Abstract
Background
Ameloblastoma (AME) is a benign odontogenic tumour of epithelial origin characterised by slow but aggressive growth, infiltration, and recurrence; it is capable of reaching large dimensions and invading adjacent structures. Stem cell research has proven to be significant in the sphere of tumour biology through these cells’ possible involvement in the aetiopathogenesis of this tumour.
Methods
Immunohistochemistry was performed on AME, dentigerous cyst (DC), and dental follicle (DF) samples, and indirect immunofluorescence was performed on the AME-hTERT cell line to determine the expression of SALL4, LIN28A, and KLF4.
Results
Expression of proteins related to cellular pluripotency was higher in AME cells than in DC and DF cells. The analysis revealed that the proteins in question were mainly expressed in the parenchyma of AME tissue samples and were detected in the nuclei of AME-hTERT cells.
Conclusions
Stem cells may be related to the origin and progression of AME.
Similar content being viewed by others
Introduction
Ameloblastomas (AME) are odontogenic tumours of epithelial origin. Although classified as a form of benign tumour, AME is characterised by aggressiveness, an infiltrative nature, and a high recurrence tendency. They can grow to large dimensions and invade adjacent structures, causing significant morbidity [1,2,3].
According to the most recent classification by the World Health Organisation (WHO), AME can be categorised into three types: conventional, unicystic, and extraosseous/peripheral. Among these, conventional AME is the most common and most aggressive [4].
The current therapeutic options include both conservative and radical approaches [5]. Conservative treatments involve enucleation and curettage [1], which avoid relevant facial deformities [5] but have recurrence rates of up to 55% [6]. More invasive treatments involve marginal resection, which is the method of choice, considering the high recurrence rates reported for AME [1]. However, this method can result in relapse rates of up to 15% in more aggressive AME types as well as significant facial deformities [7].
Although the causes of the aggressive biological behaviour and high rate of recurrence of this benign neoplasm are not fully understood, studies involving stem cells and their possible relationship with the aetiopathogenesis of neoplasms have been relevant in elucidating this behaviour [8,9,10].
Stem cells can perpetuate themselves through self-renewal mechanisms and differentiate into cells in specific tissues. These mechanisms are like those that occur in tumour cells and involve similar signalling pathways. Thus, both normal stem cells and tumorigenic cells have proliferative potential and the ability to give rise to new tissues called cancer stem cells [8].
Cancer stem cells proliferate uncontrollably, driving the formation and growth of tumors, generating heterogeneous malignant cells associated with recurrence and metastasis. It is believed that cancer cells can appropriate the self-renewal machinery that is normally expressed in normal stem cells [8].
It has been reported that AME cells originate from odontogenic stem cells located in the dental lamina [11] and that tumours are likely initiated in normal stem cells that contain a perpetual minority of cancer stem cells [12, 13].
In this context, the SALL4 (Spalt-Like Transcription Factor 4), LIN28A (LIN28 homolog A), and KLF4 (Kruppel-like factor 4) proteins, which act as essential regulators of pluripotency and embryonic self-renewal and can mediate tumour progression and differentiation, are relevant biomarkers for the analysis of stem cells [14,15,16].
SALL4 is an essential transcription factor for the maintenance of self-renewal and pluripotency of embryonic stem cells that occurs in early embryonic development [14]. Its expression is downregulated after birth and is absent in most adult human tissues. Specifically, its expression in adult tissues is restricted to germ cells [17], except for human CD34+ haematopoietic stem cells [18]. However, its high expression and dysregulation have been demonstrated in several types of cancer [14], such as leukemia, germ cell tumours, hepatocellular carcinoma, and lung cancer [19,20,21,22,23,24], where it acts as an oncogene and participates in the processes of initiation, development, and progression of cancer [14].
LIN28A is a highly conserved ribonucleic acid (RNA)-binding protein that plays a key role in cell development and pluripotency by regulating the process of cell proliferation and differentiation [25]. It is expressed in the embryos, stem cells, and embryonic carcinoma cells [26]. Its performance occurs both physiologically (i.e. through the renewal and differentiation of stem cells, tissue repair, and glucose metabolism) and pathologically, where high levels are correlated with advanced malignant tumours, poor prognosis, and increased risk of recurrence [26,27,28].
KLF4 is a transcription factor that regulates cellular processes of development, differentiation, proliferation, and apoptosis. Depending on the cell type, KLF4 acts as both a tumour suppressor and an oncogene [16]. Furthermore, KLF4 is involved in stem cell renewal and maintenance of pluripotency [29, 30].
The protein–protein interaction network was analysed for SALL4, LIN28A, and KLF4 proteins using bioinformatics with the STRING (Search Tool for Recurring Instances of Neighbouring Genes) platform [31]. According to the STRING platform, a direct association among them was demonstrated in all interactions obtained from the selected databases and was confirmed using text mining analysis. Interactions among LIN28A, KLF4, SALL4, and KLF4 were determined experimentally. Additionally, the platform demonstrated protein homology between SALL4 and KLF4, and computationally identified co-expression of LIN28A and SALL4 based on transcript-transcript interactions (see Fig. 1).
Interaction Network of SALL4, LIN28 and KLF4 proteins obtained through the STRING platform. All observed interactions between proteins (edges connecting nodes) were obtained from selected databases (light blue line) and confirmed by text mining analysis (yellow line). LIN28A and KLF4 and SALL4 and KLF4 interactions were determined experimentally (pink line). Protein homology was demonstrated between SALL4 and KLF4 (purple line), and coexpression between LIN28A and SALL4 was computationally observed from transcript–transcript interactions (black line)
Understanding the molecular mechanisms underlying AME through the expression of stem cell biomarkers can help elucidate the role of these cells in the aetiopathogenesis of this tumour. Therefore, the present study aimed to evaluate the in situ and in vitro expression of stem cell markers SALL4, LIN28A, and KLF4 in AME. This is the first work to simultaneously investigate these three proteins in this benign neoplasm.
Methods
Ethical aspects
This study was approved by the Comitê de Ética em Pesquisa com Seres Humanos – Universidade Federal do Pará, Belém, Pará, Brazil, Committee Reference No.: 5.490.937.
Samples
For the in vitro study, the cell line derived from human AME, called AME-hTERT, established at the Cell Culture Laboratory of the Faculty of Dentistry, Universidade Federal do Pará (UFPA), was used [32]. For the in situ study, 21 cases of conventional AME, ten cases of dentigerous cysts (DC), and ten cases of dental follicles (DF) were collected at the Laboratory of Pathological Anatomy and Immunohistochemistry of the Graduate Program in Dentistry, Universidade Federal do Pará, and Centro Universitário do Pará (CESUPA). In the in situ study, the cases of DC and DF were used as comparative samples, considering that DC and AME are benign odontogenic lesions but present with less aggressive behaviour and a low incidence of recurrence. Meanwhile, DF is a tissue without pathological neoplastic changes of odontogenic origin.
Cell cultivation
The cell line derived from AME, established, and characterised at the Laboratory of Cell Culture of the Graduate Program of the Universidade Federal do Pará (UFPA), was cultivated in DMEM/F-12 medium (Sigma Chemical Co., St. Louis, MO, USA), supplemented with 10% foetal bovine serum (Gibco, Carlsbad, CA, USA), 1% penicillin (Gibco®) and 0.1% antifungal (Gibco®). The cells were kept in an incubator at a temperature of 37ºC and a humid atmosphere containing 5% CO2. Cell proliferation was observed daily using an inverted phase-contrast microscope (Axiovert 40 C – Zeiss) equipped with a coupled camera (AxioCam MRc, Zeiss).
Immunohistochemistry
The immunohistochemistry technique used in the present study was performed according to the following protocol. First, deparaffinization of the slides in xylene and hydration in decreasing alcohol concentrations (100%, 90%, 80% and 70%) was conducted. Then endogenous peroxidases were blocked by immersing the slides in 3% H2O2 to methanol at a 1:1 ratio for 30 min. Antigenic recovery was conducted in a Pascal pressure chamber (Dako Cytomation, Carpinteria, CA, USA) in a citrate buffer (pH 6.0) for 30 s with a temperature of 123°C and pressure of 13.5 psi. Finally, non-specific antibodies were blocked with 1% bovine serum albumin (BSA; Sigma-Aldrich) in a phosphate-buffered saline solution for 1 h.
Incubation of primary antibodies for Anti-Sall4 (1:25, mouse, Santa Cruz Biotechnology, Santa Cruz, CA, USA) was conducted for 12–14 h (overnight) and for 1 h for the Anti-LIN-28 (1:30, mouse, Santa Cruz Biotechnology ®) and Anti-GKLF (1:100, mouse, Santa Cruz Biotechnology®) in the AME, DC, and DF samples. Secondary antibodies were incubated with an Immunoprobe Plus detection system (Advanced Biosystems, San Francisco, CA, USA) for 30 min. Diaminobenzidine chromogen (ScyTek, Logan, UT, USA) was used. The slides were counterstained with haematoxylin (Sigma-Aldrich) and mounted using Permount (Fisher Scientific, Fair Lawn, NJ, USA). Testicular seminoma samples were used as positive controls. As a negative control, the primary antibody was replaced with BSA (Sigma-Aldrich) or non-immune serum.
Immunohistochemical evaluation
The immunohistochemical (IHC) evaluation was performed by measuring the area (μm) and fraction (%) of SALL4, LIN28A, and KLF4 and labelling in the AME, DC, and DF samples. Images were obtained using an Axioscope A1 microscope (Zeiss®) equipped with an AxioCam HRC colour CCD camera (Zeiss®) with a bright field. Five areas in each sample were selected based on the quantity and morphological preservation of the parenchyma. Images were acquired using a 40x objective. Areas of the tumour parenchyma were separated and segmented using the “IHC Image Analysis Toolbox” plug in (Jie Shu, Guoping Qiu and Mohammad Ilyas, https://imagej.nih.gov/ij/plugins/ihc-toolbox/index.html) using ImageJ (public domain software developed by Wayne Rasband (NIMH, National Institutes of Health, Bethesda, MD, USA; http://rsbweb.nih.gov/ij/). After segmenting the images, the area and diaminobenzidine (DAB) staining fraction were measured, and the immunostaining differences found in AME, DC, and DF were analysed.
Indirect immunofluorescence
AME-hTERT cells were cultured on glass coverslips in 24-well plates and subjected to indirect immunofluorescence to observe the expression of SALL4, LIN28A, and KLF4. The technique involved the following steps: cell fixation in 2% paraformaldehyde for 10 min, washing with phosphate-buffered saline (PBS), permeabilisation of the membrane with a 0.5% Triton X-100 solution (Sigma®) for 15 min, wash with PBS, incubation in PBS/1% BSA for 30 min, and incubation with primary monoclonal antibodies diluted in PBS/BSA at 1% for a minimum of 12 h and a maximum of 18 h in a humid chamber at 4ºC. The primary antibodies used were Anti-Sall4 (1:50, mouse, Santa Cruz Biotechnology®), Anti-LIN-28 (1:50, mouse, Santa Cruz Biotechnology®), and anti-GKLF (1:50, mouse, Santa Cruz Biotechnology®). To detect the primary antibody, incubation was performed in a solution containing the secondary antibody conjugated to Alexa Fluor 488 (Invitrogen, Carlsbad, CA, USA) for 1 h in a humid and dark chamber at room temperature. Nuclei were stained with Hoechst 33258 (1: 2,000, Sigma) and cytoskeletons were stained with Alexa Fluor 568 phalloidin (Life Technologies, Carlsbad, CA, USA). The coverslips were immersed in PBS and distilled water and mounted using the ProLong® Gold antifade reagent (Invitrogen®). Next, the cells were analysed using a fluorescence microscope (AxioScope.A1, Zeiss) equipped with a digital camera (AxiocamMRc, Zeiss). Images of the slides were obtained for the registration of immunoexpression using a 40x objective. As a negative control, the primary antibody was replaced with a non-immune serum.
Statistical analysis
Data were analysed using GraphPad Prism 8 software (GraphPad Software Inc., San Diego, CA, USA). When parametric distribution was evidenced by the Shapiro-Wilk test, differences between groups were evaluated by one-way analysis of variance (ANOVA) followed by Tukey’s post-hoc test. When a non-parametric distribution was evidenced by the Shapiro-Wilk test, the differences between groups were evaluated by the Kruskal-Wallis test, followed by Dunn’s post-test of multiple comparisons. A 95% confidence interval (CI) was assumed (α = 0.05).
Results
Demographical and clinical data, and histopathological typing of patients with AME
In the studied sample, the mean age was 37 years. Males represented 57% of the samples. The region of greatest involvement was the mandible, totalling 95% of the cases. As for the histological types, eight cases were of the follicular type, eight of plexiform, three acanthomatous and two of granular cells (see Table 1).
Immunohistochemical staining for SALL4, LIN28a, and KLF4 in ameloblastoma, dentigerous cyst, and dental follicle
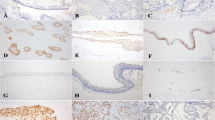
IHC staining for SALL4, LIN28A, and KLF4 was predominantly observed in the epithelial cells of the tumour islands (see Fig. 2). For SALL4, intense staining was observed in the tumour parenchyma in both the nucleus and cytoplasm of the cells, in the high columnar cells of the periphery, and in the central cells of the tumour island. LIN28A showed strong immunostaining with nuclear and cytoplasmic localisation limited to the central cells of the tumour island. Intense immunostaining was observed for KLF4 nuclear localisation in epithelial cells. The labelling was predominantly located in the nuclei of tall columnar cells at the periphery of the tumour island. Nuclear and cytoplasmic markings of SALL4, LIN28A, and KLF4 were also observed in some cases of DC and DF; however, the expression of SALL4, LIN28A, and KLF4 was significantly higher in AME samples compared to DC (p < 0.001) and DF (p < 0.001), as demonstrated in the statistical analysis (see Fig. 2).
Immunohistochemical staining for SALL4, LIN28 and KLF4 in AME, DC and DF samples. Intense SALL4 immunostaining was observed in the parenchymal cells of the plexiform AME, with nuclear and cytoplasmic location (A). Strong LIN28A immunostaining was observed in follicular-type AME, with nuclear and cytoplasmic localization limited to the central cells of the tumor island (E). Intense KLF4 immunostaining in plexiform AME was observed with a nuclear location (I). There was a subtle labeling of the three proteins in the nucleus and cytoplasm of some epithelial cells in both DC (B, F and J) and DF (C, G and K) samples. Statistical analysis of the percentage of parenchymal marking area of the three markers (D, H and L) between AME, DC and DF. (***p < 0.001). Scale bar: 20 µm. AME = Ameloblastoma; DC = Dentigerous cyst; DF = Dental follicle
AME-hTERT lineage expressed SALL4, LIN28A, and KLF4
The immunofluorescence assays revealed that the AME-hTERT strain expressed SALL4, LIN28A, and KLF4 (see Fig. 3). Neoplastic cells demonstrated nuclear and cytoplasmic expression of SALL4 (see Fig. 3A), nuclear and cytoplasmic expression, with nuclear predominance of LIN28A (see Fig. 3E) and predominantly nuclear expression of KLF4 (Fig. 3I). The immunoexpression of all proteins was granular (see Fig. 3).
Immunofluorescence photomicrographs of SALL4, LIN28 and KLF4 in AME-hTERT strains. SALL4 showed immunoexpression with a granular pattern located in the nucleus and cytoplasm (A). LIN28A showed immunoexpression with a granular pattern in the nucleus and cytoplasm, with nuclear predominance (E). Granular expression of KLF4 was observed in the nuclei, showing faint staining in the cytoplasm (I). Control group (CT) (M). The cytoskeleton was stained with phalloidin (red), and the nuclei were stained with Hoechst 33258 (blue). Overlapping images of the expression of SALL4 (D), LIN28 (H), KLF4 (L) and control group (P). Scale bar: 20 µm
Discussion
The studied proteins showed significantly higher levels of immunostaining in AME cells than in the DC and DF cells. SALL4, LIN28A, and KLF4 were expressed in the AME parenchyma, with slight staining observed in some cells of the odontogenic epithelium of DC and DF. Furthermore, immunoexpression of the studied proteins was observed in the AME-hTERT strain.
SALL4 is a transcription factor that plays a key role in maintaining pluripotency and self-renewal of embryonic stem cells [14]. It interacts with other important regulatory proteins of embryonic pluripotency — OCT4 (octamer-binding protein 4), SOX-2 (HMG-box gene 2 related to SRY), and NANOG (homeodomain protein) — forming an autoregulatory circuit in which each of these proteins regulates its own expression and that of others [33,34,35]. High expression of SOX-2, NANOG, and OCT4 has been demonstrated in AME [10], suggesting that these proteins may act together to maintain undifferentiated stem cells in this tumour.
In the present study, cells from the AME tumour islands and the AME-hTERT lineage showed nuclear and cytoplasmic expression of SALL4, corroborating the marking pattern found by other studies in oral squamous cell carcinoma that suggests that this protein plays an important role in the progression of oral cancer and may serve as a potential therapeutic target [36,37,38]. Nuclear labelling indicated the transcriptional activity of SALL4. Such activity is associated with transcriptional repression mechanisms that prevent stem cell differentiation and increase the proliferation of undifferentiated cells [35, 39]. SALL4 cytoplasmic marking has also been demonstrated in breast cancer cells and is considered a predictor of poor prognosis [40].
Studies have shown that SALL4 protein expression is negatively regulated by miRNAs (miRNAs) belonging to the Let-7 family, particularly by miR-98, which leads to a reduction in tumour cell proliferation, indicating that miR-98 acts as a tumour suppressor that inhibits SALL4 protein expression [41, 42]. It is important to emphasise that LIN28 protein can downregulate the Let-7 microRNA family through the activation of its isoforms LIN28A or LIN28B [25, 43]. This suggests that the upregulation of LIN28 leads to the inhibition of miR-98, which, in turn, leads to the upregulation of SALL4. Therefore, the co-expression of SALL4 and LIN28A in AME observed in this study may play a significant role in tumour pathogenesis.
LIN28A is a highly conserved RNA-binding protein that plays a significant role in development, glucose metabolism, and pluripotency [44,45,46]. It is highly expressed in mouse embryonic stem cells, which decreases after differentiation, and in human embryonic carcinoma cells [47]. Oral squamous cell carcinoma has been demonstrated to be associated with the regulation of the proliferative and invasive activities of this neoplasm [48]. Furthermore, LIN28A has been identified as one of the four factors that convert fibroblasts into induced pluripotent stem cells, corroborating the role of this protein in pluripotent stem cells [49].
In this study, LIN28A immunostaining showed nuclear and cytoplasmic localisation limited to the central cells of the AME tumour islands. The same expression pattern was observed for the AME-hTERT strain. LIN28A is predominantly cytoplasmic and located on ribosomes, P bodies, and cytoplasmic stress granules [50]. The cytoplasmic expression found in the present study may be associated with its performance in the recruitment of terminal uridylyl transferase (TUTase4) ZCCHC11, which inhibits Let-7 processing in the cytoplasm and selectively blocks the expression of Let-7 miRNAs and their functions tumour suppressors, acting as an oncogene and promoting tumorigenesis [25, 51]. This action has been demonstrated in embryonic stem cells, suggesting a significant role of LIN28A in inhibiting cell differentiation through miRNAs in stem cells and certain types of cancer [52].
Nuclear expression of LIN28A was observed when both RNA-binding domains were mutated [50]. A model has been proposed in which LIN28A regulates the post-transcriptional processing of its mRNA targets by first binding to these targets in the nucleus and subsequently transporting them between ribosomes, P bodies, or stress granules for translation regulation, depending on the environmental conditions [50, 53]; however, more studies are needed to better understand this process.
The central region of the AME tumour islands, which exhibits greater LIN28A labelling, is more prone to hypoxia. As the tumour progresses, the concentration of oxygen in the microenvironment around the tumour cells decreases, leading to intratumoural hypoxia [54]. In response to this condition, hypoxia-induced factor-1 alpha (HIF-1α) regulates the expression of genes that help cells adapt to this environment [55]. Studies have indicated that HIF-1α is overexpressed in AME, suggesting that hypoxia is related to proliferation and invasion of the solid areas of this tumour [56,57,58]. It has been shown that HIF-1α binds directly to the LIN28A promoter and induces its transcription [59] and that hypoxia is capable of inducing the expression of stem cell markers in cancer cell lines, thereby contributing to the dedifferentiation and reprogramming process that induces the formation of cancer stem cells [59,60,61]. From this, we can infer that the expression of LIN28A in the central cells of the AME tumour island close to the high columnar cells in the periphery may be associated with the adaptive response of tumour cells to hypoxia, inducing the dedifferentiation of peripheral cells, and thus promoting greater proliferation and invasion.
KLF4 is an essential transcription factor in the regulation of cellular processes (e.g. development, differentiation, proliferation, and apoptosis) [16] and in the renewal of stem cells and maintenance of pluripotency [29, 30]. It has been used as a reprogramming factor for fibroblasts and odontoblasts in induced pluripotent stem cells along with LIN28A [62, 63]. In different cell types, KLF4 functions as both a tumour suppressor and an oncogene [16]. Increased expression has been reported in human head and neck squamous cell carcinoma and is associated with poor prognosis and aggressiveness, corroborating its oncogenic role [64, 65]. In contrast, Land et al. [66] found an association between high KLF4 expression and a favourable prognosis. Another study reported the role of KLF4 in oral squamous cell carcinoma, in which mechanisms of action were described as both tumour suppressors and oncogenes [67]. Some scholars believe that the function of KLF4 as an oncogene or tumour suppressor is modulated by its complex interactions with several tumour microenvironments [68].
In the present study, intense nuclear immunostaining for KLF4 was observed in AME, predominantly in the tall columnar cells located on the periphery of the tumour island. In the AME-hTERT strain, nuclear expression of KLF4 with mild cytoplasmic expression was observed. KLF4 is mainly located in the nucleus, but its cytoplasmic localisation has also been reported in prostate and oral cancers [65, 68, 69]. Increased KLF4 nuclear expression has been associated with poor prognosis in patients with breast and head and neck cancer [64, 70]. However, another study suggested that the downregulation of KLF4 is associated with the progression of oral carcinoma [71]. Considering the ambiguity of this transcription factor, further studies are required to assess the role of KLF4 in AME.
The findings of the present study indicate that SALL4 and LIN28A may play a significant role in the biological behaviour of AME, suggesting a possible role for stem cells in the genesis and progression of AME. The KLF4 transcription factor plays a context-dependent role in carcinogenesis and may be up or downregulated in distinct types of cancer. Therefore, its role in AME needs to be better understood. However, considering its expression together with that of other studied proteins, we suggest its participation and interaction as an oncogene. Although these results are promising, mechanistic and in vivo studies are required to confirm these hypotheses and elucidate the underlying molecular mechanisms. Understanding these mechanisms may have significant implications for the diagnosis, prognosis, and treatment of AME, thus opening up new possibilities for personalised and effective therapies.
Conclusions
To the best of our knowledge, this is the first study to evaluate the expression of SALL4, LIN28A, and KLF4 proteins in a benign odontogenic tumour. The study results verify the expression of these stem cell markers in AME neoplastic cells by IHC and in the AME-hTERT cell line by immunofluorescence, suggesting the possible participation of stem cells in the origin, progression, and recurrence of this tumour.
Availability of data and materials
The datasets used and/or analysed in the current study are available from the corresponding author upon reasonable request.
Abbreviations
- AME:
-
Ameloblastoma
- AME-hTERT:
-
Cell line derived from human AME
- ANOVA:
-
Analysis of variance
- BSA:
-
Bovine serum albumin
- CI:
-
Confidence interval
- DAB:
-
Diaminobenzidine
- DC:
-
Dentigerous cysts
- DF:
-
Dental follicles
- HIF-1 α:
-
Hypoxia-induced factor-1 alpha
- IHC:
-
Immunohistochemistry
- KLF4:
-
Kruppel-like factor 4
- LIN28A:
-
LIN28 homolog A
- miRNAs:
-
MicroRNAs
- NANOG:
-
Homeodomain protein
- OCT4:
-
Octamer-binding protein 4
- PBS:
-
Phosphate-buffered saline
- RNA:
-
Ribonucleic acid
- SALL4:
-
Spalt-like transcription factor 4
- SOX-2:
-
HMG-box gene 2 related to SRY
- TUTase4:
-
Terminal uridylyl transferase
- UFPA:
-
Universidade Federal do Pará
- WHO:
-
World Health Organisation
References
Mendenhall WM, Werning JW, Fernandes R, Malyapa RS, Mendenhall NP. Ameloblastoma. Am J Clin Oncol. 2007;30(6):645–8. https://doi.org/10.1097/COC.0b013e3181573e59. American Journal of Clinical Oncology Database. https://journals.lww.com/amjclinicaloncology/Abstract/2007/12000/Ameloblastoma.13.aspx. Accessed 21 May 2023.
Bassey GO, Osunde OD, Anyanechi CE. Maxillofacial tumors and tumor-like lesions in a Nigerian teaching hospital: an eleven year retrospective analysis. Afr Health Sci. 2014;14(1):56–63. https://doi.org/10.4314/ahs.v14i1.9. PubMed Database. https://pubmed.ncbi.nlm.nih.gov/26060458/. Accessed 22 May 2023.
Effiom OA, Ogundana OM, Akinshipo AO, Akintoye SO. Ameloblastoma: current etiopathological concepts and management. Oral Dis. 2018;24(3):307–16. https://doi.org/10.1111/odi.12646. Oral Diseases Database. https://onlinelibrary.wiley.com/doi/full/10.1111/odi.12646. Accessed 21 May 2023.
Vered M, Wright JM. Update from the 5th Edition of the World Health Organization classification of head and neck tumors: odontogenic and maxillofacial bone tumours. Head Neck Pathol. 2022;16(1):63–75. https://doi.org/10.1007/s12105-021-01404-7. Head and Neck Pathology Database.
Dandriyal R, Gupta A, Pant S, Baweja HH. Surgical management of ameloblastoma: conservative or radical approach. Natl J Maxillofac Surg. 2011;2(1):22–7. https://doi.org/10.4103/0975-5950.85849. National Journal of Maxillofacial Surgery Database.
Almeida Rde A, Andrade ES, Barbalho JC, Vajgel A, Vasconcelos BC. Recurrence rate following treatment for primary multicystic ameloblastoma: systematic review and meta-analysis. Int J Oral Maxillofac Surg. 2016;45(3):359–67. https://doi.org/10.1016/j.ijom.2015.12.016. International Journal of Oral and Maxillofacial Surgery Database. https://www.ijoms.com/article/S0901-5027(15)01484-8/fulltext. Accessed 22 May 2023.
Masthan KM, Anitha N, Krupaa J, Manikkam S. Ameloblastoma. J Pharm Bioallied Sci. 2015;7(Suppl 1):S167–70. https://doi.org/10.4103/0975-7406.155891. Journal of Pharmacy and Bioallied Sciences Database. https://journals.lww.com/jpbs/Fulltext/2015/07001/Ameloblastoma.46.aspx. Accessed 22 May 2023.
Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414(6859):105–11. https://doi.org/10.1038/35102167. Nature Journal Database. https://www.nature.com/articles/35102167. Accessed 21 May 2023.
Silva FP, Dias A, Coelho CA, Guerra EN, Marques AE, Decurcio DA, Mantesso A, Cury SE, Silva BS. Expression of CD90 and P75NTR stem cell markers in ameloblastomas: a possible role in their biological behavior. Braz Oral Res. 2016;30(1):e109. https://doi.org/10.1590/1807-3107BOR-2016.vol30.0109. Brazilian Oral Research Database. https://www.scielo.br/j/bor/a/NFQwSWgmGTf5kfhZSYJCTcs/?lang=en. Accessed 22 May 2023.
Balbinot KM, Almeida Loureiro FJ, Chemelo GP, Alves Mesquita R, Cruz Ramos AMP, Ramos RTJ, da Costa da Silva AL, de Menezes SAF, da Silva Kataoka MS, Alves Junior SM, Viana Pinheiro JJ. Immunoexpression of stem cell markers SOX-2, NANOG AND OCT4 in ameloblastoma. PeerJ. 2023;11:e14349. https://doi.org/10.7717/peerj.14349. PeerJ Database. https://peerj.com/articles/14349/. Accessed 22 May 2023.
Harada H, Mitsuyasu T, Toyono T, Toyoshima K. Epithelial stem cells in teeth. Odontology. 2002;90(1):1–6. https://doi.org/10.1007/s102660200000. SpringerLink Database. https://link.springer.com/article/10.1007/s102660200000. Accessed 22 May 2023.
Wu C, Alman BA. Side population cells in human cancers. Cancer Lett. 2008;268(1):1–9. https://doi.org/10.1016/j.canlet.2008.03.048. Science Direct Database. https://www.sciencedirect.com/science/article/abs/pii/S0304383508002231?via%3Dihub. Accessed 22 May 2023.
O’Connor ML, Xiang D, Shigdar S, Macdonald J, Li Y, Wang T, Pu C, Wang Z, Qiao L, Duan W. Cancer stem cells: a contentious hypothesis now moving forward. Cancer Lett. 2014;344(2):180–7. https://doi.org/10.1016/j.canlet.2013.11.012.
Zhang X, Yuan X, Zhu W, Qian H, Xu W. SALL4: an emerging cancer biomarker and target. Cancer Lett. 2015;357(1):55–62. https://doi.org/10.1016/j.canlet.2014.11.037.
Yu NK, McClatchy DB, Diedrich JK, Romero S, Choi JH, Martínez-Bartolomé S, Delahunty CM, Muotri AR, Yates JR 3rd. Interactome analysis illustrates diverse gene regulatory processes associated with LIN28A in human iPS cell-derived neural progenitor cells. iScience. 2021;24(11):103321. https://doi.org/10.1016/j.isci.2021.103321.
Yadav SS, Nair RR, Yadava PK. KLF4 signalling in carcinogenesis and epigenetic regulation of hTERT. Med Hypotheses. 2018;115:50–3. https://doi.org/10.1016/j.mehy.2018.03.012.
Kohlhase J, Heinrich M, Schubert L, Liebers M, Kispert A, Laccone F, Turnpenny P, Winter RM, Reardon W. Okihiro syndrome is caused by SALL4 mutations. Hum Mol Genet. 2002;11(23):2979–87. https://doi.org/10.1093/hmg/11.23.2979.
Gao C, Kong NR, Li A, Tatetu H, Ueno S, Yang Y, He J, Yang J, Ma Y, Kao GS, Tenen DG, Chai L. SALL4 is a key transcription regulator in normal human hematopoiesis. Transfusion. 2013;53(5):1037–49. https://doi.org/10.1111/j.1537-2995.2012.03888.x.
Wang F, Zhao W, Kong N, Cui W, Chai L. The next new target in leukemia: The embryonic stem cell gene SALL4. Mol Cell Oncol. 2014;1(4):e969169. https://doi.org/10.4161/23723548.2014.969169.
Miettinen M, Wang Z, McCue PA, Sarlomo-Rikala M, Rys J, Biernat W, Lasota J, Lee YS. SALL4 expression in germ cell and non-germ cell tumors: a systematic immunohistochemical study of 3215 cases. Am J Surg Pathol. 2014;38(3):410–20. https://doi.org/10.1097/PAS.0000000000000116.
Mei K, Liu A, Allan RW, Wang P, Lane Z, Abel TW, Wei L, Cheng H, Guo S, Peng Y, Rakheja D, Wang M, Ma J, Rodriguez MM, Li J, Cao D. Diagnostic utility of SALL4 in primary germ cell tumors of the central nervous system: a study of 77 cases. Mod Pathol. 2009;22(12):1628–36. https://doi.org/10.1038/modpathol.2009.148.
Cao D, Li J, Guo CC, Allan RW, Humphrey PA. SALL4 is a novel diagnostic marker for testicular germ cell tumors. Am J Surg Pathol. 2009;33(7):1065–77. https://doi.org/10.1097/PAS.0b013e3181a13eef.
Zhou W, Zou B, Liu L, Cui K, Gao J, Yuan S, Cong N. MicroRNA-98 acts as a tumor suppressor in hepatocellular carcinoma via targeting SALL4. Oncotarget. 2016;7(45):74059–73. https://doi.org/10.18632/oncotarget.12190.
Yong KJ, Li A, Ou WB, Hong CK, Zhao W, Wang F, Tatetsu H, Yan B, Qi L, Fletcher JA, Yang H, Soo R, Tenen DG, Chai L. Targeting SALL4 by entinostat in lung cancer. Oncotarget. 2016;7(46):75425–40. https://doi.org/10.18632/oncotarget.12251.
Balzeau J, Menezes MR, Cao S, Hagan JP. The LIN28/let-7 pathway in cancer. Front Genet. 2017;8:31. https://doi.org/10.3389/fgene.2017.00031.
Feng C, Neumeister V, Ma W, Xu J, Lu L, Bordeaux J, Maihle NJ, Rimm DL, Huang Y. Lin28 regulates HER2 and promotes malignancy through multiple mechanisms. Cell Cycle. 2012;11(13):2486–94. https://doi.org/10.4161/cc.20893.
Thornton JE, Gregory RI. How does Lin28 let-7 control development and disease? Trends Cell Biol. 2012;22(9):474–82. https://doi.org/10.1016/j.tcb.2012.06.001.
Wu K, Ahmad T, Eri R. LIN28: a multifunctional versatile molecule with future therapeutic potential. World J Biol Chem. 2022;13(2):35–46. https://doi.org/10.4331/wjbc.v13.i2.35.
Schmidt R, Plath K. The roles of the reprogramming factors Oct4, Sox2 and Klf4 in resetting the somatic cell epigenome during induced pluripotent stem cell generation. Genome Biol. 2012;13(10):251. https://doi.org/10.1186/gb-2012-13-10-251. Published 2012 Oct 22.
Huang D, Wei Z, Lu W. Genome organization by Klf4 regulates transcription in pluripotent stem cells. Cell Cycle. 2013;12(21):3351–2. https://doi.org/10.4161/cc.26577.
Szklarczyk D, Gable AL, Lyon D, Junge A, Wyder S, Huerta-Cepas J, Simonovic M, Doncheva NT, Morris JH, Bork P, Jensen LJ, Mering CV. STRING v11: protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019;47(D1):D607–13. https://doi.org/10.1093/nar/gky1131.
Cruz E, Loureiro F, Silva A, Ramos RT, Kataoka M, Pinheiro J, Alves Júnior SM. Gene expression in cell lines from human ameloblastoma immortalized using hTERT and HPV16-E6/E7. Oral Dis. 2022;8:2230–8.
Lim CY, Tam WL, Zhang J, Ang HS, Jia H, Lipovich L, Ng HH, Wei CL, Sung WK, Robson P, Yang H, Lim B. Sall4 regulates distinct transcription circuitries in different blastocyst-derived stem cell lineages. Cell Stem Cell. 2008;3(5):543–54. https://doi.org/10.1016/j.stem.2008.08.004.
Zhang J, Tam WL, Tong GQ, Wu Q, Chan HY, Soh BS, Lou Y, Yang J, Ma Y, Chai L, Ng HH, Lufkin T, Robson P, Lim B. Sall4 modulates embryonic stem cell pluripotency and early embryonic development by the transcriptional regulation of Pou5f1. Nat Cell Biol. 2006;8(10):1114–23. https://doi.org/10.1038/ncb1481.
Aguila JR, Liao W, Yang J, Avila C, Hagag N, Senzel L, Ma Y. SALL4 is a robust stimulator for the expansion of hematopoietic stem cells. Blood. 2011;118(3):576–85. https://doi.org/10.1182/blood-2011-01-333641.
Kulkarni S, Solomon M, Chandrashekar C, Shetty N, Carnelio S. Spalt-like transcription factor 4 expression in oral epithelial dysplasia and oral squamous cell carcinoma: an immunohistochemical appraisal. J Carcinog. 2020;19:12. https://doi.org/10.4103/jcar.JCar_13_20.
Ota K, Shinriki S, Ando Y, Nakayama H, Shinohara M. Overexpression of the novel oncogene SALL4 in oral squamous cell carcinoma. Int J Oral Maxillofac Surg. 2015;44:e272.
Yu HH, Featherston T, Tan ST, Chibnall AM, Brasch HD, Davis PF, Itinteang T. Characterization of cancer stem cells in moderately differentiated buccal mucosal squamous cell carcinoma. Front Surg. 2016;3:46. https://doi.org/10.3389/fsurg.2016.00046.
Yang J, Aguila JR, Alipio Z, Lai R, Fink LM, Ma Y. Enhanced self-renewal of hematopoietic stem/progenitor cells mediated by the stem cell gene Sall4. J Hematol Oncol. 2011;4:38. https://doi.org/10.1186/1756-8722-4-38.
Yue X, Xiao L, Yang Y, Liu W, Zhang K, Shi G, Zhou H, Geng J, Ning X, Wu J, Zhang Q. High cytoplasmic expression of SALL4 predicts a malignant phenotype and poor prognosis of breast invasive ductal carcinoma. Neoplasma. 2015;62(6):980–8. https://doi.org/10.4149/neo_2015_119.
Liu X, Cao Y, Zhang Y, Zhou H, Li H. Regulatory effect of MiR103 on proliferation, EMT and invasion of oral squamous carcinoma cell through SALL4. Eur Rev Med Pharmacol Sci. 2019;23(22):9931–8. https://doi.org/10.26355/eurrev_201911_19559. Retraction in: Eur Rev Med Pharmacol Sci. 2021 Jan;25(2):569.
Chirshev E, Oberg KC, Ioffe YJ, Unternaehrer JJ. Let-7 as biomarker, prognostic indicator, and therapy for precision medicine in cancer. Clin Transl Med. 2019;8(1):24. https://doi.org/10.1186/s40169-019-0240-y.
Ustianenko D, Chiu HS, Treiber T, Weyn-Vanhentenryck SM, Treiber N, Meister G, Sumazin P, Zhang C. LIN28 selectively modulates a subclass of Let-7 MicroRNAs. Mol Cell. 2018;71(2):271–283.e5. https://doi.org/10.1016/j.molcel.2018.06.029.
Zhu H, Shyh-Chang N, Segrè AV, et al. The Lin28/let-7 axis regulates glucose metabolism. Cell. 2011;147(1):81–94. https://doi.org/10.1016/j.cell.2011.08.033.
Mayr F, Heinemann U. Mechanisms of Lin28-mediated miRNA and mRNA regulation–a structural and functional perspective. Int J Mol Sci. 2013;14(8):16532–53. https://doi.org/10.3390/ijms140816532.
Zhang J, Ratanasirintrawoot S, Chandrasekaran S, Wu Z, Ficarro SB, Yu C, Ross CA, Cacchiarelli D, Xia Q, Seligson M, Shinoda G, Xie W, Cahan P, Wang L, Ng SC, Tintara S, Trapnell C, Onder T, Loh YH, Mikkelsen T, Sliz P, Teitell MA, Asara JM, Marto JA, Li H, Collins JJ, Daley GQ. LIN28 regulates stem cell metabolism and conversion to primed pluripotency. Cell Stem Cell. 2016;19(1):66–80. https://doi.org/10.1016/j.stem.2016.05.009.
Moss EG, Tang L. Conservation of the heterochronic regulator Lin-28, its developmental expression and microRNA complementary sites. Dev Biol. 2003;258(2):432–42. https://doi.org/10.1016/s0012-1606(03)00126-x. Erratum in: Dev Biol. 2003 Oct 15;262(2):361.
Hayashi S, Tanaka J, Okada S, Isobe T, Yamamoto G, Yasuhara R, Irie T, Akiyama C, Kohno Y, Tachikawa T, Mishima K. Lin28a is a putative factor in regulating cancer stem cell-like properties in side population cells of oral squamous cell carcinoma. Exp Cell Res. 2013;319(8):1220–8. https://doi.org/10.1016/j.yexcr.2013.03.004.
Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin II, Thomson JA. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318(5858):1917–20. https://doi.org/10.1126/science.1151526.
Balzer E, Moss EG. Localization of the developmental timing regulator Lin28 to mRNP complexes, P-bodies and stress granules. RNA Biol. 2007;4(1):16–25. https://doi.org/10.4161/rna.4.1.4364.
Piskounova E, Polytarchou C, Thornton JE, LaPierre RJ, Pothoulakis C, Hagan JP, Iliopoulos D, Gregory RI. Lin28A and Lin28B inhibit let-7 microRNA biogenesis by distinct mechanisms. Cell. 2011;147(5):1066–79. https://doi.org/10.1016/j.cell.2011.10.039.
Viswanathan SR, Daley GQ, Gregory RI. Selective blockade of microRNA processing by Lin28. Science. 2008;320(5872):97–100. https://doi.org/10.1126/science.1154040. Epub 2008 Feb 21.
Shyh-Chang N, Daley GQ. Lin28: primal regulator of growth and metabolism in stem cells. Cell Stem Cell. 2013;12(4):395–406. https://doi.org/10.1016/j.stem.2013.03.005.
Md Hashim NF, Nicholas NS, Dart AE, Kiriakidis S, Paleolog E, Wells CM. Hypoxia-induced invadopodia formation: a role for β-PIX. Open Biol. 2013;3(6):120159. https://doi.org/10.1098/rsob.120159.
Díaz B, Yuen A, Iizuka S, Higashiyama S, Courtneidge SA. Notch increases the shedding of HB-EGF by ADAM12 to potentiate invadopodia formation in hypoxia. J Cell Biol. 2013;201(2):279–92. https://doi.org/10.1083/jcb.201209151.
de Mendonça RP, Balbinot KM, Martins BV, da Silva Kataoka MS, Mesquita RA, de Jesus Viana Pinheiro J, de Melo Alves Júnior S. Hypoxia and proangiogenic proteins in human ameloblastoma. Sci Rep. 2020;10(1):17567. https://doi.org/10.1038/s41598-020-74693-7.
da Costa NM, Fialho AD, Proietti CC, da Silva Kataoka MS, Jaeger RG, de Alves-Júnior SM, de Jesus Viana Pinheiro J. Role of hypoxia-related proteins in invasion of ameloblastoma cells: crosstalk between NOTCH1, hypoxia-inducible factor 1α, a disintegrin and metalloproteinase 12, and heparin-binding epidermal growth factor. Histopathology. 2016;69(1):99–106. https://doi.org/10.1111/his.12922.
da Costa NMM, de Siqueira AS, Ribeiro ALR, da Silva Kataoka MS, Jaeger RG, de Alves-Júnior SM, Smith AM, de Jesus Viana Pinheiro J. Role of HIF-1α and CASPASE-3 in cystogenesis of odontogenic cysts and tumors. Clin Oral Investig. 2018;22(1):141–9. https://doi.org/10.1007/s00784-017-2090-6.
Weng M, Feng Y, He Y, Yang W, Li J, Zhu Y, Wang T, Wang C, Zhang X, Qiao Y, Li Q, Zhao L, Gao S, Zhang L, Wu Y, Zhao R, Wang G, Li Z, Jin X, Zheng T, Li X. Hypoxia-induced LIN28A mRNA promotes the metastasis of colon cancer in a protein-coding-independent manner. Front Cell Dev Biol. 2021;9:642930. https://doi.org/10.3389/fcell.2021.642930.
Wang P, Wan WW, Xiong SL, Feng H, Wu N. Cancer stem-like cells can be induced through dedifferentiation under hypoxic conditions in glioma, hepatoma and lung cancer. Cell Death Discov. 2017;3:16105. https://doi.org/10.1038/cddiscovery.2016.105.
Mathieu J, Zhang Z, Zhou W, Wang AJ, Heddleston JM, Pinna CM, Hubaud A, Stadler B, Choi M, Bar M, Tewari M, Liu A, Vessella R, Rostomily R, Born D, Horwitz M, Ware C, Blau CA, Cleary MA, Rich JN, Ruohola-Baker H. HIF induces human embryonic stem cell markers in cancer cells. Cancer Res. 2011;71(13):4640–52. https://doi.org/10.1158/0008-5472.CAN-10-3320.
Xie H, Dubey N, Shim W, Ramachandra CJA, Min KS, Cao T, Rosa V. Functional odontoblastic-like cells derived from human iPSCs. J Dent Res. 2018;97(1):77–83. https://doi.org/10.1177/0022034517730026.
Weltner J, Balboa D, Katayama S, Bespalov M, Krjutškov K, Jouhilahti EM, Trokovic R, Kere J, Otonkoski T. Human pluripotent reprogramming with CRISPR activators. Nat Commun. 2018;9(1):2643. https://doi.org/10.1038/s41467-018-05067-x.
Tai SK, Yang MH, Chang SY, Chang YC, Li WY, Tsai TL, Wang YF, Chu PY, Hsieh SL. Persistent Krüppel-like factor 4 expression predicts progression and poor prognosis of head and neck squamous cell carcinoma. Cancer Sci. 2011;102(4):895–902. https://doi.org/10.1111/j.1349-7006.2011.01859.x.
Yoshihama R, Yamaguchi K, Imajyo I, Mine M, Hiyake N, Akimoto N, Kobayashi Y, Chigita S, Kumamaru W, Kiyoshima T, Mori Y, Sugiura T. Expression levels of SOX2, KLF4 and brachyury transcription factors are associated with metastasis and poor prognosis in oral squamous cell carcinoma. Oncol Lett. 2016;11(2):1435–46. https://doi.org/10.3892/ol.2015.4047.
Søland TM, Solhaug MB, Bjerkli IH, Schreurs O, Sapkota D. The prognostic role of combining Krüppel-like factor 4 score and grade of inflammation in a Norwegian cohort of oral tongue squamous cell carcinomas. Eur J Oral Sci. 2022;130(3):e12866. https://doi.org/10.1111/eos.12866.
Li W, Liu M, Su Y, Zhou X, Liu Y, Zhang X. The Janus-faced roles of Krüppel-like factor 4 in oral squamous cell carcinoma cells. Oncotarget. 2015;6(42):44480–94. https://doi.org/10.18632/oncotarget.6256.
Rowland BD, Peeper DS. KLF4, p21 and context-dependent opposing forces in cancer. Nat Rev Cancer. 2006;6(1):11–23. https://doi.org/10.1038/nrc1780.
Le Magnen C, Bubendorf L, Ruiz C, Zlobec I, Bachmann A, Heberer M, Spagnoli GC, Wyler S, Mengus C. Klf4 transcription factor is expressed in the cytoplasm of prostate cancer cells. Eur J Cancer. 2013;49(4):955–63. https://doi.org/10.1016/j.ejca.2012.09.023.
Pandya AY, Talley LI, Frost AR, Fitzgerald TJ, Trivedi V, Chakravarthy M, Chhieng DC, Grizzle WE, Engler JA, Krontiras H, Bland KI, LoBuglio AF, Lobo-Ruppert SM, Ruppert JM. Nuclear localization of KLF4 is associated with an aggressive phenotype in early-stage breast cancer. Clin Cancer Res. 2004;10(8):2709–19. https://doi.org/10.1158/1078-0432.ccr-03-0484.
Shibata M, Chiba T, Matsuoka T, Mihara N, Kawashiri S, Imai K. Krüppel-like factors 4 and 5 expression and their involvement in differentiation of oral carcinomas. Int J Clin Exp Pathol. 2015;8(4):3701–9.
Acknowledgements
Not applicable.
Funding
This research received no external funding.
Author information
Authors and Affiliations
Contributions
RAD, JJVP, and KMB: project conception and design; RAD and KMB: data acquisition; JJVP, MSSK, and SMAJ: analysis and validation of the results; JJVP: data analysis and interpretation; RAD and JJVP: conducting the survey; RAD: writing the original draft preparation, writing revision, and editing; JJVP, MSSK, and SMAJ: substantive review; and JJVP: oversight, project administration, and funding acquisition. All authors were involved in writing the manuscript and approved the submitted and published versions.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Comitê de Ética em Pesquisa com Seres Humanos – UFPA. Committee reference number: 5.490.937.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
de Albuquerque Dias, R., Balbinot, K.M., da Silva Kataoka, M.S. et al. Expression of stem cell markers SALL4, LIN28A, and KLF4 in ameloblastoma. Diagn Pathol 18, 92 (2023). https://doi.org/10.1186/s13000-023-01379-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13000-023-01379-9