Abstract
Background
Early and exclusive breastfeeding may reduce neonatal and post-neonatal mortality in low-resource settings. However, prelacteal feeding (PLF), the practice of giving food or liquid before breastfeeding is established, is still a barrier to optimal breastfeeding practices in many South Asian countries. We used a prospective cohort study to assess the association between feeding non-breastmilk food or liquid in the first three days of life and infant size at 3–5 months of age.
Methods
The analysis used data from 3,332 mother-infant pairs enrolled in a randomized controlled trial in northwestern rural Bangladesh conducted from 2018 to 2019. Trained interviewers visited women in their households during pregnancy to collect sociodemographic data. Project staff were notified of a birth by telephone and interviewers visited the home within approximately three days and three months post-partum. At each visit, interviewers collected data on breastfeeding practices and anthropometric measures. Infant length and weight measurements were used to produce length-for-age (LAZ), weight-for-age (WAZ), and weight-for-length (WLZ) Z-scores. We used multiple linear regression to assess the association between anthropometric indices and PLF practices, controlling for household wealth, maternal age, weight, education, occupation, and infant age, sex, and neonatal sizes.
Results
The prevalence of PLF was 23%. Compared to infants who did not receive PLF, infants who received PLF may have a higher LAZ (Mean difference (MD) = 0.02 [95% CI: -0.04, 0.08]) score, a lower WLZ (MD=-0.06 [95% CI: -0.15, 0.03]) score, and a lower WAZ (MD=-0.02 [95% CI: -0.08, 0.05]) score at 3–5 months of age, but none of the differences were statistically significant. In the adjusted model, female sex, larger size during the neonatal period, higher maternal education, and wealthier households were associated with larger infant size.
Conclusion
PLF was a common practice in this setting. Although no association between PLF and infant growth was identified, we cannot ignore the potential harm posed by PLF. Future studies could assess infant size at an earlier time point, such as 1-month postpartum, or use longitudinal data to assess more subtle differences in growth trajectories with PLF.
Trial registration
ClinicalTrials.gov: NCT03683667 and NCT02909179.
Similar content being viewed by others
Background
Age-appropriate breastfeeding practices provide many health benefits for infants and young children in low- and middle- income countries (LMICs), including reducing risk of infection [1,2,3], malnutrition [4, 5], and under-five mortality [6,7,8]. The World Health Organization (WHO) recommends exclusive breastfeeding for infants under six months of age and early initiation of breastfeeding within one hour of birth [9]. Breastmilk not only contains high quality macro- and micro-nutrients necessary for growth but also many distinct bioactive molecules that protect against infection and inflammation and contribute to immune maturation, organ development and healthy microbial colonization [10].
Despite the proven benefits and active advocacy efforts, adherence to age-appropriate breastfeeding remains low in many LMICs. Only half of newborn babies are put to the breast within the first hour of life and about one in three neonates in LMICs receive prelacteal feed substances during the first three days after birth [11]. In Bangladesh and other South Asia countries, prelacteal feeding (PLF), defined as “giving a newborn baby any food or liquids before breastfeeding is established” [12], is a major barrier to age-appropriate breastfeeding. Based on the 2019 Bangladesh Multiple Indicator Cluster Survey (MICS), it was estimated that only about 47% infants were breastfed within one hour of birth and only 63% infants under 6 months of age were exclusively breastfed [13]. There are two main reasons why PLF is common in South Asia. One is traditional beliefs, including a perception that feeding something sweet to the newborns is associated with strength or better luck in the future [14,15,16]. The other reason is limited knowledge about breastfeeding, ranging from negative perceptions of colostrum [17], not knowing how to position the baby while breastfeeding [18], to perceiving milk production as insufficient [16]. The most recent national estimate on prevalence of exposure to PLF within first three days of life in Bangladesh was 24% in 2019 [13].
Previous studies have shown that PLF delayed breastfeeding initiation [14, 19] and disrupted exclusive breastfeeding [20,21,22]. The early life exposure to non-breastmilk supplements might also introduce environmental contaminants [23, 24] and interfere with breastmilk’s protection against potential insults [25]. The disruption of exclusive breastfeeding and introduction of contaminants will likely put infants at higher risk of infections like diarrhea incidence, which impairs linear growth.
In many surveys, a one-day recall is used to classify breastfeeding practices at a population level. The data collected on exclusive breastfeeding lack sensitivity, in which children who consumed non-breastmilk liquid or food prior to the survey could be classified as exclusive breast feeding. This overestimation of EBF rates by 24-hour recall measure has been observed previously [26, 27].
Given the definition of PLF, the one-day recall survey failed to capture exposure to PLF, and the potential harm of PLF thus remains underappreciated. In addition, the cross-sectional nature of current studies lacks the ability to capture feeding practices as they occur right after birth. To our knowledge no studies have examined the impact of PLF on infant size using a prospective study design. The objective of this analysis was to explore the association between feeding non-breastmilk food or liquid in the first three days of life and infant size at 3 to 5 months using data from a prospective cohort of infants.
Methods
Study setting and procedure
Data for this analysis were collected at the JiVitA Research Site located in Gaibandha District in Rangpur Division in northwestern Bangladesh. The site has hosted several studies of maternal and child health [28,29,30,31,32].
A pregnancy surveillance system was launched in the site in June 2016 as part of the protocol for an mHealth Intervention trial (mCARE-II; NCT02909179). We surveyed households in the study area to identify all married women of reproductive age and obtain their consent for pregnancy surveillance. Field staff then visited households every two months to ask about their last menstrual period. Women who became pregnant were recruited into the mCARE-II trial, which documented sociodemographic, birth outcomes, and the health and nutrition of women and infants to 1 month postpartum. Infants born to women enrolled in mCARE-II who survived and whose families were still living in the study area at three months of age during the period from September 2018 through July 2019 were considered eligible for enrollment in a cluster-randomized controlled protein supplementation trial (NCT03683667) designed to address linear growth faltering in 6–12-month-old infants, hereafter referred to as JiVitA-6. Thus, enrollment for the JiVitA-6 trial was nested within the mCARE-II trial framework.
For women and infants enrolled in the JiVitA-6 trial, field staff visited the household during pregnancy, within three days post-partum (hereafter referred to as the “birth visit”), and at three months to conduct structured interviews.
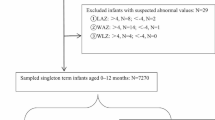
The final analyses included singleton live births. To reduce the risk of recall bias in responses, we limited the analysis to interviews conducted within 14 days of the scheduled birth visit and 60 days of the scheduled 3-mo visit [see Additional file 1 (Supplementary Fig. 1)].
Exposure
The information on PLF was collected during the birth visit, where information was collected from birth up to 3 days of life. Mother were asked: “Was the baby fed other mother’s breast milk or anything than own mother’s breastmilk in the [first 30 minutes, second 30 minutes, 2nd hour, 3rd hour, 4th hour, 5th hour, 6th hour, 7th-12th hours, 13th-24th hours, remaining hours until upcoming 6 am after completion of 24-hour, entire day 3 and night]?”
For each time interval, if the mother responded yes, data on feeding from other mother’s milk or specific foods from a list of common non-breast milk feeds (honey, water, animal milk, formula, sugar/sugar candy water, any types of drops, power/condensed milk, and others) were also collected.
Outcomes
Infant weight and length were measured by trained field staff according to standardized protocols at the birth visit and at three months of age. Undressed infants were weighed to the nearest 0.01 kg using a Tanita BD-585 Pediatric Scale. Scales were calibrated daily with 2.5 kg, 5 kg, and 10 kg weights. Infant recumbent length was measured to the nearest 0.1 cm three times on a measuring board with a sliding foot piece.
Statistical analysis
Exposure to PLF was dichotomized into responses “yes” and “no”, where “yes” was defined as reporting one or more items from the list of common non-breast milk feeds within 3 days of life at the birth visit.
The medium value was used as the final measure of infant length. Infant length and weight measurements were used to produce length-for-age (LAZ), weight-for-length (WLZ), and weight-for-age (WAZ) Z-scores using the sex-specific WHO standards [33]. Stunting, wasting, and underweight were defined as a LAZ, WLZ, and WAZ <-2, respectively. In the final analysis, we also excluded any anthropometric measures that were outside normal bounds, based on a Z-score beyond ± 6 [see Additional file 1 (Supplementary Fig. 1)] (Supplement Fig. 1).
The potential confounding variables were chosen based on previous observational studies that examined factors associated with infant growth [34,35,36,37,38]. The selected covariates were neonate size, infant sex, infant age, maternal age, maternal BMI, maternal education, maternal occupation, and household wealth. LAZ and WAZ collected at birth visits were used as indicators for neonatal size. Maternal occupation was coded as “yes” or “no” to represent maternal employment status. Maternal BMI was calculated using maternal weight and height collected at the 3-month postpartum visit. A composite Living Standards Index (LSI) based on housing material and durable asset ownership was calculated as previously described [39]. LSI, categorized into five quintiles (1–5, corresponding to the poorest to the wealthiest groups), was used as an indicator for household wealth. Categorical variables were presented as percent (n) and continuous variables presented as mean ± SD.
We used simple linear regression to assess the association between PLF and anthropometric indices (LAZ, WLZ and WAZ). We also used multiple linear regression to assess the association between PLF and anthropometric indices (LAZ, WLZ and WAZ), controlling for other covariates. All data management and statistical analysis were conducted in STATA version 15.1. Infants with missing PLF responses or anthropometric measures were excluded from the analysis [see Additional file 1 (Supplementary Fig. 1)] (Supplement Fig. 1). None of the covariates included in the multivariate models had variance inflation factors higher than 2.5, suggesting that there was no observed multicollinearity among covariates in the final models.
Results
A total of 3,332 mother and infant pairs were included in the final analysis [see Additional file 1 (Supplementary Fig. 1)] (Supplement Fig. 1). Characteristics of mothers and infants broken out by PLF status and combined are shown in Table 1. The majority of the mothers were between 20 and 29 years old (58.3%), attended school from class one to nine (73.0%), and had no occupation (70.1%). The average neonatal weight and length during birth visit were 2.8 ± 0.4 kg and 47.9 ± 2.2 cm, respectively. 25% of the infants received a birth visit on the first day of life and 75% of infants received a birth visit on the eighth day of life. The prevalence of stunting, wasting, and underweight at the three-month visit were 20.3%, 3.9%, and 16.2%, respectively. 25% of infants received a three-month visit at 3.1 months of life and 75% of infants received a three-month visit at 3.83 months of life (Table 1).
PLF were provided to 749 (22.5%) infants (Table 1). The most common PLFs were animal milk (5.1%), followed by honey (5.0%), sugar water (5.0%), any types of drops (3.6%), and formula (2.9%) (Table 2). Among the infants who received PLF, 649 (86.6%) infants received only one type of PLF, and 100 (13.4%) infants received two or more types of PLF.
In both unadjusted and adjusted analyses (Table 3), there was no association between PLF and infant LAZ, WAZ, and WLZ at 3 months of age, and point estimates for the association of PLF with anthropometry at 3 months of age were virtually unchanged between the unadjusted and adjusted models, suggesting that none of the covariates were confounding an association between PLF and size at 3 months.
Other factors were found to be associated with infant anthropometry at 3 months of age in the adjusted model. Higher neonatal LAZ (β = 0.71; 95% CI: 0.69, 0.74), female infant (β = 0.11; 95% CI: 0.06, 0.16), and higher household wealth quintile were positively associated with LAZ at 3–5 months of life. Higher neonatal WAZ (β = 0.60; 95% CI: 0.57, 0.63), maternal education of 10 years and above (β = 0.15; 95% CI: 0.03, 0.27) and higher household wealth quintile were positively associated with WAZ at 3–5 months of life. Higher neonatal WAZ (β = 0.07, 95% CI: 0.03, 0.11), younger infant age (β=-0.11, 95% CI: -0.18, -0.04), and higher maternal BMI (β = 0.02, 95% CI: 0.01, 0.04) were positively associated with WLZ at 3–5 months of age.
Discussion
We found no significant association between exposure to PLF and infant size at three to five months of age. However, infants who were exposed to PLF tended to have higher LAZ and lower WLZ and WAZ at three to five months of age. Female sex, larger size during the neonatal period, higher maternal education, and household wealth were associated with better size outcomes.
A possible reason for the null finding in our study might be because we only examine the attained size at 3 months of age, we did not capture growth interval. There may be more subtle differences in growth trajectories with PLF, such as changes in Z-score between birth and 3 months of age. There might be another intermediate risk factor like morbidity in the pathway between PLF and infant size. In addition, we assessed anthropometric measures around 3-month postpartum. We did not have data on infant size at an earlier time point, such as 1- month postpartum. It is also possible that infants in the study were exposed to pathogens through other sources in their environment, which might have attenuated the association between PLF and growth. One study in India also found an insignificant association between exposure to PLF, defined as foods other than breastmilk on the first day of life, and undernutrition among children less than 12 months old [40]. But they only conducted bivariate analysis. Another study collected data from several South Asian countries and found that provision of PLF, defined as foods other than breastmilk within first 3 days of life, increased the risk of wasting and severe wasting [41].
The main challenge of comparing the prevalence of PLF over time in a consistent manner is that there is no standard language for the questions asked to obtain the information on the exposure. Different definitions and terminologies have been used to describe the exposure. Most studies defined the exposure as any feed other than breastmilk to child during the first three days of life [16, 20, 21, 42]. Some defined the exposure as any feed other than breastmilk to children immediately after birth [24]. A number of studies did not specify the definitions [43,44,45]. The terminologies used to describe the exposure also varied across studies; the most commonly used term to describe the exposure is “prelacteal feeding”. Since most studies that defined the exposure as “any feed other than breastmilk during the first three days of life” used “prelacteal feeding” to describe the exposure, we decided to use PLF in our study to be consistent with other literature. There has been growing attention to provide better clarity in wording and definition of early exposure to non-breastmilk feed [46].
The Bangladesh Demographic and Health Surveys (DHS) consistently asked the same question— “In the first three days after delivery, was (NAME) given anything to drink other than breast milk”, and it found the prevalence of PLF decreased from 62% in 2007 [47] to 27% in 2014 [48] and increased slightly to 29% in 2018 [49]. From the JiVitA studies, using other feeds than breastmilk in the first three days of life as definition, we also observed a declining trend from 89% from data collected in 2001–2007 [20] to 24% from data collected in 2018–2019 here. Multiple Indicators Cluster Survey (MICS) conducted in 2006 asked “Did you give honey/sugar water/mustard oil/other to your child immediately after birth” and found a prevalence of 61% [50]. Later MICSs asked the same question as DHS and found the prevalence to be 23% in 2013 [51]. The most recent MICS conducted in 2019 found the prevalence to be 24% [13]. JiVitA data, like national survey data, are consistent with a decline in the PLF over the past couple decades.
A previous JiVitA study [31], carried out between 2012 and 2013, found that the prevalence of stunting, wasting, and underweight at 6 months of life were 25%, 6%, and 20%, respectively. All three prevalence estimates (20% stunted; 4% wasted; and 16% underweight) were lower at the time of our data collection between 2018 and 2019. This was consistent with the overall improvement in child nutrition status in Bangladesh in the past decades [13, 49]. In the most recent MICS conducted in 2019, it was estimated that the prevalence of stunting, wasting, and underweight for children under age 5 were 28%, 9.8%, and 22.6%, respectively [13]. We observed a slightly better nutritional status among our study population possibly because the anthropometric measurements were taken at a younger age and the participants resided in a research site where multiple projects aiming to improve maternal and newborn health have been implemented. However, compared to the WHO standard, infant size was still undesirable, where mean LAZ, WLZ, and WAZ were all below zero. When comparing to other LMICs, the estimated national prevalence of stunting among children under five in Bangladesh is still considered very high [52].
A major strength of our study was the prospective study design with a large cohort of pregnant women recruited from the community. Unlike many other studies that asked PLF questions after a couple of months, or even years after birth, our study collected the information within 14 days after birth. The responses to the PLF were comparable between birth visits conducted within 3 days (22%) vs. anytime between 4 days to 14 days after birth (23%). And more than half of the interviews (53%) were done within 3 days postpartum, which minimized the recall bias and was more likely to capture the true feeding behavior. A limitation of the current study was that in the analysis, we combined all the different types of prelacteal foods and treated them as the same exposure. This indicates the assumption that all the different types of food exert the same effects, which might not be true.
Conclusion
Prelacteal feeding is still a common practice in rural Bangladesh. Although no association between prelacteal feeding and infant size was identified within the current study, we cannot ignore the potential harm posed by PLF, both directly from environmental contaminants in non-breastmilk foods and indirectly through delaying early initiation of breastfeeding and continued non-exclusive breastfeeding. In general, a clearer definition and guidelines about PLF is necessary to make comparisons geographically and chronologically. Future studies could assess the infant size at an earlier time point, such as 1-month postpartum, or use longitudinal data to assess more subtle differences in growth trajectories with PLF.
Data availability
Data described in the manuscript, code book, and analytic code will be made available upon request pending application and approval.
Abbreviations
- DHS:
-
Demographic and Health Survey
- LAZ:
-
Length-for-age Z-score
- LMICs:
-
Low- and middle-income countries
- LSI:
-
Living Standard Index
- MD:
-
Mean difference
- MICS:
-
Multiple Indicator Cluster Survey
- PLF:
-
Prelacteal feeding
- SD:
-
Standard deviation
- WAZ:
-
Weight-for-age Z-score
- WLZ:
-
Weight-for-height Z-score
References
Lamberti LM, Fischer Walker CL, Noiman A, Victora C, Black RE. Breastfeeding and the risk for diarrhea morbidity and mortality. BMC Public Health. 2011;11(Suppl 3):15.
Horta BL, Victora C. Short-term effects of breastfeeding: a systematic review on the benefits of breastfeeding on diarrhoea and pneumonia mortality. Geneva, Switzerland: World Health Organization; 2013.
Abdulla F, Hossain MM, Karimuzzaman M, Ali M, Rahman A. Likelihood of infectious diseases due to lack of exclusive breastfeeding among infants in Bangladesh. PLoS ONE. 2022;17(2):e0263890.
Rohner F, Woodruff BA, Aaron GJ, Yakes EA, Lebanan MA, Rayco-Solon P, et al. Infant and young child feeding practices in urban Philippines and their associations with stunting, anemia, and deficiencies of iron and vitamin A. Food Nutr Bull. 2013;34(2 Suppl):17–34.
Islam M, Rahman S, Kamruzzaman, Islam M, Samad A. Effect of maternal status and breastfeeding practices on infant nutritional status - a cross sectional study in the south-west region of Bangladesh. Pan Afr Med J. 2013;16:139.
NEOVITA Study Group. Timing of initiation, patterns of breastfeeding, and infant survival: prospective analysis of pooled data from three randomised trials. Lancet Global Health. 2016;4(4):e266–e75.
Khan J, Vesel L, Bahl R, Martines JC. Timing of breastfeeding initiation and exclusivity of breastfeeding during the first month of life: effects on neonatal mortality and morbidity–a systematic review and meta-analysis. Matern Child Health J. 2015;19(3):468–79.
Lamberti LM, Zakarija-Grkovic I, Fischer Walker CL, Theodoratou E, Nair H, Campbell H, et al. Breastfeeding for reducing the risk of pneumonia morbidity and mortality in children under two: a systematic literature review and meta-analysis. BMC Public Health. 2013;13(Suppl 3):18.
WHO. Infant and young child feeding: model chapter for textbooks formedical students and allied health professionals. World Health Organization; 2009.
Ballard O, Morrow AL. Human milk composition: nutrients and bioactive factors. Pediatr Clin North Am. 2013;60(1):49–74.
Perez-Escamilla R, Tomori C, Hernandez-Cordero S, Baker P, Barros AJD, Begin F, et al. Breastfeeding: crucially important, but increasingly challenged in a market-driven world. Lancet. 2023;401(10375):472–85.
WHO Guidelines Approved by the Guidelines Review Committee. Infant and Young Child Feeding: Model Chapter for Textbooks for Medical Students and Allied Health Professionals. Geneva: World Health Organization Copyright © 2009, World Health Organization.; 2009.
Multiple Indicator Cluster Survey 2019 Survey findings Report. Dhaka: Bangladesh Bureau of Statistics, United Nations Children’s Fund; 2019.
Fikree FF, Ali TS, Durocher JM, Rahbar MH. Newborn care practices in low socioeconomic settlements of Karachi. Pakistan PubMed: Soc Sci Med. 2005;60(5):911–21.
Khanal V, Lee AH, Karkee R, Binns CW. Prevalence and factors associated with prelacteal feeding in Western Nepal. Women Birth. 2016;29(1):12–7.
Khatun H, Comins CA, Shah R, Munirul Islam M, Choudhury N, Ahmed T. Uncovering the barriers to exclusive breastfeeding for mothers living in Dhaka’s slums: a mixed method study. Int Breastfeed J. 2018;13:44.
Sharma IK, Byrne A. Early initiation of breastfeeding: a systematic literature review of factors and barriers in South Asia. Int Breastfeed J. 2016;11:17.
Rahman A, Akter F. Reasons for formula feeding among rural Bangladeshi mothers: a qualitative exploration. PLoS ONE. 2019;14(2):e0211761.
Sundaram ME, Ali H, Mehra S, Shamim AA, Ullah B, Rashid M, et al. Early newborn ritual foods correlate with delayed breastfeeding initiation in rural Bangladesh. Int Breastfeed J. 2016;11:31.
Sundaram ME, Labrique AB, Mehra S, Ali H, Shamim AA, Klemm RD, et al. Early neonatal feeding is common and associated with subsequent breastfeeding behavior in rural Bangladesh. J Nutr. 2013;143(7):1161–7.
Raihan MJ, Choudhury N, Haque MA, Farzana FD, Ali M, Ahmed T. Feeding during the first 3 days after birth other than breast milk is associated with early cessation of exclusive breastfeeding. Matern Child Nutr. 2020;16(3):e12971.
Das A, Sai Mala G, Singh RS, Majumdar A, Chatterjee R, Chaudhuri I, et al. Prelacteal feeding practice and maintenance of exclusive breast feeding in Bihar, India - identifying key demographic sections for childhood nutrition interventions: a cross-sectional study. Gates Open Res. 2019;3:1.
Nguyen P, Binns CW, Ha AVV, Chu TK, Nguyen LC, Duong DV, et al. Prelacteal and early formula feeding increase risk of infant hospitalisation: a prospective cohort study. Arch Dis Child. 2020;105(2):122–6.
Mihrshahi S, Ichikawa N, Shuaib M, Oddy W, Ampon R, Dibley MJ, et al. Prevalence of exclusive breastfeeding in Bangladesh and its association with diarrhoea and acute respiratory infection: results of the multiple indicator cluster survey 2003. J Health Popul Nutr. 2007;25(2):195–204.
Forbes JD, Azad MB, Vehling L, Tun HM, Konya TB, Guttman DS, et al. Association of exposure to formula in the hospital and subsequent infant feeding practices with gut microbiota and risk of overweight in the first year of life. JAMA Pediatr. 2018;172(7):e181161.
Arimond M, Ruel M. Progress in developing an infant and child feeding index: an Example using the Ethiopia Demographic and Health Survey 2000. International Food Policy Research Institute; 2002.
Fenta EH, Yirgu R, Shikur B, Gebreyesus SH. A single 24 h recall overestimates exclusive breastfeeding practices among infants aged less than six months in rural Ethiopia. Int Breastfeed J. 2017;12:36.
Labrique AB, Christian P, Klemm RD, Rashid M, Shamim AA, Massie A, et al. A cluster-randomized, placebo-controlled, maternal vitamin a or beta-carotene supplementation trial in Bangladesh: design and methods. Trials. 2011;12:102.
West KP Jr., Christian P, Labrique AB, Rashid M, Shamim AA, Klemm RD, et al. Effects of vitamin A or beta carotene supplementation on pregnancy-related mortality and infant mortality in rural Bangladesh: a cluster randomized trial. JAMA. 2011;305(19):1986–95.
Merrill RD, Shamim AA, Ali H, Jahan N, Labrique AB, Schulze K, et al. Iron status of women is associated with the iron concentration of potable groundwater in rural Bangladesh. J Nutr. 2011;141(5):944–9.
Christian P, Shaikh S, Shamim AA, Mehra S, Wu L, Mitra M, et al. Effect of fortified complementary food supplementation on child growth in rural Bangladesh: a cluster-randomized trial. Int J Epidemiol. 2015;44(6):1862–76.
Pasqualino MM, Shaikh S, McGready J, Islam MT, Ali H, Ahmed T, et al. An egg intervention improves dietary intakes but does not fill intake gaps for multiple micronutrients among infants in rural Bangladesh. J Nutr. 2023;153(4):1199–210.
de Onis M, Garza C, Victora CG, Onyango AW, Frongillo EA, Martines J. The WHO Multicentre Growth Reference Study: planning, study design, and methodology. Food Nutr Bull. 2004;25(1 Suppl):15–26.
Saha KK, Frongillo EA, Alam DS, Arifeen SE, Persson LA, Rasmussen KM. Appropriate infant feeding practices result in better growth of infants and young children in rural Bangladesh. Am J Clin Nutr. 2008;87(6):1852–9.
Marriott BP, White AJ, Hadden L, Davies JC, Wallingford JC. How well are infant and young child World Health Organization (WHO) feeding indicators associated with growth outcomes? An example from Cambodia. Matern Child Nutr. 2010;6(4):358–73.
Arifeen SE, Black RE, Caulfield LE, Antelman G, Baqui AH. Determinants of infant growth in the slums of Dhaka: size and maturity at birth, breastfeeding and morbidity. Eur J Clin Nutr. 2001;55(3):167–78.
Owais A, Schwartz B, Kleinbaum DG, Suchdev PS, Faruque AS, Das SK, et al. Minimum acceptable diet at 9 months but not exclusive breastfeeding at 3 months or timely complementary feeding initiation is predictive of infant growth in rural Bangladesh. PLoS ONE. 2016;11(10):e0165128.
Jones AD, Ickes SB, Smith LE, Mbuya MN, Chasekwa B, Heidkamp RA, et al. World Health Organization infant and young child feeding indicators and their associations with child anthropometry: a synthesis of recent findings. Matern Child Nutr. 2014;10(1):1–17.
Gunnsteinsson S, Labrique AB, West KP Jr., Christian P, Mehra S, Shamim AA, et al. Constructing indices of rural living standards in Northwestern Bangladesh. J Health Popul Nutr. 2010;28(5):509–19.
Meshram, II, Mallikharjun Rao K, Balakrishna N, Harikumar R, Arlappa N, Sreeramakrishna K, et al. Infant and young child feeding practices, sociodemographic factors and their association with nutritional status of children aged <3 years in India: findings of the National Nutrition Monitoring Bureau survey, 2011–2012. Public Health Nutr. 2019;22(1):104– 14.
Harding KL, Aguayo VM, Webb P. Birthweight and feeding practices are associated with child growth outcomes in South Asia. Matern Child Nutr. 2018;14(Suppl 4):e12650.
Talukder S, Farhana D, Vitta B, Greiner T. In a rural area of Bangladesh, traditional birth attendant training improved early infant feeding practices: a pragmatic cluster randomized trial. Matern Child Nutr. 2017;13(1):e12237.
Rasheed S, Frongillo EA, Devine CM, Alam DS, Rasmussen KM. Maternal, infant, and household factors are associated with breast-feeding trajectories during infants’ first 6 months of life in Matlab, Bangladesh. J Nutr. 2009;139(8):1582–7.
Akhtar K, Haque ME, Islam MZ, Yusuf MA, Sharif AR, Ashan AI. Feeding pattern and nutritional status of under two years slum children. J Shaheed Sunhrawardy Med Coll. 2012;4(1):3–6.
Joshi PC, Angdembe MR, Das SK, Ahmed S, Faruque ASG, Ahmed T. Prevalence of exclusive breastfeeding and associated factors among mothers in rural Bangladesh: a cross-sectional study. Int Breastfeed J. 2014;9:7.
Meeting Report: Reconsidering, Refining, and Extending the World Health Organization Infant and Young Child Feeding Indicators. New York; 2017.
Bangladesh Demographic and Health Survey. 2007. Dhaka, Bangladesh and Calverton, Maryland, USA: National Institute of Population Research and Training, Mitra and Associates, and Macro International. National Institute of Population Research and Training, Mitra and Associates, and Macro International.; 2009.
Bangladesh Demographic and Health Survey. 2014. Dhaka, Bangladesh, and Rockville, Maryland, USA: National Institute of Population Research and Training (NIPORT), Mitra and Associates, and ICF International; 2016.
Bangladesh Demographic and Health Survey 2017-18. Dhaka, Bangladesh, and Rockville, Maryland, USA: National Institute of Population Research and Training (NIPORT), and ICF; 2020.
Multiple Indicator Cluster Survey Progotir Pathey. 2006. Bangladesh Bureau of Statistics, United Nations Children’s Fund; 2007.
Bangladesh Multiple Indicator Cluster Survey 2012–2013, ProgotirPathey: final report. Dhaka, Bangladesh: Bangladesh Bureau of Statistics (BBS) and UNICEF Bangladesh; 2014.
United Nations Children’s Fund (UNICEF), World Health Organization, Bank TW. Levels and trends in child malnutrition: key findings of the 2021 edition of the joint child malnutrition estimates. Geneva: World Health Organization; 2021.
Acknowledgements
We are grateful to the mothers and infants for participation in the study. The following senior staff and management team of the JiVitA Project are acknowledged: Azaduzzaman and Rezwanul Huque.
Funding
This research was funded by the Bill and Melinda Gates Foundation (OPP1163259), Johnson & Johnson, and the UBS Optimus Foundation.
Author information
Authors and Affiliations
Contributions
HT, ATL, ACP conceptualized the study and designed the data analysis; HT analyzed data and wrote the manuscript draft; ACP, ATL, MMP, KS provided critical feedback on the manuscript draft; All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The protocol for the mCARE-II trial was reviewed and approved by institutional review board at the Bloomberg School of Public Health at Johns Hopkins University and Bangladesh Medical Research Council, Dhaka, Bangladesh (IRB No. 00006469, approved on August 27, 2015). The protocol for the JiVitA-6 trial was reviewed and approved by the institutional review board at the Bloomberg School of Public Health at Johns Hopkins University (IRB No. 00008000, approved on July 11, 2018) and the International Centre for Diarrhoeal Disease Research, Bangladesh (#PR-17119, approved on 21 April 2018).
Consent for publication
Not applicable.
Compete interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Tong, H., Thorne-Lyman, A., Palmer, A.C. et al. Prelacteal feeding is not associated with infant size at 3 months in rural Bangladesh: a prospective cohort study. Int Breastfeed J 19, 15 (2024). https://doi.org/10.1186/s13006-024-00621-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13006-024-00621-4