developed and utilized to detect some common aneuploids irrespective of heterozygosity. We screened 17 specific SSR markers covering all loquat linkage groups and redesigned 6 pairs of primers for SSR markers that can detect loquat chromosome aneuploidies. The SSR-qPCR detection results obtained for hybrid progeny and open-pollination progeny of triploid loquat showed diagnostic accuracies of 88.9% and 62.5%, respectively, compared with the chromosome preparation results.
Conclusion
SSR-qPCR can detect loquat aneuploids and be used to construct the entire molecular karyotypes of aneuploid individuals. Therefore, this method offers a novel alternative for the detection of chromosome aneuploidies.
Similar content being viewed by others
Background
Aneuploidy can result in partial genome duplication, which involves the imbalance between the relative dosages of chromosomes [1]. Aneuploidy is usually lethal in plants [2,3,4], and the surviving individuals frequently exhibit novel phenotypic traits specific to the molecular karyotypes. Each of the 12 Datura trisomies results in a different phenotype, depending on which type of chromosome has trisomy [2]. Henry et al. [1] demonstrated that certain traits are strongly associated with the dosages of specific chromosome types and that chromosomal effects can be additive in Arabidopsis thaliana. Some aneuploids are useful for crop production. Guava, pear and loquat aneuploids have been studied in relation to rootstock selection for dwarfing [5,6,7,8,9]. Moreover, aneuploidy in plants is associated with some unique and desirable traits, such as multiple petals, male sterility, tolerance of cold or drought or resistance to disease [10,11,12]. Therefore, research on aneuploidy is of great significance for crop production. The identification of aneuploids and the construction of their complete molecular karyotypes are fundamental and crucial for such research.
Many techniques have been widely used for the detection of chromosome copy number changes in aneuploid individuals. The unquestionable and unique merit of classic cytogenetics and fluorescence in situ hybridization (FISH) is their abilities to produce complete karyotypes and reveal balanced major rearrangements; however, these techniques are time consuming and labour intensive [13]. Flow cytometry does not accurately detect aneuploidy, but simple sequence repeat (SSR) and single nucleotide polymorphism (SNP) markers are advantageous for the reliable identification of aneuploidy [14]. Many recently developed DNA-based methods are faster, less expensive and achieve a higher resolution compared with the previously available methods. Quantitative fluorescent polymerase chain reaction (QF-PCR) is widely applied in humans for the prenatal diagnosis of trisomy of chromosomes 21, 18, 13, X, and Y [15, 16]. SNP arrays have been used as high-throughput genotyping tools for polyploid wheat, potato, sugarcane, and strawberry crops [17]. Quantitative real-time polymerase chain reaction (qPCR) has also been used for detection of the trisomy of chromosomes 21 (Down syndrome) and 11 [18]. qPCR, due to its specificity, sensitivity and replicability, is widely used for gene expression analysis and is one of the most reliable tools for the detection of nucleic acid sequence copy numbers [13, 19]. However, the application of qPCR for the detection of aneuploid plant molecular karyotypes has rarely been reported. Previously, aneuploid and polyploid A. thaliana have been detected by measurement of the relative allelic ratios of the heterozygous genotype [3]. The details of polyploidization have been described in Malus, with particular emphasis on aneuploidy, via detection of the types of heterozygous genotypes and the corresponding frequencies of occurrence [12]. Both of these methods are limited to heterozygous genotypes; in particular, the detection of the occurrence frequencies of heterozygous genotypes using statistical methods requires large numbers of codominant SSR markers. Similarly, high-resolution melting (HRM) and multiplex ligation-dependent probe amplification (MLPA) are also limited to heterozygous genotypes. SSR markers are randomly distributed throughout the plant genome and are reproducible, codominant, and transferable [20]. Microsatellite markers have been widely used in genetic and paternal studies [21, 22], but they are rarely used in molecular karyotypic studies. Codominant markers can be amplified from allelic loci simultaneously by qPCR. When allelic loci are homozygous, the ratios between markers from different loci are constant between euploids. If there are appropriate SSR markers in each linkage group (LG) used for qPCR, accurate detection of aneuploidy based on the whole-chromosome dosage can be achieved through the use of qPCR combined with SSR markers to distinguish aneuploidy irrespective of heterozygosity. Notably, with the publication of the genome sequences of major crops, more SSR markers have become available.
Loquat (Eriobotrya japonica (Thunb.) Lindl.) originated in Southwest China, and among the 20 species belonging to the genus Eriobotrya, only loquat is used for commercial fruit production [23, 24]. Many triploid lines that can be derived from unreduced gametes have been selected from progeny of diploid germplasms [25], and some aneuploid plants can be derived from triploids [1, 4]. These aneuploid loquat plants are useful for research on the effects of chromosome dosage on the genomic expression and phenotypes of plants. At present, the whole molecular karyotype of aneuploid loquat is helpful for genomics-based research.
In this study, we found, for the first time, that a combination of SSR markers and qPCR (SSR-qPCR) can be used to construct the entire molecular karyotypes of aneuploid individuals of E. japonica. This approach does not require large numbers of SSR markers or multiple types of fluorescent markers. In addition, this DNA-based qPCR method does not require additional reference genes, and SSR markers in 17 LGs can be selected as cross-references. The approach is also not restricted to heterozygous genotypes. Moreover, SSR-qPCR allows the construction of molecular karyotypes without whole-genome sequence data, and then validated in the offspring populations of triploid lines, and more than 88% of the offspring of triploids were found to be aneuploid. These data will be critical for the development of molecular tools and strategies for loquat breeding programmes.
Methods
Plant material
Twenty-three loquat lines with different ploidies were used as the materials in this study (Table 1): 2 Sichuan Province cultivars (‘Dawuxing’ and ‘Longquan No. 1’), 2 Zhejiang Province cultivars (‘Ruantiaobaisha’ and ‘Ninghaibai’), 1 Fujian Province cultivar (‘Changbai No. 1’) and 18 excellent strains selected at Southwest University (A313, A322, B350, B352, B353, B356, B431, B456, B460, B432, ‘Wuheguoyu’, H424, H39, 77-1, K474, ‘Huabai No. 1’, Q24 and ‘Huayuwuhe No. 1’). All of these lines were obtained from the polyploid loquat germplasm resource nursery at Southwest University, Chongqing, China. In addition, to produce aneuploid strains, we performed hybridization of Q24 (3x) × ‘Huabai No. 1’ (2x), and we selected some hybrids and elucidated their genetic relationships (Fig. 1). Moreover, we found that two triploid loquat strains (A313 and A322) produced seeds by open pollination, and we selected their progeny as additional research materials (Fig. 1).
DNA extraction and examination
Genomic DNA isolation was performed based on the cetyltrimethylammonium bromide method of Roche et al. [26]. After isolation, the quality was checked on a 1.0% agarose gel, and all genomic DNA was diluted to 50 ng/μL for SSR-PCR and SSR-qPCR analysis.
SSR primers
A total of 209 SSRs derived from loquat (16), pear (42), apple (144), peach (5), apricot (1) and plum (1) were used in this study (Table 2). Some of the SSRs were located in the 17 LGs of bronze loquat [27], and some were also located on genetic linkage maps of the Japanese pears ‘Housui’ or the European pear ‘Bartlett’ and ‘La France’ [28,29,30,31]. Almost all loquat LGs were aligned with the pear consensus map by using at least two apple or pear SSRs [27]. The primers were synthesized by Shanghai Invitrogen™ Life Technologies.
PCR amplification and electrophoresis of SSRs
SSR reactions were performed in a final volume of 20 μL, containing 2.0 μL of 10× PCR buffer (Mg2+ free), 1.5 mM MgCl2, 0.0375 mM each dNTP, 0.25 µM each of the forward and reverse primer, 50 ng of genomic DNA and 1 unit of Taq DNA polymerase (Takara Biotechnology Company, Dalian, China). The amplification was performed using a C1000 Touch PCR System thermal cycler (Bio-Rad) with the following temperature conditions: 94 °C for 2.5 min, followed by 35 cycles of 94 °C for 30 s, 60 °C for 30 s and 72 °C for 30 s and a final step of 72 °C for 10 min. The PCR products were mixed with 15% loading buffer (36% glycerol, 30 mM ethylenediaminetetraacetic acid (EDTA), and 0.05% bromophenol blue and xylene cyanol). Three microliters of each mixture was loaded into 8% denaturing polyacrylamide gels (7 M urea) in 1× TBE buffer (89 mM Tris–borate, 1 mM EDTA, pH 8.0). The gels were run at 220 W for 50 min and silver stained [32].
Chromosome preparation
Chromosomes at mitotic metaphase were prepared from root tip materials according to methods reported by Chen et al. [33]. Root tip tissue with a length of approximately 1 cm was immersed in 0.002 mol/L 8-hydroxyquinoline aqueous solution for 4 h and fixed overnight in Carnoy’s solution (methanol: glacial acetic acid = 3:1). The apical meristems were cut into tissue blocks of approximately 1 mm3, rinsed in deionized water, and then incubated in mixed enzyme solution containing 3% cellulose (SCR, China) and 0.3% pectinase (Yakult, Japan). After 3 h, the enzyme solution was removed, the apical meristems were soaked in deionized water for 10 min, and placed in Carnoy’s solution. At last, apical meristems were dispersed on slides with tweezers and dried over the alcohol burner flame. Chromosomes were colorized with 5% Giemsa staining, dried in air, and visualized under a microscope (Olympus, Tokyo, Japan).
qPCR
qPCR was performed using a StepOne real-time PCR instrument (ABI Corporation). The PCR amplifications were performed in a 10 μL reaction system containing genomic DNA (50 ng), 0.2 µM each primer, corresponding ROX Reference Dye I (0.2 μL), and 2 × NovoStart® SYBR qPCR SuperMix Plus (Novoprotein Scientific Inc.) (5 μL). The PCR began at 95 °C for 1 min and then progressed to 40 cycles of 95 °C for 20 s and 60 °C for 1 min. This programme was followed by 95 °C for 15 s and 60 °C for 1 min. The fluorescence was measured at 60 °C for 40 cycles.
Data analysis
We first obtained the PCR product amount (ΔRn value) ratios of the 17 LGs in known euploid strains as controls. Known aneuploid strains and unknown loquat strains were then examined and compared with the controls. A limited number of ΔRn values were expected according to the following aneuploidy and euploidy ΔRn scale: 1 (2x, 3x, and 4x karyotypes), 0.5 (2x − 1 karyotype), 1.5 (2x + 1 karyotype), 0.75 (4x − 1 karyotype) and 1.25 (4x + 1 karyotype).
StepOne v2.1 software (ABI Corporation) was used to calculate the ΔRn values of each segmental duplication. Each LG had one SSR marker, and the fluorescence intensity of each SSR marker in a single material was calculated. The ΔRn values were calculated based on the method of Zimmermann et al. [34]. To the extent possible, parallel amplification curves were obtained during the exponential growth period to ensure equal amplification of the 17 sites. The ΔRn values with the cycle threshold (CT) values between 16 and 24 were selected, and the ΔRn ratios were examined at three separate points (bottom, middle and top) along the amplification curve in the exponential phase. Samples that deviated were disregarded. All ΔRn values of the SSR markers were standardized with the ΔRn of NZmsCO754252 as the reference standard. The proportions of ΔRn values for whole chromosomes were used to discriminate aneuploid individuals and euploid individuals. Analysis of variance was performed by using SPSS 19.0.
Results
SSR analysis
Our goal was to obtain 17 amplified stable SSR markers covering all loquat LGs. Of all the 209 SSR markers derived from loquat, pear, apple, peach, apricot and plum, 102 SSRs (approximately half) were applicable to loquat, of which 28 (approximately 13.4% of the total) showed polymorphism (Table 2).
Screening of 17 SSR markers covering all loquat LGs by qPCR
We aimed to develop a rapid and reliable method for studying aneuploid karyotypic abnormalities. For the detection of aneuploidy, 17 SSR markers were selected from a previously published high-density genetic linkage map [27], and primers for 6 of the 17 SSR markers were redesigned according to the expressed sequences, with at least one well-conserved locus from each LG (Additional file 1: Fig. S1). These 17 SSR markers were amplified with the same efficiency during the exponential phase of PCR and used as target sequences to detect the number of chromosomes in each LG. Table 3 presents the primer sequences and product lengths.
After qPCR detection, each of the dissolution curves of the 17 pairs of SSR primers showed a single peak (Additional file 2: Fig. S2). The CT values of the 17 pairs of SSR primers were between 15 and 25, and the negative control had no CT value, which indicated that the PCR primer was specific.
Detection of molecular karyotype abnormalities in aneuploid loquat H39 by SSR-qPCR
We included some known euploid strains, such as ‘Ruantiaobaisha’, ‘Wuheguoyu’ and H424, as controls in the detection of aneuploid loquat H39 (2n = 2x + 5 = 39) karyotype variation. The means and standard deviations of ΔRn of the bottom, middle and top of the exponential phase are shown in Table 4. The results showed that the relative intensities of ΔRn values of the 17 SSR markers were similar among ‘Ruantiaobaisha’, ‘Wuheguoyu’ and H424, whereas the ΔRn values of H39 were altered in five LGs. The ΔRn values obtained for H39 in LG3, LG8, LG10, LG16 and LG17 were approximately 41.8% higher than those obtained for the euploids. The results implied that LG3, LG8, LG10, LG16 and LG17 have one more chromosome in H39 than in the diploid ‘Ruantiaobaisha’. In other words, H39 might have five more chromosomes than the diploid ‘Ruantiaobaisha’. This result was consistent with the result obtained with conventional cytological methods [9]. The statistical means and standard deviations of the ΔRn values obtained for the 17 loquat LGs are shown in Table 4.
Application of molecular karyotypes to triploid offspring populations
To verify the method for the detection of aneuploid karyotype variation, 9 hybrid offspring of Q24× ‘Huabai No. 1’, 7 open-pollination progeny of the triploid loquat A313 and 9 open-pollination progeny of the triploid loquat A322 were obtained, and these included a total of 22 aneuploids and 3 euploids. We detected their chromosome numbers using conventional cytological methods (Table 5, Figs. 2, 3).
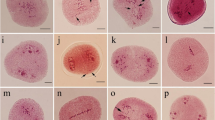
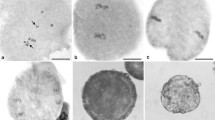
Mitotic metaphase chromosomes of 9 hybrid offspring of Q24× ‘Huabai No. 1’. The strain name of each chromosome preparation corresponds to that in the genetic diagram shown in Fig. 1, and the underlined chromosome numbers are consistent with the SSR-qPCR results
Mitotic metaphase chromosomes of 16 open-pollination progeny of triploid loquat strains (A313 and A322). The strain name of each chromosome preparation corresponds to that in the genetic diagram shown in Fig. 1, and the underlined chromosome numbers are consistent with the SSR-qPCR results
To quantify the agreement rate between the SSR-qPCR and conventional cytological methods, the SSR-qPCR method was used to estimate and describe the level of aneuploidy in the 9 hybrid offspring of Q24× ‘Huabai No. 1’ (Additional file 3: Table S2). We included some known euploid strains, such as ‘Huabai No. 1’, ‘Changbai No. 1’, Q24 and ‘Huayuwuhe No. 1’ (Additional file 3: Table S1) as controls in the SSR-qPCR analysis of the detection of aneuploid molecular karyotype variation in the offspring of Q24× ‘Huabai No. 1’. The detection results showed that the ΔRn values of the control strains were between 0.84 and − 1.08, and 88.9% of the SSR-qPCR results for the 9 hybrids, including 8 trisomic samples, were consistent with the results obtained using conventional cytological methods. Furthermore, the LGs of the added chromosomes in 8 loquat aneuploids were located (Fig. 2a–e, g–i; Table 6). Eight trisomic samples were detected to have trisomy mainly in LG1, LG2, LG7, LG10, LG13, and LG14.
The SSR-qPCR method was also used to estimate and describe the level of aneuploidy in 16 open-pollination progeny of triploid loquat strains (A313 and A322) (Additional file 3: Table S3). In the SSR-qPCR analysis of the detection of aneuploid molecular karyotype variation in the offspring of A313 and A322, we included 22 known euploid loquat strains as controls (Additional file 3: Table S1). The detection results showed that the ΔRn values of the control strains were between 0.81 and − 1.15, and 62.5% of the SSR-qPCR results for 16 open-pollination progeny, including 2 tetraploid samples (Fig. 3h, j), 3 trisomic samples (Fig. 3b, e, f) and 5 pentasomic samples (Fig. 3k, m–p), were consistent with the results obtained using conventional cytological methods.
Discussion
SSR-qPCR is a fast, convenient and accurate method for the molecular karyotypes of aneuploids
Our results demonstrate that dosage abnormalities of chromosomes and complete molecular karyotypes of loquat aneuploids can be detected by SSR-qPCR. The 17 SSR markers that used in this study were sequentially located in 17 LGs according to a high-density genetic linkage map [27]. We used 17 SSR markers without polymorphisms and detected them by qPCR. The analysis of the relative proportions of the ΔRn values of the 17 SSR markers (Table 4) allowed the detection of 17 loquat LGs and corresponding molecular karyotypes associated with aneuploidy. Aneuploidy-associated dosage abnormalities produce different ratios of the corresponding amplicons, which result in ΔRn values that are notably different from euploid individuals sample. When performing qPCR to diagnose abnormalities of any given chromosomes in samples, SSR markers for other autosomes can be selected as standards [13]. Our method could clearly differentiate changes in the chromosome dosage as low as 1.16-fold for trisomic diagnosis, and the ΔRn values of the 22 known euploid loquat strains were 1.00 ± 0.05. Furthermore, the changes of the chromosome copy number in 17 loquat LGs were identified through assessment of the ΔRn ratios between 17 SSR markers.
Similar to conventional cytological methods, SSR-qPCR has several advantages: reliability, low sample demand, ease of performance, and ability to produce precise data. The PCR analysis can directly generate data for analysis without the need for gel electrophoresis or other processing steps, which saves considerable time. Notably, karyotyping through conventional cytological analysis is difficult for some species, such as A. thaliana, due to the lack of chromosome-specific probes and the small sizes of chromosomes [3]. Kiwifruit (2n = 2x = 58) chromosomes are small and numerous, and the average length of each chromosome is 0.6–1.5 μm [51,52,53]. Liang et al. analysed the karyotypes of 10 diploid varieties of the genus Malus, all of which have small chromosomes, and found that the difference between the longest and shortest chromosomes was often very small [54]. These factors make conventional karyotype analyses relatively difficult. During the detection of aneuploid individuals in a polyploid population, the difficulty of classic cytological chromosome counting increases with the number of polyploid chromosomes. Many major cultivars are polyploid, such as allotetraploid cotton (Gossypium hirsutum and Gossypium barbadense) [55], octoploid strawberry (Fragaria × ananassa) [56], and the octoploid and decaploid Actinidia. arguta var. giraldii [57]. In addition, seedling population experiments do not provide sufficient material for distinguishing chromosomes, and cutting the root tip of a seedling is likely to lead to death; however, these difficulties can be avoided through the use of SSR-qPCR. SSR-qPCR also has other advantages in contrast to conventional cytology: genomic DNA in all organs of an individual is identical at all periods of growth, and the SSR-qPCR technique is not limited to the tips of roots and stems. qPCR using SSR markers based on genomic DNA is more convenient and stable than classic cytology, and SSR markers of 17 LGs were selected in this study as cross-references. The method is convenient and produces data that are easy to analyse. Thus, SSR-qPCR provides a novel alternative for the detection of aneuploid individuals. Of course, SSR-qPCR also has some limitations. For a certain species, we must screen appropriate markers in each LG according to a high-density genetic linkage map based on markers, and unexpected SNPs or copy number variations (CNVs) can lead to errors in judgement. Replacing markers or increasing the number of markers can minimize these errors in judgement.
Similar to microarray-based comparative genomic hybridization (array-CGH), SSR-qPCR can be used to detect changes in fluorescence intensity caused by chromosomal abnormalities, and changes in the chromosome copy number in the whole-genome can be detected through just one experiment. Array-CGH detects abnormalities in the initial chromosome copy number of a sample and requires the use of different fluorescent labels. The method is complicated and extremely expensive in terms of chip cost; therefore, array-CGH is not suitable for large-scale experiments [58]. SSR-qPCR amplifies the fluorescence intensity through PCR amplification of the initial chromosome copy number. All chromosomes are subjected to qPCR using the SYBR Green I dye. The advantages of relatively low cost and simple operation make SSR-qPCR suitable for large-scale experiments. Additionally, when whole-genome sequences are used, the molecular karyotypes obtained by SSR-qPCR and array-CGH correspond to the karyotypes obtained using conventional cytological methods, and the application potential is higher.
Moreover, qPCR directly quantifies the fluorescence intensity, which is directly proportional to the amount of PCR product amplified. The examination of ΔRn values might be more accurate than a method that relies solely on CT values [34]. Therefore, the SSR-qPCR method will yield more accurate and detailed information on molecular karyotypes compared with other methods. In this study, SSR-qPCR was used to construct a molecular karyotype of all chromosomes by detecting the gene dosage balance in the exponential phase (Table 6). In addition, this method allows the simultaneous processing of all LGs of one sample, which will greatly improve the accuracy of the analysis while reducing costs. We calculated the cost of producing a single aneuploid individual karyotype using the SSR-qPCR method to be less than $10.
SSR-qPCR is suitable for both heterozygous and homozygous alleles
Detection using the SSR-qPCR method is based on the amount of the PCR product amplified. Some studies based on QF-PCR have shown that small changes in the length of the sequences do not affect the amount of the PCR product amplified. For example, the sequence of Chr13 differs from that of Chr6 by 3 bp but this difference does not affect the trisomy detection of Chr13 by QF-PCR [16]. In addition, QF-PCR of 12 loci can be used for the simultaneous detection of aneuploid and polyploid karyotypes of A. thaliana [3]. The aneuploidy detection rate in the prenatal diagnosis of 22,504 samples has been determined to be 98.6% using QF-PCR [59]. The peak ratios of capillary electrophoresis in these studies are consistent with the theoretical results. QF-PCR can accurately detect the chromosomes with trisomiy to describe the molecular karyotypes of partial aneuploids and is widely used for prenatal detection in humans [15, 16]. These studies indicate that QF-PCR based on codominant markers is suitable for heterozygous alleles. Detection using both the QF-PCR and SSR-qPCR methods are based on the amount of the PCR product amplified. Similarly, SSR-qPCR can also be applied to heterozygous alleles.
Our method is more suitable for homozygous alleles than heterozygous alleles. The 17 SSR markers used in this study produced single bands and were not polymorphic. We found that these 17 SSR markers were relatively conserved in 23 known loquat strains. However, these 17 SSR markers might not be suitable for some unknown loquat varieties. The genetic heterozygosity of fruit trees is generally high. In this study, the accuracy of SSR-qPCR for 16 open-pollination progeny of triploid loquat strains (A313 and A322) was only 62.5%, whereas the accuracy of SSR-qPCR for 9 hybrid offspring of Q24× ‘Huabai No. 1’ was 88.9%. SSR markers are randomly distributed throughout plant genomes, and the flanking regions of SSR markers are generally relatively conserved single-copy sequences. SSR markers are short tandem repeats. Tandem repeats are usually found in pericentromeres, centromeres or telomeres, and the tandem repeats near centromeres are more conserved than those in other locations [60]. Tandem repeats are suitable cytogenetic markers for molecular karyotyping and chromosome identification [61]. After the loquat genome sequences are published, subsequent studies should select SSR markers located as close to the centromere as possible. Alternatively, it would be equally feasible to exploit other codominant markers without polymorphism on each chromosome.
SSR-qPCR allows the application of molecular karyotyping to more species
We were able to detect the complete molecular karyotypes of aneuploid individuals using the SSR-qPCR method, which requires a high-density genetic linkage map. SSR markers are codominant, polymorphic, and suitable for the construction of high-density genetic maps [27]. At present, the high-density genetic linkage maps based on SSR markers are available for apple [43], pear [62], loquat [27, 63], grape [64], kiwifruit [65], strawberry [66], papaya [67], longan [68], tomato [69], cucumber [70], spinach [71], cabbage [72], lettuce [73], pepper [74], rape [75], sorghum [76], cranberry [77], tobacco [78], soybean [79, 80], mungbean [81], wheat [82], maize [83], willow [84], orchardgrass [85], oil palm [86], zoysiagrass [87], carnation [88], cotton [89], spruce [90], rice [91], and tall fescue [92]. The studies that produced these maps allow the possibility of constructing complete molecular karyotypes for these species. Whole-genome sequences of apple [93, 94], pear [95, 96], strawberry [97], peach [98], Prunus mume [99], sweet orange [100], jujube [101], pineapple [102], and kiwifruit [103], as well as those of rice, maize, wheat, soybean [104], have been published; therefore, we can obtain large numbers of conserved SSR markers located in the centromeres and telomeres from these whole-genome sequences. Therefore, molecular karyotyping using SSR markers and qPCR is a very useful tool for basic genetic research and accurate breeding. Moreover, this method can also be used for euploid populations, in which it is an effective tool for screening aneuploids and measuring the proportion of chromosomal abnormalities at the population level. Additionally, this method involves a substantially shorter operation time and lower assay complexity compared with many conventional methods. The combination of classic cytological karyotyping with molecular karyotyping based on genomic data will drive the development of breeding from the field of cytology to the field of molecular biology.
Impacts of aneuploidization on plant inheritance and evolution
The impacts of aneuploidization on speciation and evolution have long been ignored. In humans, trisomy is mainly found on chromosomes 21, 18, 13, 11, X, and Y [15, 16, 18]. In Malus, aneuploidization of chromosomes mainly occurs in 15 LGs, excluding LG1 and LG8 [12]. We found a new model of aneuploidization in loquat, because trisomies were found in 17 LGs (Table 6). Aneuploidization can result in loquat speciation with both even and odd basic chromosome numbers (Table 5). Aneuploids with extra chromosomes are often used for analyses of chromosomes or genic dosage effects in evolution and genetics studies.
Aneuploidy greatly exceeds euploidy in crosses between a triploid and a diploid parent [105,106,107,108,109]. In this study, 88% of the progeny of triploids were aneuploids (Table 5). Trisomy of the offspring of Q24× ‘Huabai No. 1’ was mainly detected in LG1, LG7, LG10, LG13, and LG14. The regulation of chromosomal gain or loss contributing to aneuploidy might be controlled by different genes; therefore, the effects of the genes on the adaptation and survival of aneuploids might be very different [105, 107, 109]. Spermatozoa in aneuploids and triploids are mostly sterile, but their ova are usually fertile [105, 107, 109,110,111]. We found that some triploid loquat strains (‘Wuheguoyu’, A313, A322 and Q24) could produce offspring as female parents. Therefore, aneuploids and triploids might be useful as new alternative male-sterile materials in commercial seed crop breeding programmes.
Aneuploidy can initiate special gene expression. For example, dwarfing tree systems, male sterility, multiple petals, tolerance of drought or cold or resistance to disease can result from ‘super-dominant expression’ due to chromosomal gain or ‘pseudo-dominant expression’ due to chromosomal loss in aneuploids [10,11,12]. Therefore, aneuploidization increases the diversity and breadth of the foundation for natural selection. However, aneuploids are usually less reproductively stable than euploids [105, 107, 109]. Thus, the advantages and disadvantages of aneuploidization should be appropriately evaluated, and their impacts on speciation and evolution should be properly determined.
Conclusions
In summary, SSR-qPCR can be used to construct molecular karyotypes of loquat aneuploids. This study provides the first demonstration that a strategy using SSR markers and qPCR can be used to successfully describe complete molecular karyotypes of aneuploids. This technique provides a novel alternative for the detection of chromosome aneuploidies. Marker-assisted breeding using SSR markers with stable amplification will effectively accelerate the breeding of loquat aneuploids. The influences of aneuploidization on speciation and evolution have previously been ignored. The greater genetic diversity in aneuploids than in euploids might provide a broader basis for natural selection. Aneuploids and euploids should be more accurately and strongly applied in studies of breeding, genetics and evolutionary biology. This is the first study to provide reliable molecular evidence for aneuploidy in triploid progeny. This study provides a reliable strategy for further exploration of aneuploidy and the enhancement of polyploid breeding programmes for other species.
Availability of data and materials
The datasets supporting the conclusions and a description of the complete protocol are included within the article.
Abbreviations
- array-CGH:
-
Microarray-based comparative genomic hybridization
- CNVs:
-
Copy number variations
- CT:
-
Cycle threshold
- FISH:
-
Fluorescence in situ hybridization
- EDTA:
-
Ethylenediaminetetraacetic acid
- HRM:
-
High-resolution melting
- LG:
-
Linkage group
- QF-PCR:
-
Quantitative fluorescent polymerase chain reaction
- MLPA:
-
Multiplex ligation-dependent probe amplification
- qPCR:
-
Quantitative real-time polymerase chain reaction
- ΔRn:
-
Amount of PCR product
- SNP:
-
Single nucleotide polymorphism
- SSR:
-
Simple sequence repeat
- Tm:
-
Melting temperature
References
Henry IM, Dilkes BP, Miller ES, Burkart-Waco D, Comai L. Phenotypic consequences of aneuploidy in Arabidopsis thaliana. Genetics. 2010;186(4):1231–45.
Blakeslee AF. Variations in Datura due to changes in chromosome number. Am Nat. 1922;56:16–31.
Henry IM, Dilkes BP, Comai L. Molecular karyotyping and aneuploidy detection in Arabidopsis thaliana using quantitative fluorescent polymerase chain reaction. Plant J. 2006;48(2):307–19.
Kikuchi S, Iwasuna M, Kobori A, Tsutaki Y, Yoshida A, Murota Y, et al. Seed formation in triploid loquat (Eriobotrya japonica) through cross-hybridization with pollen of diploid cultivars. Breeding Sci. 2014;64(2):176–82.
Feungehum S. Growth potential of aneuploid guava (Psidium guaiava L.) in relation to rootstock selection. New Delhi: Indian Agricultural Research Institute (IARI), Division of Fruits and Horticultural Technology; 1975.
Sharma YK, Goswami AM, Sharma RR. Effect of dwarfing aneuploid guava rootstock in high density orcharding. Indian J Hortic. 1992;49(2):31–6.
Simard MH, Demilly D. Ploidy level evaluation of rootstock selections and Pyrus genus accessions. Acta Hortic. 2005;671:267–73.
Simard MH, Guisnel R, Daguin F, Demilly D, Billy B, Honore D. Is dwarfing in pear rootstocks due to aneuploid genetic structures? Acta Hortic. 2011;909:59–66.
Dang JB, Wu TR, Liang GL, Wu D, He Q, Guo QG. Identification and characterization of a loquat aneuploid with novel leaf phenotypes. HortScience. 2019;54(5):804–8.
Harding J, Singh F, Mol JNM. Genetics and breeding of ornamental species. Dordrecht-Boston-London: Kluwer Academic Publishers; 1991.
Vainstein A. Breeding for ornamentals: classical and molecular approaches. The Netherlands: Kluwer Academic Publishers; 2002.
Considine MJ, Wan YZ, D'Antuono MF, Zhou Q, Han MY, Gao H, et al. Molecular genetic features of polyploidization and aneuploidization reveal unique patterns for genome duplication in diploid Malus. PLoS ONE. 2012;7(1):57–65.
Dudarewicz L, Holzgreve W, Jeziorowska A, Jakubowski L, Zimmermann B. Molecular methods for rapid detection of aneuploidy. J Appl Genet. 2005;46(2):207–15.
Wang L, Han DG, Gao C, Wang Y, Zhang XZ, Xu XF, et al. Paternity and ploidy segregation of progenies derived from tetraploid Malus xiaojinensis. Tree Genet Genomes. 2012;8(6):1469–76.
de Moraes RW, de Burlacchini Carvalho MH, de Amorim-Filho AG, Vieira Francisco RP, Romao RM, Levi JE, et al. Validation of QF-PCR for prenatal diagnoses in a Brazilian population. Clinics. 2017;72(7):400–4.
Sun L, Fan ZQ, Long J, Weng XJ, Tang WJ, Pang WR. Rapid prenatal diagnosis of aneuploidy for chromosomes 21, 18, 13, X, and Y using segmental duplication quantitative fluorescent PCR (SD-QF-PCR). Gene. 2017;627:72–8.
You Q, Yang XP, Peng Z, Xu LP, Wang JP. Development and applications of a high throughput genotyping tool for polyploid crops: single nucleotide polymorphism (SNP) array. Front Plant Sci. 2018;9:104.
Sun L, Fan ZQ, Weng XJ, Ye XH, Long J, Fu KP, et al. Rapid detection of Down's syndrome using quantitative real-time PCR (qPCR) targeting segmental duplications on chromosomes 21 and 11. Gene. 2014;552(2):272–6.
Huang K, Mellor KE, Paul SN, Lawson MJ, Mackey AJ, Timko MP. Global changes in gene expression during compatible and incompatible interactions of cowpea (Vigna unguiculata L.) with the root parasitic angiosperm Striga gesnerioides. BMC Genomics. 2012;13:402.
Kalia RK, Rai MK, Kalia S, Singh R, Dhawan AK. Microsatellite markers: an overview of the recent progress in plants. Euphytica. 2011;177(3):309–34.
He Q, Li XW, Liang GL, Ji K, Guo QG, Yuan WM, et al. Genetic diversity and identity of chinese loquat cultivars/accessions (Eriobotrya japonica) using apple SSR markers. Plant Mol Biol Rep. 2011;29(1):197–208.
Dakin EE, Avise JC. Microsatellite null alleles in parentage analysis. Heredity. 2004;93(5):504–9.
Lin SQ, Sharpe RH, Janick J. Loquat: botany and horticulture. Hortic Rev. 1999;23:233–76.
Yang XH, Kodjo G, Lin SQ, Hu YL, He YH, Nhung NTC, et al. Taxa of plants of genus Eriobotrya around the world and native to Southeastern Asia. J Fruit Sci. 2005;22(1):55–9.
Liang GL, Wang WX, Li XL, Guo QG, Xiang SQ, He Q. Selection of large-fruited triploid plants of loquat. Acta Hortic. 2011;887:95–100.
Roche P, Alston FH, Maliepaard C, Evans KM, Vrielink R, Dunemann F, et al. RFLP and RAPD markers linked to the rosy leaf curling aphid resistance gene (Sd1) in apple. Theor Appl Genet. 1997;94(3–4):528–33.
Fukuda S, Ishimoto K, Sato S, Terakami S, Hiehata N, Yamamoto T. A high-density genetic linkage map of bronze loquat based on SSR and RAPD markers. Tree Genet Genomes. 2016;12(4):80.
Yamamoto T, Kimura T, Terakami S, Nishitani C, Sawamura Y, Saito T, et al. Integrated reference genetic linkage maps of pear based on SSR and AFLP markers. Breeding Sci. 2007;57(4):321–9.
Yamamoto T, Terakami S, Moriya S, Hosaka F, Kurita K, Kanamori H, et al. DNA markers developed from genome sequencing analysis in Japanese pear (Pyrus pyrifolia). Acta Hortic. 2013;976:477–83.
Nishitani C, Terakami S, Sawamura Y, Takada N, Yamamoto T. Development of novel EST-SSR markers derived from Japanese pear (Pyrus pyrifolia). Breeding Sci. 2009;59(4):391–400.
Terakami S, Kimura T, Nishitani C, Sawamura Y, Saito T, Hirabayashi T, et al. Genetic linkage map of the Japanese pear ‘Housui’ identifying three homozygous genomic regions. J Jpn Soc Hortic Sci. 2009;78(4):417–24.
Bassam BJ, Caetano-Anolles G, Gresshoff PM. Fast and sensitive silver staining of DNA in polyacrylamide gels. Anal Biochem. 1991;196(1):80–3.
Chen RY, Song WQ, Li XL. A new method for preparing mitotic chromosomes from plant. Acta Bot Sin. 1979;21:297–8.
Zimmermann B, Holzgreve W, Wenzel F, Hahn S. Novel real-time quantitative PCR test for trisomy 21. Clin Chem. 2002;48(2):362–3.
Gisbert AD, Lopez-Capuz I, Soriano JM, Llacer G, Romero C, Badenes ML. Development of microsatellite markers from loquat, Eriobotrya japonica (Thunb.) Lindl. Mol Ecol Resour. 2009;9(3):803–5.
Yamamoto T, Kimura T, Sawamura Y, Manabe T, Kotobuki K, Hayashi T, et al. Simple sequence repeats for genetic analysis in pear. Euphytica. 2002;124(1):129–37.
Yamamoto T, Kimura T, Shoda M, Ban Y, Hayashi T, Matsuta N. Development of microsatellite markers in the Japanese pear (Pyrus pyrifolia Nakai). Mol Ecol Notes. 2002;2(1):14–6.
Yamamoto T, Kimura T, Shoda M, Imai T, Saito T, Sawamura Y, et al. Genetic linkage maps constructed by using an interspecific cross between Japanese and European pears. Theor Appl Genet. 2002;106(1):9–18.
Inoue E, Matsuki Y, Anzai H, Evans K. Isolation and characterization of microsatellite markers in Japanese pear (Pyrus pyrifolia Nakai). Mol Ecol Notes. 2007;7(3):445–7.
Liebhard R, Gianfranceschi L, Koller B, Ryder CD, Tarchini R, Van de Weg E, et al. Development and characterisation of 140 new microsatellites in apple (Malus × domestica Borkh.). Mol Breeding. 2002;10(4):217–41.
Silfverberg-Dilworth E, Matasci CL, Van de Weg WE, Van Kaauwen MPW, Walser M, Kodde LP, et al. Microsatellite markers spanning the apple (Malus × domestica Borkh.) genome. Tree Genet Genomes. 2006;2(4):202–24.
Guilford P, Prakash S, Zhu JM, Rikkerink E, Gardiner S, Bassett H, et al. Microsatellites in Malus × domestica (apple): abundance, polymorphism and cultivar identification. Theor Appl Genet. 1997;94(2):249–54.
Celton JM, Tustin DS, Chagne D, Gardiner SE. Construction of a dense genetic linkage map for apple rootstocks using SSRs developed from Malus ESTs and Pyrus genomic sequences. Tree Genet Genomes. 2009;5(1):93–107.
Moriya S, Iwanami H, Kotoda N, Haji T, Okada K, Terakami S, et al. Aligned genetic linkage maps of apple rootstock cultivar ‘JM7’ and Malus sieboldii ‘Sanashi 63’ constructed with novel EST-SSRs. Tree Genet Genomes. 2012;8(4):709–23.
Hokanson SC, Szewc-McFadden AK, Lamboy WF, McFerson JR. Microsatellite (SSR) markers reveal genetic identities, genetic diversity and relationships in a Malus × domestica borkh. core subset collection. Theor Appl Genet. 1998;97(5–6):671–83.
Vinatzer BA, Patocchi A, Tartarini S, Gianfranceschi L, Sansavini S, Gessler C. Isolation of two microsatellite markers from BAC clones of the Vf scab resistance region and molecular characterization of scab-resistant accessions in Malus germplasm. Plant Breeding. 2004;123(4):321–6.
Yamamoto T, Mochida K, Imai T, Shi YZ, Ogiwara I, Hayashi T. Microsatellite markers in peach [Prunus persica (L.) Batsch] derived from an enriched genomic and cDNA libraries. Mol Ecol Notes. 2002;2(3):298–301.
Dirlewanger E, Cosson P, Tavaud M, Aranzana MJ, Poizat C, Zanetto A, et al. Development of microsatellite markers in peach [Prunus persica (L.) Batsch] and their use in genetic diversity analysis in peach and sweet cherry (Prunus avium L.). Theor Appl Genet. 2002;105(1):127–38.
Lopes MS, Sefc KM, Laimer M, Machado AD. Identification of microsatellite loci in apricot. Mol Ecol Notes. 2002;2(1):24–6.
Mnejja M, Garcia-Mas J, Howad W, Badenes ML, Arus P. Simple-sequence repeat (SSR) markers of Japanese plum (Prunus salicina Lindl.) are highly polymorphic and transferable to peach and almond. Mol Ecol Notes. 2004;4(2):163–6.
Bowden WM. A list of chromosome numbers in higher plants I. Acanthaceae to Myrtaceae. Am J Bot. 1945;32(2):81–92.
Mcneilage MA, Considine JA. Chromosome studies in some Actinidia taxa and implications for breeding. New Zeal J Bot. 1989;27(1):71–81.
Watanabe K, Takahashi B, Shirato K. Chromosome numbers in kiwifruit (Actinidia deliciosa) and related species. J Jpn Soc Hortic Sci. 1990;58(4):835–40.
Liang GL, Li YN, Li XL. Cytogenetic studies on the origin of basic chromosome numbers X=17 in the genus Malus. J Fruit Sci. 1994;11(4):216–20.
Wang MJ, Tu LL, Yuan DJ, Zhu D, Shen C, Li JY, et al. Reference genome sequences of two cultivated allotetraploid cottons, Gossypium hirsutum and Gossypium barbadense. Nat Genet. 2019;51(2):224–9.
Edger PP, Poorten TJ, VanBuren R, Hardigan MA, Colle M, McKain MR, et al. Origin and evolution of the octoploid strawberry genome. Nat Genet. 2019;51(4):765.
Zhang Y, Zhong CH, Liu YF, Zhang Q, Sun XR, Li DW. Agronomic trait variations and ploidy differentiation of kiwiberries in Northwest China: implication for breeding. Front Plant Sci. 2017;8:711.
Cheung SW, Bi WM. Novel applications of array comparative genomic hybridization in molecular diagnostics. Expert Rev Mol Diagn. 2018;18(6):531–42.
Nicolini U, Lalatta F, Natacci F, Curcio C, Bui TH. The introduction of QF-PCR in prenatal diagnosis of fetal aneuploidies: time for reconsideration. Hum Reprod Update. 2004;10(6):541–8.
Sharma S, Raina SN. Organization and evolution of highly repeated satellite DNA sequences in plant chromosomes. Cytogenet Genome Res. 2005;109(1–3):15–26.
Kato A, Vega JM, Han FP, Lamb JC, Birchler JA. Advances in plant chromosome identification and cytogenetic techniques. Curr Opin Plant Biol. 2005;8(2):148–54.
Wang L, Li XG, Wang L, Xue HB, Wu J, Yin H, et al. Construction of a high-density genetic linkage map in pear (Pyrus communis × Pyrus pyrifolia nakai) using SSRs and SNPs developed by SLAF-seq. Sci Hortic. 2017;218:198–204.
Gisbert AD, Martinez-Calvo J, Llacer G, Badenes ML, Romero C. Development of two loquat [Eriobotrya japonica (Thunb.) Lindl.] linkage maps based on AFLPs and SSR markers from different Rosaceae species. Mol Breeding. 2009;23(3):523–38.
Vezzulli S, Troggio M, Coppola G, Jermakow A, Cartwright D, Zharkikh A, et al. A reference integrated map for cultivated grapevine (Vitis vinifera L.) from three crosses, based on 283 SSR and 501 SNP-based markers. Theor Appl Genet. 2008;117(4):499–511.
Fraser LG, Tsang GK, Datson PM, De Silva HN, Harvey CF, Gill GP, et al. A gene-rich linkage map in the dioecious species Actinidia chinensis (kiwifruit) reveals putative X/Y sex-determining chromosomes. BMC Genomics. 2009;10:102.
Isobe SN, Hirakawa H, Sato S, Maeda F, Ishikawa M, Mori T, et al. Construction of an integrated high density simple sequence repeat linkage map in cultivated strawberry (Fragaria × ananassa) and its applicability. DNA Res. 2013;20(1):79–92.
Blas AL, Yu QY, Chen CX, Veatch O, Moore PH, Paull RE, et al. Enrichment of a papaya high-density genetic map with AFLP markers. Genome. 2009;52(8):716–25.
Guo YS, Zhao YH, Liu CJ, Ren PR, Huang TL, Fu JX, et al. Construction of a molecular genetic map for longan based on RAPD, ISSR, SRAP and AFLP markers. Acta Hortic Sinica. 2009;36(5):655–62.
Shirasawa K, Asamizu E, Fukuoka H, Ohyama A, Sato S, Nakamura Y, et al. An interspecific linkage map of SSR and intronic polymorphism markers in tomato. Theor Appl Genet. 2010;121(4):731–9.
Zhang WW, Pan JS, He HL, Zhang C, Li Z, Zhao JL, et al. Construction of a high density integrated genetic map for cucumber (Cucumis sativus L.). Theor Appl Genet. 2012;124(2):249–59.
Khattak JZK, Torp AM, Andersen SB. A genetic linkage map of Spinacia oleracea and localization of a sex determination locus. Euphytica. 2006;148(3):311–8.
Wang WX, Huang SM, Liu YM, Fang ZY, Yang LM, Hua W, et al. Construction and analysis of a high-density genetic linkage map in cabbage (Brassica oleracea L. var. capitata). BMC Genomics. 2012;13:523.
Truco MJ, Antonise R, Lavelle D, Ochoa O, Kozik A, Witsenboer H, et al. A high-density, integrated genetic linkage map of lettuce (Lactuca spp.). Theor Appl Genet. 2007;115(6):735–46.
Zhang XF, Sun HH, Xu Y, Chen B, Yu SC, Geng SS, et al. Development of a large number of SSR and InDel markers and construction of a high-density genetic map based on a RIL population of pepper (Capsicum annuum L.). Mol Breeding. 2016;36:92.
Suwabe K, Tsukazaki H, Iketani H, Hatakeyama K, Kondo M, Fujimura M, et al. Simple sequence repeat-based comparative genomics between Brassica rapa and Arabidopsis thaliana: The genetic origin of clubroot resistance. Genetics. 2006;173(1):309–19.
Menz MA, Klein RR, Mullet JE, Obert JA, Unruh NC, Klein PE. A high-density genetic map of Sorghum bicolor (L.) Moench based on 2926 AFLP, RFLP and SSR markers. Plant Mol Biol. 2002;48(5):483–99.
Schlautman B, Covarrubias-Pazaran G, Diaz-Garcia LA, Johnson-Cicalese J, Iorrizo M, Rodriguez-Bonilla L, et al. Development of a high-density cranberry SSR linkage map for comparative genetic analysis and trait detection. Mol Breeding. 2015;35(8):177.
Bindler G, Plieske J, Bakaher N, Gunduz I, Ivanov N, Van der Hoeven R, et al. A high density genetic map of tobacco (Nicotiana tabacum L.) obtained from large scale microsatellite marker development. Theor Appl Genet. 2011;123(2):219–30.
Song QJ, Marek LF, Shoemaker RC, Lark KG, Concibido VC, Delannay X, et al. A new integrated genetic linkage map of the soybean. Theor Appl Genet. 2004;109(1):122–8.
Xia ZJ, Tsubokura Y, Hoshi M, Hanawa M, Yano C, Okamura K, et al. An integrated high-density linkage map of soybean with RFLP, SSR, STS, and AFLP markers using a single F2 population. DNA Res. 2007;14(6):257–69.
Zhao D, Cheng XZ, Wang LX, Wang SH, Ma YL. Integration of mungbean (Vigna radiata) genetic linkage map. Acta Agron Sinica. 2010;36(6):932–9.
Wu QH, Chen YX, Fu L, Zhou SH, Chen JJ, Zhao XJ, et al. QTL mapping of flag leaf traits in common wheat using an integrated high-density SSR and SNP genetic linkage map. Euphytica. 2016;208(2):337–51.
Sharopova N, McMullen MD, Schultz L, Schroeder S, Sanchez-Villeda H, Gardiner J, et al. Development and mapping of SSR markers for maize. Plant Mol Biol. 2002;48(5):463–81.
Hanley S, Barker JHA, Van Ooijen JW, Aldam C, Harris SL, Ahman I, et al. A genetic linkage map of willow (Salix viminalis) based on AFLP and microsatellite markers. Theor Appl Genet. 2002;105(6–7):1087–96.
Zhao XX, Huang LK, Zhang XQ, Wang JP, Yan DF, Li J, et al. Construction of high-density genetic linkage map and identification of flowering-time QTLs in orchardgrass using SSRs and SLAF-seq. Sci Rep. 2016;6:29345.
Gan ST, Wong WC, Wong CK, Soh AC, Kilian A, Low ETL, et al. High density SNP and DArT-based genetic linkage maps of two closely related oil palm populations. J Appl Genet. 2018;59(1):23–34.
Li ML, Yuyama N, Hirata M, Han JG, Wang YW, Cai HW. Construction of a high-density SSR marker-based linkage map of zoysiagrass (Zoysia japonica Steud.). Euphytica. 2009;170(3):327–38.
Yagi M, Shirasawa K, Waki T, Kume T, Isobe S, Tanase K, et al. Construction of an SSR and RAD marker-based genetic linkage map for carnation (Dianthus caryophyllus L.). Plant Mol Biol Rep. 2017;35(1):110–7.
Yu JW, Yu SX, Lu CR, Wang W, Fan SL, Song MZ, et al. High-density linkage map of cultivated allotetraploid cotton based on SSR, TRAP, SRAP and AFLP markers. J Integr Plant Biol. 2007;49(5):716–24.
Achere V, Faivre-Rampant P, Jeandroz S, Besnard G, Markussen T, Aragones A, et al. A full saturated linkage map of Picea abies including AFLP, SSR, ESTP, 5S rDNA and morphological markers. Theor Appl Genet. 2004;108(8):1602–13.
McCouch SR, Teytelman L, Xu YB, Lobos KB, Clare K, Walton M, et al. Development and mapping of 2240 new SSR markers for rice (Oryza sativa L.). DNA Res. 2002;9(6):257–79.
Saha MC, Mian R, Zwonitzer JC, Chekhovskiy K, Hopkins AA. An SSR- and AFLP-based genetic linkage map of tall fescue (Festuca arundinacea Schreb.). Theor Appl Genet. 2005;110(2):323–36.
Velasco R, Zharkikh A, Affourtit J, Dhingra A, Cestaro A, Kalyanaraman A, et al. The genome of the domesticated apple (Malus × domestica Borkh.). Nat Genet. 2010;42(10):833–9.
Daccord N, Celton JM, Linsmith G, Becker C, Choisne N, Schijlen E, et al. High-quality de novo assembly of the apple genome and methylome dynamics of early fruit development. Nat Genet. 2017;49(7):1099–106.
Wu J, Wang ZW, Shi ZB, Zhang S, Ming R, Zhu SL, et al. The genome of the pear (Pyrus bretschneideri Rehd.). Genome Res. 2013;23(2):396–408.
Dong XG, Wang Z, Tian LM, Zhang Y, Qi D, Huo HL, et al. De novo assembly of a wild pear (Pyrus betuleafolia) genome. Plant Biotechnol J. 2020;18:581–95.
Shulaev V, Sargent DJ, Crowhurst RN, Mockler TC, Folkerts O, Delcher AL, et al. The genome of woodland strawberry (Fragaria vesca). Nat Genet. 2011;43(2):109–16.
Verde I, Abbott AG, Scalabrin S, Jung S, Shu SQ, Marroni F, et al. The high-quality draft genome of peach (Prunus persica) identifies unique patterns of genetic diversity, domestication and genome evolution. Nat Genet. 2013;45(5):487–94.
Zhang QX, Chen WB, Sun LD, Zhao FY, Huang BQ, Yang WR, et al. The genome of Prunus mume. Nat Commun. 2012;3:1318.
Xu Q, Chen LL, Ruan XA, Chen DJ, Zhu AD, Chen CL, et al. The draft genome of sweet orange (Citrus sinensis). Nat Genet. 2013;45(1):59–66.
Liu MJ, Zhao J, Cai QL, Liu GC, Wang JR, Zhao ZH, et al. The complex jujube genome provides insights into fruit tree biology. Nat Commun. 2014;5:5315.
Ming R, VanBuren R, Wai CM, Tang HB, Schatz MC, Bowers JE, et al. The pineapple genome and the evolution of CAM photosynthesis. Nat Genet. 2015;47(12):1435–42.
Huang SX, Ding J, Deng DJ, Tang W, Sun HH, Liu DY, et al. Draft genome of the kiwifruit Actinidia chinensis. Nat Commun. 2013;4:2640.
Morrell PL, Buckler ES, Ross-Ibarra J. Crop genomics: advances and applications. Nat Rev Genet. 2011;13(2):85–96.
Bretagnolle F, Thompson JD. Tansley Review No 78 Gametes with the somatic chromosome number: mechanisms of their formation and role in the evolution of autopolyploid plants. New Phytol. 1995;129(1):1–22.
Ramanna MS, Jacobsen E. Relevance of sexual polyploidization for crop improvement: a review. Euphytica. 2003;133(1):3–18.
Cai XW, Xu SS. Meiosis-driven genome variation in plants. Curr Genomics. 2007;8(3):151–61.
Senchina DS, Alvarez I, Cronn RC, Liu B, Rong JK, Noyes RD, et al. Rate variation among nuclear genes and the age of polyploidy in Gossypium. Mol Biol Evol. 2003;20(4):633–43.
Cifuentes M, Grandont L, Moore G, Chevre AM, Jenczewski E. Genetic regulation of meiosis in polyploid species: new insights into an old question. New Phytol. 2010;186(1):29–36.
Wood TE, Takebayashi N, Barker MS, Mayrose I, Greenspoon PB, Rieseberg LH. The frequency of polyploid speciation in vascular plants. PNAS. 2009;106(33):13875–9.
Dzialuk A, Chybicki I, Welc M, Sliwinska E, Burczyk J. Presence of triploids among oak species. Ann Bot-London. 2007;99(5):959–64.
Acknowledgements
Not applicable.
Funding
This work was supported by National Key Research and Development Program of China (No. 2019YFD1000200), Fundamental Research Funds for the Central Universities (XDJK2019AA001 and XDJK2020B058), Innovation Research Group Funds for Chongqing Universities (CXQT19005), Chongqing Science and Technology Commission (cstc2018jscx-mszdX0054).
Author information
Authors and Affiliations
Contributions
GW and JD designed the experiments. GW, JW and JD selected the material. GW performed the experiments. GW, JD, PJ and ZX analysed the data. GW and JW wrote the paper. JD, QG and GL commented on the manuscript and revised the text and structure. QG and GL provided project management and acquired the project funding. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors have consented to publication of this article.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1: Fig. S1.
Seventeen pairs of SSR primers detected in 23 known loquat strains by polyacrylamide gel electrophoresis.
Additional file 2: Fig. S2.
qPCR melting curves for the 17 pairs of SSR primers.
Additional file 3: Table S1.
ΔRn values for the 17 pairs of SSR primers in 22 euploid loquat strains. Table S2. ΔRn values for the 17 pairs of SSR primers in 9 hybrid offspring of Q24 × ‘Huabai No. 1’. Table S3. ΔRn values for the 17 pairs of SSR primers in 16 open-pollination progeny of triploid loquat strains (A313 and A322).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wen, G., Dang, J., Xie, Z. et al. Molecular karyotypes of loquat (Eriobotrya japonica) aneuploids can be detected by using SSR markers combined with quantitative PCR irrespective of heterozygosity. Plant Methods 16, 22 (2020). https://doi.org/10.1186/s13007-020-00568-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13007-020-00568-7