Abstract
Background
Herbal medicine has been used for the treatment of human and livestock ailments since ancient times. Numerous rural and urban communities in Ethiopia practice traditional medicine and transfer the knowledge verbally from generation to generation. Thus, this study was conducted to document the traditional medicinal plants and associated indigenous knowledge in Dibatie district, Metekel zone, Benishangul Gumuz Regional State, western Ethiopia.
Methods
Three hundred seventy-four (374) informants from 11 kebeles (the smallest administrative units) were selected and participated in the data delivery. The ethnobotanical data collection was carried out using semi-structured interviews, preference ranking, direct matrix ranking, field observation, market surveys, and focus group discussions, including voucher specimen collections. The ethnobotanical data were analyzed using descriptive statistics (frequency and percentage), ranking, comparison, and quantitative ethnobotanical techniques such as informant consensus factor, fidelity level index, Jaccard’s coefficient of similarity, and use value index.
Results
A total of 170 plant species were recorded to treat 79 human and 29 livestock ailments. Fabaceae (with 20 species) and Asteraceae (with 18 species) were the most dominant medicinal plant families in the area. Most remedial plants were herbs (61 species, 35.88%), followed by shrubs (39 species, 22.94%). The majority (135 species, 79.41%) of medicinal plants were harvested from wild sources and mainly possessed multiple remedy parts (41.17%) that are usually prescribed in fresh form (60.13%). The most commonly reported human ailment was snake venom, while blackleg was mostly reported among livestock diseases. The herbal medicines were mostly administered orally (52.20%), followed by dermal (17.62%) application. Embelia schimperi Vatke, Glinus lotoides L., Haplosciadium abyssinicum Hochst., Mucuna melanocarpa Hochst. ex A. Rich., and Phragmanthera macrosolen (Steud. ex A. Rich.) M.G.Gilbert had the highest fidelity level values (100%) against the corresponding ailments.
Conclusion
The study area is rich in a diversity of potential medicinal plants and associated indigenous knowledge. Thus, appropriate conservation actions and careful utilization are essential to counteract the rise of anthropogenic threats and to ensure the continuity of plants with the related indigenous knowledge. Additionally, the medicinal plants should be validated through experimentation to integrate local knowledge with modern medications.
Similar content being viewed by others
Background
Traditional medicine has been widely used worldwide since ancient times. Traditional medicine is the use of any plant, animal, or mineral product, either independently or in combination, for the treatment of human or animal ailments [1]. Approximately 60% of the world’s population is estimated to use traditional medicine for the treatment of various health problems [2]. Especially, there has been an increase in the demand for herbal medicines in some developed and developing countries, owing to different reasons such as their biological and pharmacological activities, high safety, and low costs [3]. For this purpose, the wide use of herbal medicine has been concerned with multi-herbal formulations or herbal interactions with conventional drugs, which may have synergistic or antagonistic effects on efficacy [4].
In developing countries, where the practice of modern medicine is rare, traditional medicine is a crucial resource for the population to meet their primary healthcare needs [5]. Like other developing countries, most Ethiopians (about 80%) [6, 7] and about 90% of their livestock [8, 9] are treated using traditional medicine, of which more than 95% is prepared from plant products [9]. This is due to the lack of adequate modern health services [10, 11], the habits of cultural interaction, the ease of accessibility, the relative efficacy against certain diseases, and the low cost of using traditional medicines [2, 12]. Hence, many rural populations in Ethiopia were stated to use several medicinal plants for the treatment of various human as well as livestock ailments [11, 12]. Additionally, some urban populations employ traditional medicinal plants for their health care, although urbanization poses a great impact on the use of traditional medicine and associated indigenous knowledge [13].
Moreover, Ethiopian people have rich indigenous knowledge with respect to the use of medicinal plants for the treatment of various communicable and noncommunicable human diseases. The rural community also use medicinal plants to treat several livestock diseases like blackleg, diarrhea, Newcastle disease, colic, listeriosis, leech, eye disease, rabies, swelling, wounds, pasteurellosis, coughing, anthrax, footrot, and so on [9, 14, 15]. The people obtain indigenous knowledge of using medicinal plants through verbal transmission from parents, healers, neighbors, or friends, frequent observations, experiences, and trial-and-error practices [8]. The medicinal plants are usually harvested from natural forests, home gardens, farmlands, coffee agroforestry, stream sides, road sides, along valleys, wetland areas, and other microhabitats [16]. The local population use medicinal plant parts such as roots, stems, leaves, fruits, seeds, flowers, bark, buds, twigs, latex, bulbs, and/or whole parts as traditional medicine alone or in combinations [17,18,19].
In terms of the people in Dibatie district of the Metekel zone, different ethnic groups in the area (Agaw, Amhara, Gumuz, Oromo, and Shinasha) rely on traditional medicines, mostly in the past during the shortage of modern health centers. Even today, residents in the Dibatie district of the Metekel zone widely use traditional medicines, mainly from plant sources. Recently, however, medicinal plants have been declining along with the associated indigenous knowledge due to various factors such as the expansion of urbanization, settlements, agriculture, and the better availability of modern drugs, as well as a lack of awareness about their conservation. Moreover, the traditional medicinal plants in the Dibatie district of the Metekel zone are not documented yet, and the related local knowledge is transferred orally from generation to generation. This calls for written documentation of medicinal plants and associated indigenous knowledge for sustainable use in the future. Hence, this study was aimed at documenting the traditional medicinal plants used to treat various human and livestock ailments with the associated indigenous knowledge of the local community in Dibatie district of Metekel zone, Benishangul Gumuz Regional State, western Ethiopia.
Methods
Description of the study area
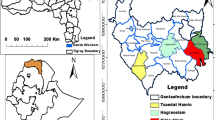
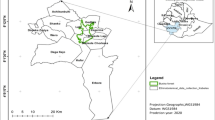
The study was conducted in Dibatie district, Metekel zone, Benishangul Gumuz Regional State, western Ethiopia (Fig. 1). Dibatie district is one of the seven administrative districts in Metekel zone. It is bordered by Mandura district in the north, Bulen district in the west, Kamashi zone in the south and southeast, and Amhara Regional State in the east. The district consists of 30 kebeles (the lowest administrative units), with five towns and 25 rural kebeles. Out of these, 11 kebeles such as Dibatie-02, Parzeyit, Lega-buna, Berber, Jan, Donben, Gipho, Tuski-gambela, Galessa, Qorqa, and Sombo-sire kebeles were selected as representative study sites (Fig. 1 and Table 1). There are five ethnic groups, namely Amhara, Gumuz, Shinasha, Oromo, and Agaw, in the district. These ethnic groups speak mother-tongue languages such as Amharic (in the Semitic family), Gumuzigna (in the Nilo-Saharan family), Shinashigna (in the Omotic family), Afaan Oromoo and Agawgna (in the Cushitic family), respectively. However, residents of the Gumuz ethnic group were not included in this study due to the ethnic conflict in the area during data collection.
The study area is characterized by highland, semi-highland, and lowland agro-ecological types [20]. The area is categorized as Combretum-Terminalia woodland vegetation, including broad-leaved deciduous woodland, Vachellia woodland, Boswellia woodland, riparian woodland, and bamboo thicket [21].
Sampling techniques
The study sites (kebeles) were selected purposefully based on the geographic variations, native ethnic group composition, security of the area, and road access to the study site. Out of the total of 9879 households, 374 informants were selected as representative respondents using the formula developed by Cochran’s (1977) with a 95% confidence level and ± 5% precision [22]. Then, 34 informants participated in the data delivery from each kebele. Households of 200 general informants were selected randomly and participated in the ethnomedicinal data collection. Besides, 174 key informants, such as knowledgeable community elders and traditional healers, were selected purposefully based on their knowledge of medicinal plants in the community.
Sociodemographic characteristics of informants
Household members of ages greater than 20 participated in the data delivery as representative respondents. Most (41.71%) informants in the present study were in the age range of 41-60 years. Out of 374 informants, the majority (67.38%) were males and 32.62% were females (Table 1).
Methods of data collection
The ethnobotanical data related to medicinal plants were collected using semi-structured interviews, preference ranking, direct matrix ranking [23], field observation, market surveys [24], and focus group discussions [25].
The interview was carried out in the appropriate languages (Afaan oromoo, Agawgna, Amharic, and Shinashigna) of the informants, with the help of translators when required. The interview was focused on remedy parts, types of human and/or livestock ailments treated, use condition, preparation method, additive agents, routes of administration, dosage, side effects and antidotes, and additional uses of the medicinal plants. The interview also included recording the local names of plants, demographic characteristics (e.g., age, gender, ethnicity, religion, occupation, literacy level, etc.) of informants, marketability, threats to, and management practices of medicinal plants in the study area. The field survey was performed, including guided field observation, focus group discussions, and the collection of voucher specimens for identification. Voucher specimens were identified and deposited in the National Herbarium (ETH) of the Addis Ababa University, following the permission letter (reference number DPBBM/CNS/487/13/2020) provided from the Department of Plant Biology and Biodiversity Management at Addis Ababa University. Botanical names were confirmed by experts in the herbarium and checked on the website Plants of the World Online (POWO) for taxonomic updates.
Methods of data analysis
The collected ethnobotanical data were analyzed through descriptive statistics like frequency and percentage, ranking, and comparison. Accordingly, informant consensus factor (ICF) was calculated to differentiate the agreement of informants on the medicinal plants reported to treat ailment categories [26]. The ailments were categorized according to the International Classification of Primary Care (ICPC-2) [27]. Then, the informants’ consensus factor was calculated using the formula: ICF \(=\frac{\text{Nur}-\text{Nt}}{\text{Nur}-1}\) where Nur is the number of use citations for a particular ailment category and Nt is the number of medicinal plant species used to treat a particular ailment category by all informants.
Preference ranking [23] was applied to assess the degree of preference of 10 medicinal plant species based on their efficacy to treat hemorrhoids in humans. A direct matrix ranking was conducted according to [23] to compare the multiple uses of selected eight (8) medicinal plants based on the eight (8) use categories, such as beehive trees, charcoal, construction, fodder, food, fuel wood, medicine, and household utensils. For this purpose, seven (7) key informants were selected and asked to rate each plant species, assigning the use values of each attribute using integer numbers from 5 (most frequently used) to 0 (not used). At the end, the average values of each plant species were summed up and ranked.
The index of fidelity level (FL) was computed to determine the relative healing potential of the medicinal plants against major human ailments [28]. The percentage of fidelity level (FL%) was computed for 25 medicinal plants against the most frequently reported ailments to be treated with them. It was calculated using the formula: \( FL\% = Ip/Iu \, \times 100, \) where Ip is the number of respondents who independently stated the use of a species for the same major ailments and Iu is the total number of respondents who indicated the plant for any ailments.
Jaccard’s coefficient of similarity (JCS) [29] was computed to determine the medicinal plant species use similarity between the ethnic groups of Dibatie district. The JCS was calculated using the formula: \(\text{JCS}=\frac{c}{(a+ b + c)}\) where JCS is the Jaccard’s coefficient of similarity, a is the plant species used by ethnic group A, b is the plant species used by ethnic group B, and c is the common plant species used by both ethnic groups A and B. Then, the percentage of proximity was obtained by multiplying the similarity coefficient by 100 [30].
The use value (UV) index was computed to assess the degree of importance of medicinal plant species using the formula: \( UV = \left( {\Sigma Ui} \right)/n \), modified from Phillips and Gentry (1993a, 1993b) [31,32,33], where Ui is the number of uses mentioned by each informant for a specific species and n is the total number of informants. The statistical analysis was carried out using Ms-Excel (version 2013), Statistical Package for the Social Sciences (SPSS) version 21.0, and R-statistical packages (ggplot2, scales, and dplyr).
Results and discussion
Taxonomic diversity of medicinal plants
A total of 170 medicinal plants were identified from the study area, which belong to 144 genera and 65 families. Fabaceae and Asteraceae were the most dominant medicinal plant families represented by 20 and 18 species, respectively, followed by Euphorbiaceae and Solanaceae (eight species each), Cucurbitaceae (seven species), Lamiaceae (six species), Apocynaceae and Vitaceae (five species each), Apiaceae, Malvaceae, Poaceae, and Rubiaceae (four species each), Acanthaceae, Asparagaceae, Moraceae, Polygonaceae, Ranunculaceae, and Rutaceae (three species each), and the remaining 47 families were represented by two or less medicinal plant species each (Table 2). Genus Solanum was represented by four species, followed by Cyphostemma, Echinops, Euphorbia, Ficus, and Rumex genera, represented by three species each, and the other remaining genera had two or one medicinal plant species each.
Consistent with the current study, other studies conducted by [30, 34, 35] in different parts of Ethiopia reported the dominance of medicinal plants within the Fabaceae and Asteraceae plant families. This might be because of the phytochemicals shared among medicinal plants in the same taxonomic categories [36]. Hence, medicinal plants in the Fabaceae and Asteraceae families could be rich in bioactive chemical compounds that contribute to their therapeutic roles. In contrast, some researchers [37, 38] justified the point of view that Fabaceae and Asteraceae are the most widely abundant families in the Flora regions of Ethiopia than other plant families.
Growth habit of the medicinal plants
The most dominant growth habits of medicinal plants in the study area were herbs (61 species, 35.88%), followed by shrubs (39 species, 22.94%), trees (38 species, 22.35%), and climbers (22 species, 12.94%), while subshrub growth forms were found to be the least (10 species, 5.88%) (Fig. 2). In agreement with the current study, previous studies [34, 37, 39,40,41,42] conducted in different areas of Ethiopia reported the dominance of herbaceous medicinal plants. This might be due to the more widespread distribution of herbaceous plants than plants of other growth habits [35, 41]. However, other studies in Suro Barguda district, southern Ethiopia [38], and in Kebridehar and Shekosh districts, southeast Ethiopia [43] revealed the dominance of shrub growth forms of medicinal plants. In this sense, the habits of medicinal plants in these areas might be different from the current study area due to the dominance of shrubby vegetation types and their geographic locations in semiarid or arid areas of the country.
Cultivation status of medicinal plants in the study area
Results showed that most (135 species, 79.41%) medicinal plants in the current study area were harvested from wild habitats. While some (32 species, 18.82%) medicinal plants were found as cultivated plants, a few others (three species, 1.76%) were found to be semi-wild (Fig. 3). This indicates that the cultivation of medicinal plants in the present study area is very low. Similarly, previous studies elsewhere in Ethiopia [9, 15, 30, 35, 37, 44, 45] reported that medicinal plants are usually harvested from wild sources and rarely available as cultivated plants or as both cultivated and wild plants. The abundance of medicinal plants in the wild habitat increases their exposure to different threats like overexploitation [15, 46], deforestation, and habitat destruction [47]. Thus, the practice of domesticating medicinal plants is important to access the plants easily and to ensure their survival in the future.
Proportion of medicinal plants used to treat human and livestock ailments
Out of the recorded medicinal plants, 105 species (61.76%) were used to treat only human ailments, 13 species (7.65%) were used to treat only livestock illnesses, and 52 species (30.59%) were used to treat both human and livestock ailments (Fig. 4). The documented medicinal plants were traditionally used for the treatment of 79 types of human ailments and 29 kinds of livestock ailments. The most commonly treated human ailments were snake venom, wounds, diarrhea, general malaise, impotency, toothache, tonsillitis, boils, evil eye, hemorrhoids, placenta retention, rabies, abdominal pain, hepatitis, lymphadenitis, rectal prolapse, swelling, leishmaniasis, ascaris, cough, choking, gastritis, Bell's palsy, gonorrhea, arthritis, scorpion venom, eye disease, tapeworm, tinea versicolor, and vomiting consecutively, among others based on informant citation. While the commonly cured livestock ailments were found to be blackleg, rabies, wound worms, diarrhea, placenta retention, breast swelling, eye disease, hemorrhoids, snake venom, Newcastle disease, tick infestation, constipation, denying of milk, milk deficiency, swelling, wounds, ascaris, cattle thinness, colic, choking, difficulty of excretion, febrile disease, chicken lice, leech infestation, lumpy skin, mouth wound, respiratory disorder, sneezing, and difficulty of urine flow successively (Additional file 1).
The number of human ailments traditionally treated by the medicinal plants in the present study area was comparable to the number of human ailments (81) reported in Asagirt district, northeastern Ethiopia [34]. The number of human ailments stated to be treated in the present study was greater than those of earlier studies [17, 37, 39, 42, 45, 48,49,50] in different regions of Ethiopia. On the other hand, the number of livestock ailments reported to be treated by the medicinal plants in the current study exceeds those of previous findings [9, 14, 15, 17, 39, 45, 49], but is exceeded by the study [51] conducted in the Adea Berga district of West Shewa zone, central Ethiopia. The current results showed that multiple ailments could be treated by a single plant species, and more than one plant species could also be used to treat a single ailment, in line with the previous reports [17, 34] in Ethiopia. The results clearly indicate the rich medicinal plant diversity and strong relationships between the plants and residents in the current study area with respect to human and livestock health care. This might be because of the predominance of mixed agriculture (crop cultivation and animal husbandry) and the remoteness of the area for access to modern health care.
Medicinal parts of the plants in the study area
In the study area, most (70 species, 41.17%) medicinal plants provide multiple medicinal parts from a single plant, followed by medicinal plants with leaves (29 species, 17.06%), roots (19 species, 11.18%), bark (14 species, 8.24%), tubers (11 species, 6.47%), seeds (eight species, 4.71%), and whole plants (eight species, 4.71%) as the common remedy parts. The others contribute medicinal parts such as fruits (four species, 2.35%), exudate (three species, 1.76%), aerial parts (two species, 1.18%), and stems (two species, 1.18%) (Fig. 5).
The current results showed that multiple plant parts were prepared as remedies from a single species, consistent with studies in other countries [52, 53] and less frequently in Ethiopia [30, 37, 54]. This indicates the pharmaceutical potential of the plants due to the presence of bioactive phytocompounds in many parts. Besides, the present study revealed most (29 species) medicinal plants with leaves as remedy parts next to plants with more than one remedy part (70 species). Similarly, leaves were reported as the most commonly used medicinal plant parts in different regions of Ethiopia [34, 37, 39, 44, 46, 55] and other countries [52, 53, 56]. The regular use of leaves as traditional medicine might be due to the ease of preparations and the excess of bioactive compounds that promote their efficacy [42, 52]. The use of leaves instead of other plant parts can minimize risks related to the loss of medicinal plants [44]. Nevertheless, frequent utilization of whole plants [57,58,59] and roots [43, 60,61,62,63] has been reported in different parts of the world, including in Ethiopia. However, extensive use of whole plants and roots of medicinal plants can pose adverse effects on their survival and continuity in the future [52, 64, 65] though the use of rhizomes, bulbs, flowers, bark, and stems may also cause destructive effects relative to the harvesting of leaves [42, 66, 67].
Use conditions of the medicinal plants
The medicinal plant parts in the present study were mostly used in fresh form (60.13%), followed by either fresh or dry condition (31.28%), and sometimes utilized in dry state (8.59%) (Fig. 6). Likewise, several researchers [34, 37, 41, 42, 44,45,46] reported the fresh preparation of traditional medicinal plants elsewhere in the country. A fresh prescription was often performed to give a remedy immediately to the patient. Utilization of fresh medicinal plants may have the advantage of reducing the loss of bioactive phytochemicals upon drying, thus enhancing their efficacy [39]. However, the frequent use of fresh remedies has been considered one of the threats to medicinal plants since they are not preserved for later usage [38]. The current finding also verified that some medicinal plants were prescribed in dry conditions and could be stored for months or a year with efficacy in healing. This might be due to the fact that dry remedy preparation increases the shelf life of the medicine [42].
Preparation methods of the medicinal plants
Results showed that local communities prepare herbal remedies mostly by crushing (25.49%), followed by pounding (19.12%), chewing (11.11%), powdering (8.50%), squeezing (8.17%), heating (6.54%), boiling (4.58%), rubbing (4.41%), exuding (3.92%), cutting/slicing (2.61%), burning (2.12%), roasting (1.63%), and sousing (0.98%), and there was also direct consumption (0.82%) of some medicinal plant parts without processing (Fig. 7). The present findings agreed with numerous ethnobotanical studies [11, 30, 34, 37, 41, 43,44,45, 50, 68], which reported crushing medicinal plants as a common means of traditional remedy preparation in many areas of the country. However, other studies reported grinding [49], pounding [38, 42, 46], and squeezing [9] as the most commonly employed methods of remedy preparation in different parts of Ethiopia. The remedy preparation method might vary depending on the nature of medicinal plant parts and ease of processing [34, 39], as well as the kind of illness and its site on the body parts [43]. For instance, the remedies taken orally and those applied topically may require different processing mechanisms to be administered safely. Additionally, fresh and dried medicinal plant parts may not need similar preparation methods. That is, dry plant materials are suitable for grinding or powdering, while fresh plant parts are good for crushing, pounding, and squeezing.
Application routes of the medicinal plants
The informants in the present study area described that herbal medicines were mainly applied orally (52.20%), followed by dermal (17.62%), through fumigation (9.69%), and topical (9.69%) methods. In addition, informants confirmed that there were multiple ways (4.41%) of application for a specific ailment and ocular (1.98%), nasal (1.76%), external (1.54%), and aural (1.10%) means of administration (Fig. 8). The current results were consistent with several ethnobotanical findings in different regions of Ethiopia [30, 34, 37, 40, 41, 44,45,46] and other countries [3, 52, 58, 69, 70]. However, other studies [39, 71, 72] reported dermal remedy administration as the most frequently applied in some areas of Ethiopia. The present study also reported the practice of using more than one route of administration to treat a single disease type, which is in line with previous findings [11]. This might be essential to the synergistic effect of remedies in healing an ailment through a variety of applications. The preference for oral remedy administration might be due to its simplicity [46] or the fast reaction of the medicine to take part in healing [30]. The routes of administration may also be dependent on the type of illness and the location of affected body parts [44]. Accordingly, internal ailments need to be treated through oral modes of remedy application [34, 45], while cutaneous diseases require dermal routes of administration [71, 72].
Additives or solvents of the medicinal plants
The herbal remedies in the current study area were applied with or without additives. Indeed, the majority (32.72%) of medicinal plants were administered without additive ingredients. However, some herbal medicines were applied with additives such as water (29.70%), salt (9.23%), local alcoholic and nonalcoholic drinks (‘Tella,’ ‘Kenneto,’ or ‘Areqe’) (5.70%), honey (4.70%), porridge or syrup (4.53%), chicken stew (3.69%), butter (2.85%), sugar (2.35%), milk, yogurt, or cheese (1.34%), bread or ‘Injera’ (0.84%), spices (0.67%), hot drinks (tea or coffee) (0.67%), soot (0.34%), egg yolk (0.34%), and Hippo's skin ash (0.34%) (Table 3). A large proportion of traditional remedies in the present study were administered with no additives, similar to the previous findings [39, 46] in the country. On the other hand, the current findings are in line with other studies [40, 45, 73], which reported the use of additives like coffee, milk, honey, water, salt, tea, red ‘teff’ powder, sugar, butter, eggs, bread (‘Injera’), and local alcoholic beverages (‘Tella’ and ‘Areqe’). However, none of the previous studies have claimed the use of soot and Hippo's skin ash as additives to herbal medicine. This implies the diversity of indigenous knowledge among ethnic groups in Dibatie district with respect to the utilization of traditional medicines. The local people may use additives or solvents in traditional medicine in order to reduce the toxicity, soften the remedy, or improve the taste [10, 30, 45]. Informants in the present study stated that additives were also believed to be vital for increasing the healing efficacy of the remedies, consistent with the previous reports [38, 43] in their respective study areas. This may need extra investigation to prove whether the healing potential is from additives, medicinal plants, or the synergistic effects of both ingredients. Water is the most frequently used vehicle in the preparation of herbal medicine in the present study as well as other ethnomedicinal studies [45, 46] in Ethiopia. This might be because of the easy access to water compared to other ingredients or solvents in the locality.
Adverse effects and antidotes for the traditionally used medicinal plants
In the current study area, no adverse effects were reported for most (97 species, 57.06%) medicinal plants. Out of the documented medicinal plants, 35 species (20.59%) were reported to have adverse effects related to overdosage, and 23 species (13.53%) were stated to have negative effects due to misuse of prescription. Other medicinal plants were reported to cause diarrhea (20 species, 11.76%), vomiting (17 species, 10.00%), body irritation (16 species, 9.41%), gastric inflammation (nine species, 5.29%), effect on pregnancy (six species, 3.53%), bad mouth feeling (five species, 2.94%), effect on eyesight (five species, 2.94%), skin damage (five species, 2.94%), and tooth crushing (two species, 1.18%). Local people described numerous antidotes (milk, butter, egg yolk, porridge, syrup, linseed, ‘Injera,’ honey, sugar, coffee, and water) and prevention methods that could be employed to counteract adverse effects (Table 4).
In agreement with the present study, a study [46] conducted in the Artuma Fursi district of northeast Ethiopia reported that most (81.25%) medicinal plants have no complaints of adverse side effects. On the other hand, the current study aligns with other studies that described some adverse effects such as overdose problems [41], diarrhea, vomiting [37, 41, 42, 74, 75], bad mouth taste [42], gastric or stomach inflammation [37, 42], and skin irritation [75] in different parts of Ethiopia. Participants in the present study reported that the occurrence of diarrhea, vomiting, and internal inflammation was usually linked with an overdosage of orally applied medicines. Reports from similar investigations [16, 39] indicated the dosage effect associated with oral prescriptions. This might be correlated with the highly sensitive nature of the internal body parts compared to the external body.
Local communities in the present study area and across the country [10, 38, 39, 41] were reported to use various antidotes like milk, butter, honey, coffee, linseed, and local drinks to reduce the risks related to the negative effects of traditional medicines. For instance, overdosage of Euphorbia abyssinica J. F. Gmel. exudate was reported to cause diarrhea and weaken the patient when taken orally to treat gonorrhea; hence, milk or porridge was given as an antidote by traditional practitioners. Informants also indicated that E. abyssinica exudate was formulated with butter and creamed dermally to treat Tinea versicolor as it wounds the skin alone. Local people in the present study area also indicated that doses of herbal medicines were adjusted based on the age and physical condition of the patients, which is found to be consistent with previous report [10] in Gubalafto district, northern Ethiopia. Although some negative side effects were recorded, herbal medicines have recently been mentioned as safer than conventional drugs [68, 76]. Therefore, medicinal plant remedies and associated local knowledge need to be preserved as low-risk indigenous medicines.
Informant consensus factor (ICF)
The informant consensus factor (ICF) was calculated to assess the homogeneity of informants’ traditional knowledge on the use of medicinal plants against the broadly classified human ailments in the study area. Accordingly, the ICF value ranges from 0.45 for pregnancy-related ailments (abortion, erythroblastosis fetalis, infertility, milk deficiency, and placenta retention) to 0.88 for psychosocial problems (enemy protection, nightmares, and human relationships), as shown in Table 5. The highest ICF value indicates a good degree of agreement among informants on the utility of the reported medicinal plants to cure a particular ailment category, and vice versa [44, 49]. As a result, the highest agreement was observed between the respondents with respect to the medicinal plants used to treat psychosocial problems, followed by that of general and unspecified ailments such as acute febrile illness, chest pain, evil eye, general malaise, internal swelling, leech infestation, malaria, rabies, splenomegaly, trauma, and typhoid fever (ICF = 0.72). Here, the ICF value was different for each disease type involved in a specific disease category. This is because the current result was computed for a combination of disease types in a certain disease category.
Preference ranking of plants used to cure hemorrhoids in humans
Results of the preference ranking performed by ten (10) key informants on 10 medicinal plants used to treat hemorrhoids showed that Euphorbia abyssinica was the most preferred (ranked 1st) to heal the disease, followed by Calotropis procera (Aiton) W. T. Aiton (2nd). The remaining medicinal plants were ranked from 3rd to 10th, as illustrated in Table 6. Here, informants gave the highest score (number 7) for the medicinal plant that they thought was most effective and the lowest (number 1) for the least effective medicinal plant species. This indicates that local communities prefer one medicinal plant over the other, while multiple plant species were also suggested to cure a particular ailment.
The results of the preference ranking showed that E. abyssinica was the most preferred medicinal plant to treat hemorrhoids. In support of this finding, earlier ethnomedicinal studies [42, 45] have reported its traditional utility to cure hemorrhoids in different parts of Ethiopia. In addition, E. abyssinica was reported to treat other human ailments, including gastritis [44], gonorrhea [40], hepatitis [77], jaundice [78], leishmaniasis, malaria, stomachache [45], rabies [78, 79], and swelling [80] across the country. A second plant, C. procera, was also reported to treat different human ailments such as breast cancer [81], hemorrhoids [41, 46], wart [55], breast swelling, dyspepsia, herpes zoster, typhoid [25], and wounds [73]. This indicates their important role in the primary health care of various communities in Ethiopia.
Fidelity level (FL) index
Results showed that Embelia schimperi Vatke, Glinus lotoides L., Haplosciadium abyssinicum Hochst., Mucuna melanocarpa Hochst. ex A. Rich., and Phragmanthera macrosolen (Steud. ex A. Rich.) M.G.Gilbert had the highest fidelity level values (100%) against the corresponding ailments, followed by Cassia arereh Delile, Justicia schimperiana (Hochst. ex Nees) T. Anderson., Phytolacca dodecandra L'Hér., and Acmella caulirhiza Delile with the fidelity level values of 92%, 88%, 85%, and 80%, respectively (Table 7). This may indicate that these plants were effective against the corresponding ailments explained to be treated with them. The plants mentioned for the same ailment by many respondents will have the greatest FL% values, while those reported for many ailments exhibit the least [44]. In the present study, for example, Croton macrostachyus Hochst. ex Delile exhibited the lowest (21%) fidelity level value against gonorrhea since it was reported for the healing of several (14) diseases compared to others. The medicinal plants with the highest FL% values were claimed to be helpful in getting a hint of their phytochemical constituents for further investigation of bioactive compounds [34].
The results of the fidelity level showed that E. schimperi and G. lotoides were effective in curing tapeworm in the study area. Similarly, other studies elsewhere in Ethiopia revealed the traditional use of E. schimperi [42, 82,83,84,85] and G. lotoides [8, 42, 73, 86] for the treatment of tapeworm. This indicates the pharmaceutical potential of both E. schimperi and G. lotoides to treat intestinal parasites in different parts of the country. Consequently, these plants and associated indigenous knowledge need to be recognized by the Ministry of Health for sustainable health care throughout the country.
Jaccard’s similarity coefficient on local knowledge among ethnic groups
The results of Jaccard’s similarity coefficient revealed that the traditional utilization of medicinal plants in Oromo and Shinasha ethnic groups showed the highest (44%) resemblance, followed by Amhara and Shinasha ethnic groups accounting for 29% (Table 8). This may indicate the greatest share of indigenous knowledge between Oromo and Shinasha people relative to the other communities in the area.
Taboos related to medicinal plants in the study area
In the current study, some informants verified that they do not put medicinal plants, even in their containers, on the ground. They believe the remedy loses its efficacy if placed on the ground. Informants also indicated that a snake-bitten person should not be allowed to get into the old traditional house and should not be allowed to sleep until recovery. This is because local people thought a person could not wake up after sleeping, thinking he or she might die. Traditional healers usually collect herbal medicines early in the morning before washing their hands and eating breakfast, hiding themselves from humans or animals. Some healers justified that morning hands were perceived to have the power to heal patients. Healers also verified that they hide themselves for the sake of efficacy, though it might be in order not to show their remedies to other people. About 10% of traditional healers indicated that they keep themselves away from sexual intercourse during the days of harvesting, preparing, and applying the herbal remedies. Healers also pointed out that patients restrict themselves from sexual intercourse while taking the remedies. In addition, informants confirmed that Monday, Wednesday, and Friday were the preferred days for remedy harvesting, preparation, and administration. They believed that a remedy would lose its effectiveness if the rules were violated.
Additional uses of medicinal plants in the study area
The medicinal plants in the study area were used by the local residents for a variety of purposes beyond their medicinal uses. Even if all plants are ecologically important, almost all (168 species, 98.82%) medicinal plants in the present study were reported to have extra uses other than their use in herbal medicines. In this perspective, they were reported to be used as bee forage, fodder, food, fuel wood, fence, shade, household utensil, construction, cleaning material, beehive tree, land demarcation, tooth brush, fumigating material, farm material, fishing implement, traditional soap, beehive raw material, charcoal, local beverage, soil fertilizer, timber, bread wrap, hot drink, handling stick, ornament, stimulating drug, lubricant, and plate softening (Additional file 1). Similar findings elsewhere in the country [15, 30, 38, 41, 43, 46, 49] revealed extra uses of medicinal plants in addition to their therapeutic values.
The direct matrix ranking results of multipurpose medicinal plants showed that Ficus sycomorus L. was ranked first and Cordia africana Lam. was second for eight use types such as beehive trees, charcoal, construction, fodder, food, fuel wood, medicine, and household utensils. The remaining multipurpose plants, Ficus sur Forssk., Stereospermum kunthianum Cham., Croton macrostachyus, Grewia mollis Juss., Breonadia salicina (Vahl) Hepper & J. R. I.Wood, and Terminalia laxiflora Engl. & Diels, were ranked 3rd, 4th, 5th, 6th, 7th, and 8th, respectively. Besides, the comparison of use categories revealed that the use of these plants for construction ranked 1st, followed by the uses for fuel wood, medicine, fodder, charcoal, household utensils, beehive trees, and food, which ranked 2nd to 8th, respectively (Table 9). This result indicates that multipurpose medicinal plants in the present study area have been highly used for construction purposes compared to other uses. This in turn may lead to the depletion of primarily used plant species unless proper awareness is created in the community for the wise use and sustainable management practices of the plants. For example, informants confirmed that Breonadia salicina, Stereospermum kunthianum, and Terminalia laxiflora were highly required for construction due to their high termite resistance, and Cordia africana was extensively needed for timber production. It is evidenced that those widely used multipurpose medicinal plants are often threatened owing to overexploitation for different purposes [15, 39, 41, 46]. The loss of multipurpose medicinal plants leads to the loss of associated indigenous knowledge, increasing the incidence of human and livestock health problems [48]. Therefore, these multipurpose medicinal plants need to be used wisely and given appropriate conservation considerations in the current study area and the country as a whole.
Use value (UV) index
The use value was calculated for each plant species to determine the relative importance of the recorded medicinal plants in the study area. In this perspective, Breonadia salicina, Ficus sycomorus, and Terminalia laxiflora had the highest importance in the community, accounting for UV of 5.00 each, followed by Cordia africana with UV = 4.24, among others (Table 10). The use value of each of the recorded medicinal plants in the study area was provided in Additional file 2. The use value is crucial in estimating the most locally useful plant species and assessing the possible usages of a particular plant [31]. However, the technique of UV measures only some aspects of human and plant interaction since it is influenced by the number of uses and the consensus of informants [32].
In the current study area, B. salicina was among the medicinal plants with the highest use values within the communities. It is an evergreen, riverine tree reported to be essential for water conservation, refreshing air sources, and a shelter for wild life like birds, reptiles, and monkeys. In the present study, F. sycomorus was also one of the most important medicinal plants, with crucial environmental and cultural roles. In this sense, it was reported to serve as a shade for animals during sunny times, and local people usually conduct meetings under its shade. In line with the current study, a study conducted by [87] reported that F. sycomorus was a culturally respected tree under which certain ritual ceremonies and meetings are held in the Oromia region of Ethiopia. In addition, B. salicina and F. sycomorus were among the indigenous trees traditionally used for beehive hanging and transferred from father to son along the generations. This implies that these plants are critical from environmental and cultural perspectives within the communities and need to be conserved for future generations.
Moreover, Cordia africana and Phoenix reclinata were medicinal plants culturally important in the present study area. Indeed, C. africana was reported as a source of timber used for the preparation of traditional furniture, household utensils, and doors or windows in traditional houses. In support of the current finding, a study conducted by [88] revealed the cultural significance of C. africana in the local communities of Gozamin district, East Gojjam zone, Northwest Ethiopia. In the present study area, P. reclinata was also reported to be used for the preparation of traditional mats and baskets. Furthermore, Portulaca quadrifida L. was a cultural food plant in Shinasha ethnic groups, and it was usually consumed as homemade stew or in hotels due to its role as an appetizer and gastric reliever. In this regard, some medicinal plants play crucial roles in cultural heritage through material cultures or foods.
Marketability of medicinal plants in the study area
The market survey and interview showed that most (133 species, 78.24%) medicinal plants in the study area were not marketable. Only few (two species, 1.18%) medicinal plants were reported to be sold for medicinal purpose. Some medicinal plants were vended for other purposes, such as food or local drinks (24 species, 14.12%), stimulant drugs (two species, 1.18%), and traditional plate softening (one species, 0.59%), but they were also used as traditional medicine. Others were marketable for the purposes of construction (three species, 1.76%), making household utensils (three species, 1.76%), milk containers’ fumigation (one species, 0.59%), and timber (one species, 0.59%).
Similar ethnomedicinal studies [9, 35, 37, 42, 72] reported that the majority of traditional medicinal plants in Ethiopia were not marketable for medicinal purposes. Instead, they were reported to be sold usually for spice, food, firewood, construction, beverage, and as stimulants [37, 42], consistent with the reports of the present study. This indicates that medicinal plants are less marketable in the open market across the country. It might be due to the habits of the local residents, who search for medicinal plants only when required for medication [35]. As a result, it is important to promote the trading of medicinal plants to improve income generated from them at local and national levels. In the present study area, however, traditional healers were reported to take payments at the moment of treatment, though the amount of payment varied from one healer to another. Similarly, an earlier study [40] reported the trading of medicinal plants by healers at home instead of selling them on the open market. The reason is that most traditional practitioners prefer to hide their medicinal plants [72].
Threats to and conservation practice of medicinal plants
The results of ethnobotanical survey revealed that medicinal plants in the study area were mainly threatened by deforestation accounting for 33.70% of the total threats reported. Overgrazing or browsing was the second-cited threat, accounting for 18.75%. The other threats to medicinal plants in the area were drying (12.50%), overexploitation (10.33%), agricultural expansion (8.83%), herbicides (6.79%), wildfire (3.53%), uprooting as a weed (3.40%), destruction by animals (1.49%), and insect or fungal pests (0.68%).
The local people in the present study area cut medicinal plants to fulfill their various livelihood needs, such as shelter construction for themselves or domestic animals, fuel wood, fodder, fences, household materials, charcoal, and so on. A number of researchers [15, 34, 37, 42, 43] reported the destruction of medicinal plants for different purposes elsewhere in Ethiopia. On the other hand, other similar studies [37, 40, 44, 45] described agricultural expansion as the most commonly threatening factor for medicinal plants in their respective study sites. In the present study, overgrazing was also mentioned as a common threat to medicinal plants. This might be linked with the mixed agricultural lifestyle of the residents, which involves the livestock husbandry parallel to crop production. In this respect, farmers cut some medicinal plants for their cattle during dry season beyond free grazing. Informants also claimed that drying was the third most threatening factor to medicinal plants. The months from January to April were usually dry in the study area. Hence, annual herbs, including herbaceous medicinal plants, have disappeared during these months. This is in line with the previous studies [9, 39, 43], which identified drying and overgrazing as serious threats to medicinal plants in different parts of Ethiopia.
Overexploitation for multiple uses was reported to be a cause for the current depletion of medicinal plants, consistent with the earlier reports [15, 39, 41, 46] in the country. In the present study area, for example, Albizia gummifera (J. F.Gmel.) C. A. Sm. was debarked by fishermen, and its bark was added to water, collecting a fainted fish. This may lead to the death and reduction of plant species if the activity continues for years. Besides, the use of herbicides and the uprooting of medicinal plants as weeds were reported in the farming areas, similar to the findings [39] in Adwa district, northern Ethiopia. This might be because of the low awareness of the local community about the ecological, economic, and health benefits of medicinal plants. In addition, wildfire, destruction by animals, and insect or fungal attacks on medicinal plants were described in the study area. This calls for urgent conservation measures to ensure the continued availability of medicinal plants in the current study area and across the country.
Out of the reported 170 medicinal plant species, most (112 species, 65.88%) were not conserved in the study area. Some (28 species, 16.47%) medicinal plants were cultivated by the local communities as staple foods, spices, fruit plants, or cash crops. Some others (19 species, 11.18%) were conserved by traditional healers for medicine, and a few (11 species, 6.47%) were preserved by the local communities near home gardens or farmland for various purposes, such as construction, timber, live fences, shade, land demarcation, and beehive hanging trees.
In the current study area, the conservation practice of medicinal plant resources was very minimal. Similar studies [25, 35, 41, 50] also reported the poor conservation practices of medicinal plants in different regions of Ethiopia. This might be due to the fact that the majority of medicinal plants in the country are from wild natural vegetation [44, 45]. However, a little bit of effort toward medicinal plant conservation was started by traditional healers and some local communities in the present study area. For example, some originally wild medicinal plants, such as Acmella caulirhiza, Aloe benishangulana Sebsebe & Tesfaye, Brucea antidysenterica J. F. Mill., Calpurnia aurea (Aiton) Benth., Cissus quadrangularis L., Euphorbia abyssinica, Kalanchoe densiflora Rolfe, Ocimum gratissimum L., Rumex nepalensis Spreng., Rumex nervosus Vahl, Withania somnifera (L.) Dunal, and Verbena officinalis L. were observed being protected in the gardens of traditional healers for medicinal purposes. This is in alignment with the reports of previous findings [34, 35, 42] in different regions of Ethiopia. Recently, administrative leaders and development agents have progressed legally to inhibit the cutting of trees, especially riverine trees like Breonadia salicina and Syzygium guineense (Wild.) DC. subsp. guineense, for the preservation of the plants as well as the rivers. In addition, local residents were reported to apply certain indigenous conservation measures by protecting some plants near homesteads, on farmlands, as live fences, and as beehive hanging trees. The beehive hanging trees like B. salicina, C. macrostachyus, and F. sycomorus have been transferred from a father to a son, just like permanent wealth in the area. Likewise, previous study [42] conducted in the Ensaro district of northern Ethiopia reported the indigenous conservation methods of useful medicinal plant species. As a result, the conservation initiative habits of the local communities should be encouraged, and in situ and ex situ conservation strategies should be applied for sustainable use of medicinal plant resources, preserving the associated indigenous knowledge throughout the country.
Conclusions
This study documented 170 traditional medicinal plant species used for the treatment of 79 human and 29 livestock ailments. The study area was rich in potential medicinal plant diversity and associated indigenous knowledge that can be used to alleviate various human and domestic animal health problems. For example, Bell's palsy, boils, diarrhea, general malaise, gonorrhea, hemorrhoids, hepatitis, herpes, impotency, leishmaniasis, lymphadenitis, rabies, rectal prolapse, tumors, tapeworm, and wounds were among the human diseases reported to be traditionally cured by medicinal plants in the Dibatie district. Blackleg, rabies, wound worm, diarrhea, placenta retention, breast swelling, eye disease, hemorrhoids, Newcastle disease, tick infestation, febrile disease, leech infestation, and lumpy skin were examples of livestock ailments treated by traditional practitioners. Nevertheless, the beneficial medicinal plant species in the study area were predominantly threatened by anthropogenic factors due to cutting or deforestation for the purposes of construction, fuel wood, fodder, timber, charcoal, fences, household utensils, farm materials, and so on. The conservation practices of medicinal plants in the study area were found to be very low, though some conservation habits were observed from traditional healers and some community members. Therefore, immediate conservation practices, proper management, and careful utilization of medicinal plants should be employed by the local community and the office of natural resource management in the Dibatie district to sustain the health benefits and transfer them to the next generation along with the associated indigenous knowledge. Moreover, appropriate registration and recognition should be given to the traditional healers by concerned bodies like the health office in Dibatie district, the Ethiopian Public Health Institute, and the Armauer Hansen Research Institute in order to encourage them and preserve their indigenous knowledge. Furthermore, the recorded medicinal plants need to be validated through experimental studies by the researchers of different institutions in the country to integrate traditional medicine with modern medication systems and improve healthcare services.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request, and some datasets are provided within the supplementary information files.
Abbreviations
- ETH:
-
National Herbarium
- FL:
-
Fidelity level
- ICF:
-
Informant consensus factor
- ICPC:
-
International Classification of Primary Care
- JCS:
-
Jaccard’s coefficient of similarity
- POWO:
-
Plants of the World Online
- SPSS:
-
Statistical Package for the Social Sciences
- UV:
-
Use value
References
World Health Organization. WHO global report on traditional and complementary medicine 2019. Geneva: World Health Organization; 2019. https://apps.who.int/iris/handle/10665/312342. Accessed 26 Aug 2023.
Aschale Y, Tegegne BA, Yihunie W. Medicinal plants used for the management of hepatitis over the past 15 years in Ethiopia: a systematic review. HMER. 2023;15:11–9.
Nakibuuka MM, Mugabi R. Ethnobotanical study of indigenous nutri-medicinal plants used for the management of HIV/AIDS opportunistic ailments among the local communities of central Uganda. Sci African. 2022;16:e01245.
Enioutina EYu, Salis ER, Job KM, Gubarev MI, Krepkova LV, Sherwin CMT. Herbal Medicines: challenges in the modern world. Part 5. Status and current directions of complementary and alternative herbal medicine worldwide. Expert Rev Clin Pharmacol. 2016;1–12.
Oyebode O, Kandala N-B, Chilton PJ, Lilford RJ. Use of traditional medicine in middle-income countries: a WHO-SAGE study. Health Policy Plan. 2016;31:984–91.
Hailu F, Cherie A, Gebreyohannis T, Hailu R. Determinants of traditional medicine utilization for children: a parental level study in Tole District, Oromia, Ethiopia. BMC Complement Med Ther. 2020;20:125.
Aragaw TJ, Afework DT, Getahun KA. Assessment of knowledge, attitude, and utilization of traditional medicine among the communities of Debre Tabor Town, Amhara Regional State, North Central Ethiopia: a Cross-Sectional Study. Evid-Based Complementary Alternative Med. 2020;2020:1–10.
Moges A, Moges Y. Ethiopian common medicinal plants: Their parts and uses in traditional medicine - ecology and quality control. In: Gonzalez A, Rodriguez M, Gören Sağlam N, editors. Plant science - structure, anatomy and physiology in plants cultured in vivo and in vitro. 2020. https://www.intechopen.com/books/plant-science-structure-anatomy-and-physiology-in-plants-cultured-in-vivo-and-in-vitro/ethiopian-common-medicinal-plants-their-parts-and-uses-in-traditional-medicine-ecology-and-quality-c. Accessed 25 Aug 2023.
Abebe M. The study of ethnoveterinary medicinal plants at Mojana Wodera district, central Ethiopia. Ishtiaq M, editor. PLoS ONE. 2022;17:e0267447.
Chekole G. Ethnobotanical study of medicinal plants used against human ailments in Gubalafto District, Northern Ethiopia. J Ethnobiol Ethnomed. 2017;13:55.
Demie G, Negash M, Awas T. Ethnobotanical study of medicinal plants used by indigenous people in and around Dirre Sheikh Hussein heritage site of South-eastern Ethiopia. J Ethnopharmacol. 2018;220:87–93.
Tolossa K, Debela E, Athanasiadou S, Tolera A, Ganga G, Houdijk JG. Ethno-medicinal study of plants used for treatment of human and livestock ailments by traditional healers in South Omo, Southern Ethiopia. J Ethnobiol Ethnomed. 2013;9:32.
Teshome-Bahiru W. Impacts of urbanisation on the traditional medicine of Ethiopia. Anthropologist. 2006;8:43–52.
Assefa A, Bahiru A. Ethnoveterinary botanical survey of medicinal plants in Abergelle, Sekota and Lalibela districts of Amhara region, Northern Ethiopia. J Ethnopharmacol. 2018;213:340–9.
Asfaw A, Lulekal E, Bekele T, Debella A, Debebe E, Sisay B. Medicinal plants used to treat livestock ailments in Ensaro District, North Shewa Zone, Amhara Regional State, Ethiopia. BMC Vet Res. 2022;18:235.
Kassa Z, Asfaw Z, Demissew S. An ethnobotanical study of medicinal plants in Sheka Zone of Southern Nations Nationalities and Peoples Regional State, Ethiopia. J Ethnobiol Ethnomed. 2020;16:7.
Kidane L, Gebremedhin G, Beyene T. Ethnobotanical study of medicinal plants in Ganta Afeshum District, Eastern Zone of Tigray, Northern Ethiopia. J Ethnobiol Ethnomed. 2018;14:64.
Chekole G, Asfaw Z, Kelbessa E. Ethnobotanical study of medicinal plants in the environs of Tara-gedam and Amba remnant forests of Libo Kemkem District, northwest Ethiopia. J Ethnobiol Ethnomed. 2015;11:4.
Wondimu T, Asfaw Z, Kelbessa E. Ethnobotanical study of medicinal plants around ‘Dheeraa’ town, Arsi Zone, Ethiopia. J Ethnopharmacol. 2007;112:152–61.
Anbessa B, Lulekal E, Getachew P, Hymete A. Ethnobotanical study of wild edible plants in Dibatie district, Metekel zone, Benishangul Gumuz Regional State, western Ethiopia. J Ethnobiol Ethnomed. 2024;20:27.
Girmay G, Arega B, Tesfaye D, Berkvens D, Muleta G, Asefa G. Community-based tsetse fly control significantly reduces fly density and trypanosomosis prevalence in Metekel Zone, Northwest, Ethiopia. Trop Anim Health Prod. 2016;48:633–42.
Cochran WG. Sampling techniques, 3rd ed. New York, USA: Wiley; 1977.
Martin GJ. Ethnobotany: a methods manual, 1st ed. London ; New York: Chapman & Hall; 1995.
Alexiades MN. Collecting ethnobotanical data: an introduction to basic concepts and techniques. Adv Econ Bot. 1996;10:53–94.
Teklehaymanot T. An ethnobotanical survey of medicinal and edible plants of Yalo Woreda in Afar regional state, Ethiopia. J Ethnobiol Ethnomed. 2017;13:40.
Heinrich M, Ankli A, Frei B, Weimann C, Sticher O. Medicinal plants in Mexico: healers’ consensus and cultural importance. Soc Sci Med. 1998;47:1859–71.
Staub PO, Geck MS, Weckerle CS, Casu L, Leonti M. Classifying diseases and remedies in ethnomedicine and ethnopharmacology. J Ethnopharmacol. 2015;174:514–9.
Friedman J, Yaniv Z, Dafni A, Palewitch D. A preliminary classification of the healing potential of medicinal plants, based on a rational analysis of an ethnopharmacological field survey among Bedouins in the Negev Desert, Israel. J Ethnopharmacol. 1986;16:275–87.
Hoft M, Barik SK, Lykke AM. Quantitative ethnobotany, applications of multivariate and statistical analyses in ethnobotany, people and plants. Working Paper 6. Unesco Paris; 1999. p. 3–3.
Bekele M, Woldeyes F, Lulekal E, Bekele T, Demissew S. Ethnobotanical investigation of medicinal plants in Buska Mountain range, Hamar district, Southwestern Ethiopia. J Ethnobiol Ethnomed. 2022;18:60.
Zenderland J, Hart R, Bussmann RW, Paniagua Zambrana NY, Sikharulidze S, Kikvidze Z, et al. The use of “Use Value”: Quantifying importance in ethnobotany. Econ Bot. 2019;73:293–303.
Albuquerque UP, Lucena RF, Monteiro JM, Florentino AT, Cecília de Fátima CBR. Evaluating two quantitative ethnobotanical techniques. Ethnobotany Res Appl. 2006;4:051–60.
Rossato SC, Leitão-Filho H de F, Begossi A. Ethnobotany of caiçaras of the Atlantic Forest coast (Brazil). Econ Botany. 1999;53:387–95.
Tahir M, Asnake H, Beyene T, Van Damme P, Mohammed A. Ethnobotanical study of medicinal plants in Asagirt District, Northeastern Ethiopia. Trop Med Health. 2023;51:1.
Tamene S, Negash M, Makonda FB, Chiwona-Karltun L, Kibret KS. Ethnobotanical study on medicinal plant knowledge among three ethnic groups in peri-urban areas of south-central Ethiopia. J Ethnobiol Ethnomed. 2023;19:55.
Reinaldo R, Albuquerque U, Medeiros P. Taxonomic affiliation influences the selection of medicinal plants among people from semi-arid and humid regions: a proposition for the evaluation of utilitarian equivalence in Northeast Brazil. PeerJ. 2020;8:e9664.
Tadesse T, Teka A. Ethnobotanical study on medicinal plants used by the local communities of Ameya District, Oromia Regional State, Ethiopia. Zhang F, editor. Evidence-Based Complementary Altern Med. 2023;2023:1–10.
Eshete MA, Molla EL. Cultural significance of medicinal plants in healing human ailments among Guji semi-pastoralist people, Suro Barguda District, Ethiopia. J Ethnobiol Ethnomed. 2021;17:61.
Tahir M, Gebremichael L, Beyene T, Van Damme P. Ethnobotanical study of medicinal plants in Adwa District, Central Zone of Tigray Regional State, Northern Ethiopia. J Ethnobiol Ethnomed. 2021;17:71.
Megersa M, Nedi T, Belachew S. Ethnobotanical study of medicinal plants used against human diseases in Zuway Dugda District, Ethiopia. Gorzalczany S, editor. Evidence-based complementary and alternative medicine. 2023;2023:1–22.
Megersa M, Tamrat N. Medicinal plants used to treat human and livestock ailments in Basona Werana District, North Shewa Zone, Amhara Region, Ethiopia. Kung W-M, editor. Evidence-Based Complementary and Alternative Medicine. 2022;2022:1–18.
Teshome M, Kebede F, Yohannes T. An ethnobotanical survey of indigenous knowledge on medicinal plants used by communities to treat various diseases around Ensaro District, North Shewa Zone of Amhara Regional State, Ethiopia. Wani I, editor. Scientifica. 2023;2023:1–19.
Wubu KA, Ngatie AH, Haylie TA, Osman AD. Ethnobotanical study of traditional medicinal plants in Kebridehar and Shekosh districts, Korahi zone, Somali Region. Ethiopia Heliyon. 2023;9:e22152.
Bogale M, Sasikumar JM, Egigu MC. An ethnomedicinal study in tulo district, west hararghe zone, oromia region. Ethiopia Heliyon. 2023;9:e15361.
Mekonnen AB, Mohammed AS, Tefera AK. Ethnobotanical study of traditional medicinal plants used to treat human and animal diseases in Sedie Muja District, South Gondar, Ethiopia. Bourhia M, editor. Evidence-Based Complementary and Alternative Medicine. 2022;2022:1–22.
Yimam M, Yimer SM, Beressa TB. Ethnobotanical study of medicinal plants used in Artuma Fursi district, Amhara Regional State, Ethiopia. Trop Med Health. 2022;50:85.
Berhanu M, Tintagu T, Fentahun S, Giday M. Ethnoveterinary survey of medicinal plants used for treatment of animal diseases in Ambo District of Oromia Regional State of Ethiopia. Andrade-Cetto A, editor. Evidence-Based Complementary and Alternative Medicine. 2020;2020:1–12.
Agize M, Asfaw Z, Nemomissa S, Gebre T. Ethnobotany of traditional medicinal plants and associated indigenous knowledge in Dawuro Zone of Southwestern Ethiopia. J Ethnobiol Ethnomed. 2022;18:48.
Tefera BN, Kim Y-D. Ethnobotanical study of medicinal plants in the Hawassa Zuria District, Sidama zone, Southern Ethiopia. J Ethnobiol Ethnomed. 2019;15:25.
Jima TT, Megersa M. Ethnobotanical study of medicinal plants used to treat human diseases in Berbere District, Bale Zone of Oromia Regional State, South East Ethiopia. Evid-Based Complementary Altern Med. 2018;2018:1–16.
Feyisa M, Kassahun A, Giday M. Medicinal plants used in ethnoveterinary practices in Adea Berga District, Oromia Region of Ethiopia. Ali Shtayeh MS, editor. Evidence-Based Complementary and Alternative Medicine. 2021;2021:1–22.
Hani N, Baydoun S, Nasser H, Ulian T, Arnold-Apostolides N. Ethnobotanical survey of medicinal wild plants in the Shouf Biosphere Reserve, Lebanon. J Ethnobiol Ethnomed. 2022;18:73.
Jadid N, Kurniawan E, Himayani CES, Andriyani, Prasetyowati I, Purwani KI, et al. An ethnobotanical study of medicinal plants used by the Tengger tribe in Ngadisari village, Indonesia. Ahmad KS, editor. PLoS ONE. 2020;15:e0235886.
Giday M, Teklehaymanot T, Animut A, Mekonnen Y. Medicinal plants of the Shinasha, Agew-awi and Amhara peoples in northwest Ethiopia. J Ethnopharmacol. 2007;110:516–25.
Osman A, Sbhatu DB, Giday M. Medicinal plants used to manage human and livestock ailments in Raya Kobo District of Amhara Regional State, Ethiopia. Satou T, editor. Evidence-based complementary and alternative medicine. 2020;2020:1–19.
Axiotis E, Halabalaki M, Skaltsounis LA. An Ethnobotanical study of medicinal plants in the Greek Islands of North Aegean Region. Front Pharmacol. 2018;9:409.
Bai Y, Zhang Q, He X, Wang H, Li W, Zhu J, et al. An ethnobotanical study on medicinal plants of Shexian Dryland Stone Terraced System in northern China. J Ethnobiol Ethnomed. 2022;18:62.
Huang S-S, Huang C-H, Ko C-Y, Chen T-Y, Cheng Y-C, Chao J. An ethnobotanical study of medicinal plants in Kinmen. Front Pharmacol. 2022;12:681190.
Hu R, Lin C, Xu W, Liu Y, Long C. Ethnobotanical study on medicinal plants used by Mulam people in Guangxi, China. J Ethnobiol Ethnomed. 2020;16:40.
Jin S, Zhang SS, Shad N, Naeem A, Yang YD, Wu SK. Ethnobotanical investigation of medicinal plants used in Lingchuan county, Shanxi, China. Braz J Biol. 2022;82:e260774.
Tshikalange TE, Mophuting BC, Mahore J, Winterboer S, Lall N. An ethnobotanical study of medicinal plants used in villages under Jongilanga Tribal Council, Mpumalanga, South Africa. Afr J Tradit Complement Altern Med. 2016;13:83–9.
Ndhlovu PT, Asong JA, Omotayo AO, Otang-Mbeng W, Aremu AO. Ethnobotanical survey of medicinal plants used by indigenous knowledge holders to manage healthcare needs in children. Šiler BT, editor. PLoS ONE. 2023;18:e0282113.
Megersa M, Jima TT, Goro KK. The use of medicinal plants for the treatment of toothache in Ethiopia. Evidence-Based Complementary Altern Med. 2019;2019:1–16.
Lulekal E, Kelbessa E, Bekele T, Yineger H. An ethnobotanical study of medicinal plants in Mana Angetu District, southeastern Ethiopia. J Ethnobiol Ethnomed. 2008;4:10.
Kefalew A, Asfaw Z, Kelbessa E. Ethnobotany of medicinal plants in Ada’a District, East Shewa Zone of Oromia Regional State. Ethiopia J Ethnobiol Ethnomed. 2015;11:25.
Belayneh A, Asfaw Z, Demissew S, Bussa NF. Medicinal plants potential and use by pastoral and agro-pastoral communities in Erer Valley of Babile Wereda, Eastern Ethiopia. J Ethnobiol Ethnomed. 2012;8:42.
Asnake S, Teklehaymanot T, Hymete A, Erko B, Giday M. Survey of medicinal plants used to treat malaria by Sidama People of Boricha District, Sidama Zone, South Region of Ethiopia. Evid-Based Complementary Altern Med. 2016;2016:1–9.
Siraj J, Belew S, Suleman S. Ethnobotanical assessment and physicochemical properties of commonly used medicinal plants in Jimma Zone, Southwest Ethiopia: traditional healers based cross-Sectional Study. JEP. 2020;12:665–81.
Karaköse M. An ethnobotanical study of medicinal plants in Güce district, north-eastern Turkey. Plant Diversity. 2022;44:577–97.
Aparicio H, Hedberg I, Bandeira S, Ghorbani A. Ethnobotanical study of medicinal and edible plants used in Nhamacoa area, Manica province–Mozambique. S Afr J Bot. 2021;139:318–28.
Woldeamanuel MM, Geda MK, Mohapatra S, Bastia TK, Rath P, Panda AK. Ethnobotanical study of endemic and non-endemic medicinal plants used by indigenous people in environs of Gullele botanical garden Addis Ababa, central Ethiopia: a major focus on Asteraceae family. Front Pharmacol. 2022;13:1020097.
Giday M, Asfaw Z, Woldu Z, Teklehaymanot T. Medicinal plant knowledge of the Bench ethnic group of Ethiopia: an ethnobotanical investigation. J Ethnobiol Ethnomed. 2009;5:34.
Getaneh S, Girma Z. An ethnobotanical study of medicinal plants in Debre Libanos Wereda, Central Ethiopia. Afr J Plant Sci. 2014;8:366–79.
Shimels A, Atinafu K, Akalu M, Getachew M. Ethnobotanical study of medicinal plants used by agro pastoralist Somali people for the management of human ailments in Jeldesa Cluster, Dire Dawa Administration, Eastern Ethiopia. J Med Plants Res. 2017;11:171–87.
Nigussie S, Godana A, Birhanu A, Abdeta T, Demeke F, Lami M, et al. Practice of traditional medicine and associated factors among residents in Eastern Ethiopia: a community-based cross-sectional study. Front Public Health. 2022;10:915722.
Faye G, Birhanu T, Belete T. Survey and antimicrobial activity study of ethnomedicinal plants in selected districts of North Shewa Zone, Oromia, Ethiopia. IDR. 2021;14:5511–20.
Zemede J, Mekuria T, Ochieng CO, Onjalalaina GE, Hu G-W. Ethnobotanical study of traditional medicinal plants used by the local Gamo people in Boreda Abaya District, Gamo Zone, southern Ethiopia. J Ethnobiol Ethnomed. 2024;20:28.
Amsalu N, Bezie Y, Fentahun M, Alemayehu A, Amsalu G. Use and conservation of medicinal plants by indigenous people of Gozamin Wereda, East Gojjam Zone of Amhara Region, Ethiopia: an ethnobotanical approach. Evid-Based Complementary Altern Med. 2018;2018:1–23.
Alemneh D. Ethnobotanical study of plants used for human ailments in Yilmana Densa and Quarit Districts of West Gojjam Zone, Amhara Region, Ethiopia. Imran A, editor. BioMed Res Int. 2021;2021:1–18.
Giday K, Lenaerts L, Gebrehiwot K, Yirga G, Verbist B, Muys B. Ethnobotanical study of medicinal plants from degraded dry afromontane forest in northern Ethiopia: species, uses and conservation challenges. J Herbal Med. 2016;6:96–104.
Tesfaye S, Belete A, Engidawork E, Gedif T, Asres K. Ethnobotanical study of medicinal plants used by traditional healers to treat cancer-like symptoms in eleven districts, Ethiopia. Evid-Based Complementary Altern Med. 2020;2020:1–23.
Lulekal E, Rondevaldova J, Bernaskova E, Cepkova J, Asfaw Z, Kelbessa E, et al. Antimicrobial activity of traditional medicinal plants from Ankober District, North Shewa Zone, Amhara Region, Ethiopia. Pharmaceutical Biol. 2014;52:614–20.
Giday M, Asfaw Z, Woldu Z. Ethnomedicinal study of plants used by Sheko ethnic group of Ethiopia. J Ethnopharmacol. 2010;132:75–85.
Mekebib Weldearegay E, Awas T. Ethnobotanical study in and around Sirso natural forest of Melokoza District, Gamo Goffa Zone, Southern Ethiopia. Ethnobot Res App. 2021. https://ethnobotanyjournal.org/index.php/era/article/view/3029. Accessed 21 Mar 2024.
Abebe BA, Chane Teferi S. Ethnobotanical study of medicinal plants used to treat human and livestock ailments in Hulet Eju Enese Woreda, East Gojjam Zone of Amhara Region, Ethiopia. Ghayur MN, editor. Evidence-Based Complementary and Alternative Medicine. 2021;2021:1–11.
Abera B. Medicinal plants used in traditional medicine by Oromo people, Ghimbi District, Southwest Ethiopia. J Ethnobiol Ethnomed. 2014;10:40.
Gemedo-Dalle T, Maass BL, Isselstein J. Plant biodiversity and ethnobotany of Borana pastoralists in southern Oromia, Ethiopia. Econ Bot. 2005;59:43–65.
Yinebeb M, Lulekal E, Bekele T. Composition of homegarden plants and cultural use in an indigenous community in Northwest Ethiopia. J Ethnobiol Ethnomed. 2022;18:47.
Acknowledgements
We would like to thank the administrative leaders, informants, field assistants, and residents of Dibatie district who participated directly or indirectly in the ethnobotanical data collection. The authors are also grateful to the Office of the Research Directorate, College of Natural and Computational Sciences, as well as the Department of Plant Biology and Biodiversity Management of Addis Ababa University for funding this work through the thematic project entitled ‘Ethnobotany of the medicinal and wild edible plants of the Dibatie people and antimicrobial activity study of plants against infectious diseases of the Dibatie district, Metekel zone, Benishangul Gumuz Regional State, western Ethiopia.’ Besides, the Ethiopian Public Health Institute is appreciated for its partial financial support during field data collection.
Funding
This study was funded by the Office of the Directorate for Research in Addis Ababa University and partially by the Ethiopian Public Health Institute.
Author information
Authors and Affiliations
Contributions
B.A. designed the research, conducted data collection and analysis, identified plants, and wrote the initial and final draft of the manuscript. E.L. supervised the data collection, confirmed plant identification, and reviewed the manuscript. A.D. facilitated the fund and supervised the data collection. A.H. supervised the data collection and reviewed the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The field data collection was conducted following the consent letters given by the Department of Plant Biology and Biodiversity Management at Addis Ababa University (reference number DPBBM/CNS/478/13/2020) and the Administrative Office of the Dibatie District.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Anbessa, B., Lulekal, E., Debella, A. et al. Ethnobotanical study of medicinal plants in Dibatie district, Metekel zone, Benishangul Gumuz Regional State, western Ethiopia. J Ethnobiology Ethnomedicine 20, 85 (2024). https://doi.org/10.1186/s13002-024-00723-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13002-024-00723-7