Abstract
Background
Progressive familial intrahepatic cholestasis (PFIC) is a rare group of autosomal recessive hereditary hepatic diseases. There are three types of PFIC, classified according to the mutated gene. For example, PFIC type 3 (PFIC3) is due to mutations in the ABCB4 gene (encoding multidrug-resistant protein 3 [MDR3]).
Case presentation
We present a 19-year-old Chinese female patient who had a 2-year history of recurrent liver dysfunction, with mainly elevated alkaline phosphatase and γ-glutamyl transpeptidase(γ-GT) levels. After excluding other causes of abnormal liver function and cholestasis, the final diagnosis of PFIC3 was confirmed by histopathological examination and gene detection. The immunohistochemical results showed no MDR3 protein expression in the bile duct membrane. Genetic sequencing analysis revealed a novel heterozygous 2137G > A; p. V713M mutation (Exon 17) and a synonymous 504C > T; p. N168N mutation (Exon 6) in ABCB4.
Conclusions
Our patient with long-term liver dysfunction demonstrated that elevated alkaline phosphatase and γ-GT levels should be associated with the diagnosis of PFIC3, and gene detection is the key to diagnosis. From our in silico analysis, the novel mutation p. V713M in Exon 17 was predicted to affect protein function, with a SIFT (Sorting Intolerant from Tolerant) score of 0.02, indicating a deleterious effect. Further studies are necessary to investigate the impact of the novel heterozygous 2137G > A; p. V713M mutation (Exon 17) on functional defects of MDR3 and PFIC3.
Similar content being viewed by others
Background
Progressive familial intrahepatic cholestasis (PFIC) is a rare group of autosomal recessive hereditary hepatic diseases. Gene mutations result in bile secretion or excretion disorders, and the main clinical manifestation of patients is intrahepatic cholestasis. Without intervention, patients would progress to liver cirrhosis or failure eventually [1]. There are three types of PFIC, classified according to the mutated gene as follows: PFIC type 1 (PFIC1) is due to mutations in the ATP8B1 gene (encoding FIC1); PFIC type 2 (PFIC2), mutations in the ABCB11 gene (encoding bile salt export pump); and PFIC type 3 (PFIC3), mutations in the ABCB4 gene (encoding multidrug-resistant protein 3 [MDR3]). Patients with PFIC1 and PFIC2 have normal or low serum γ-GT levels, but patients with PFIC3 are characterized by high serum γ-GT levels [2, 3]. Patients with PFIC3 usually develop cholestasis in late infancy (one third of cases) to adolescent age group. Gastrointestinal bleeding due to cirrhosis and portal hypertension may be the first presentation in older children or young adults. The disease usually progresses from chronic cholestasis with or without jaundice to portal hypertension and end stage liver disease [3].
We present the case of a 19-year-old female patient who had a 2-year history of recurrent liver dysfunction, with mainly elevated alkaline phosphatase andγ-GT levels. After excluding other causes of abnormal liver function and cholestasis, the final diagnosis of PFIC3 was confirmed by gene detection. Genetic sequencing analysis revealed a novel heterozygous 2137G > A; p. V713M mutation (Exon 17) and a synonymous 504C > T; p. N168N mutation (Exon 6) in ABCB4.
Case presentation
A 19-year-old female patient, a high school student, presented with abnormal liver function with unknown etiology for 2 years and was admitted to our hospital on October 17, 2018. Two years before, she was found to have abnormal liver function with no symptom in a physical examination but with mainly elevated alkaline phosphatase and γ-GT levels (Table 1). Her liver function improved slightly after taking some liver-protective drugs (Ursodeoxycholic Acid and Bicyclol) at the outpatient department. The subsequent follow-up liver function tests still yielded abnormal results; thus, we suggested that the patient be hospitalized to identify the causes of her abnormal liver function. However, because she was asymptomatic and busy in her senior high school year, she refused hospitalization. She revisited our hospital outpatient clinic again on October 17, 2018. This time, she was admitted to our hospital, because liver ultrasonography revealed cirrhosis.
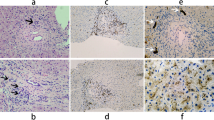
After admission to our department, we found that the patient had no jaundice, pruritus, asthenia, anorexia, and other discomforts. She denied a history of alcohol intake and use of any hepatotoxic drugs. She also had no family history of liver disease. On physical examination, mild splenomegaly was observed. The results of the rest of the examinations were unremarkable. Her laboratory data (October 18, 2018) showed the following values: alanine aminotransferase, 62 U/L; aspartate aminotransferase 45 U/L; alkaline phosphatase, 300 U/L; γ-GT, 119 U/L; total bilirubin, 14.9 μmol/L; direct bilirubin, 4.7 μmol/L; and albumin, 43.9 g/L. The hepatitis virus markers (hepatitis A, B, C, and E) were negative. Antibodies against the cytomegalovirus and Epstein-Barr virus were also negative. Her ceruloplasmin and serum copper levels were normal, and no Kayser-Fleischer ring was observed upon examination by an experienced ophthalmologist. The qualitative urinary porphyrin test result was negative. All autoimmune antibodies, including antimitochondrial, antinuclear, and antineutrophil cytoplasmic antibodies, were negative. The serum α-1-antitrypsin concentration, thyroid function, coagulation function, and other laboratory investigation results were normal. Abdominal magnetic resonance imaging revealed liver cirrhosis, portal hypertension, splenomegaly, and cholestasis. No obstructions of the intrahepatic and extrahepatic bile ducts were found after further examination with magnetic resonance cholangiopancreatography (Fig. 1). With the consent of the patient, we performed a liver pathological examination. Histological analysis revealed that the MDR3 protein staining decreased significantly as compared with that in healthy persons (Fig. 2).
Expression of the multidrug-resistant protein 3 in liver biopsy specimens. a: Liver biopsy specimens from the patient: MDR3 protein staining decreased significantly (immunohistochemical staining; original magnification × 200). b: Liver biopsy specimens from a healthy person showing strong staining and normal expression of the MDR3 protein (immunohistochemical staining; original magnification × 200)
Combined with the patient’s medical history and results of related tests, we considered the possibility of PFIC3. With her consent, we performed gene mutation detection. Genomic DNA was purified from peripheral blood samples. All 27 coding exons of ABCB4, together with at least 100 bp of the adjacent intronic sequence, were amplified by polymerase chain reaction and directly sequenced. The result revealed a synonymous mutation on Exon 6 (c.504C > T; p.N168N; heterozygous) and a novel missense mutation on Exon 17 (c.2137G > A; p.V713M; heterozygous). Based on these data, a diagnosis of PFIC3 was made.
After finding the cause of the disease, the patient was treated with ursodeoxycholic acid 750 mg/d. Considering that the patient had progressed to cirrhosis, we suggested that she should be treated with partial external biliary diversion or liver transplantation. However, her family rejected our suggestion because of the high cost, so she was discharged on November 11, 2018. The liver function test results during the course of the disease are shown in Table 1.
Discussion
PFIC3 is a rare disease, and most cases are sporadic. Hence, the incidence of the disease has not been accurately reported. A survey showed that the mean incidence of PFIC3 was 1 in 500,000 people [4]. In China, in our literature search, reports of such cases in last 10 years are limited [5,6,7]. Increasing the coverage of PFIC3 is meaningful and thus can improve the current understanding of this disease.
In this study, we present the case of a 19-year-old Chinese female patient with a novel mutation in ABCB4 who experienced intrahepatic cholestasis of unknown etiology 2 years before. After excluding other etiologies such as biliary atresia, sclerosing cholangitis, Alagille syndrome, primary biliary cirrhosis, and drug-induced liver injury by laboratory, radiographic, and histological examinations, we highly suspected that this disease might be PFIC3 according to her high γ-GT level. Thus, we performed a gene mutation analysis, which revealed a novel missense heterozygous 2137G > A; p. V713M mutation and a synonymous 504C > T; p. N168N mutation in ABCB4. On the basis of these data, the patient was diagnosed as having PFIC3.
PFIC3 is a disease caused by a genetic defect in the ABCB4 gene encoding the MDR3 protein, which is located on the canalicular membrane of the hepatocyte. The MDR3 protein acts as a phospholipid translocator that transfers phospholipids from hepatocytes to the bile ducts, which is the rate-limiting step of phospholipid secretion in the bile duct [4]. Normally, the phospholipids synthesized by hepatocytes are transported to the bile by MDR3, and phospholipids combine with bile salts to form microparticles, thereby increasing the hydrophilicity of bile salts, reducing the detergency of bile salts, and protecting the cholangiocytes from the bile salts [8]. Mutations in the ABCB4 gene lead to the decrease in the secretion of the phospholipid translocator MDR3 protein. The phospholipid in the bile is deficient, and the mixture of bile salts and phospholipids cannot be formed. The bile canaliculi and biliary epithelium get injured because of continuous exposure to hydrophobic bile salts. The detergent effects of bile salts are no longer being countered by phospholipids, thereby leading to cholangitis, hyperplasia, and inflammatory infiltration of the small bile duct. The disease then gradually progresses to portal area fibrosis, cirrhosis, and liver failure [1, 9].
In our patient, liver immunostaining for MDR3 showed a markedly attenuated, discontinuous, and disordered canalicular staining with apparent spread to the basolateral membrane. This led us to the diagnosis of PFIC3. However, normal liver immunostaining of MDR3 does not preclude a gene defect, as a mutation may induce loss of function but indicate normal synthesis and expression [10]. Thus, a clear diagnosis still requires genetic analysis for ABCB4. By using data from the human gene mutation database (http://www.hgmd.org/), we confirmed that > 150 types of diseases are related with the ABCB4 gene mutations, 50 types of mutations are associated with PFIC3. The mutation types include missense mutation, nonsense mutations, deletion, and small base insert fragments. Illness severity is related to the gene mutation type, and nonsense mutation and deletion easily manifest severe clinical symptoms [6].
The genetic sequencing analysis of ABCB4 in our patient revealed a synonymous 504C > T; p. N168N mutation and a novel heterozygous 2137G > A; p. V713M mutation. Although synonymous mutations do not change the translated protein products, more and more studies have found that synonymous mutations in germ cells and somatic cells may be harmful, leading to genetic diseases and cancers [11]. The database of harmful synonymous mutations (dbdsm, http://bioinfo.ahu.edu.cn: 8080 / dbdsm / index. JSP) is the first database of harmful synonymous mutations in the human genome. We tried to search this synonymous mutation in the database, and found no 504C > T; p. N168N mutation, however, this synonymous mutation has been reported in patients with intrahepatic cholestasis of pregnancy [12]. The relationship between 504C > T; p. N168N mutation and PFIC3 needs further study.
No other novel heterozygous 2137G > A; p. V713M mutation has been reported yet. To evaluate the functional effects of the novel mutation p. V713M, polymorphism phenotyping v2 (PolyPhen-2) and SIFT (Sorting Intolerant from Tolerant) were used. PolyPhen-2 is a tool that predicts the possible impact of an amino acid substitution on the structure and function of a human protein by using straightforward physical and comparative considerations. SIFT predicts whether an amino acid substitution affects protein function. SIFT prediction is based on the degree of conservation of amino acid residues in sequence alignments derived from closely related sequences, collected through PSI-BLAST. SIFT predicts substitutions with scores of < 0.05 as deleterious. Mutation p. V713M is predicted to be damaging at a score of 0.995 (sensitivity: 0.68; specificity: 0.97) by PolyPhen-2. SIFT scores showed that the novel mutation p. V713M was predicted to affect protein function at a SIFT score of 0.02, which indicates a deleterious effect.
Conclusions
Our patient with long-term liver dysfunction had elevated alkaline phosphatase and γ-GT levels, which should be associated with the diagnosis of PFIC3. Gene detection is the key to diagnosis. From our in silico analysis, we predicted that the novel mutation p. V713M in Exon 17 affects protein function at a SIFT score of 0.02, which indicates a deleterious effect. Further studies are necessary to investigate the impact of the novel heterozygous 2137G > A; p. V713M mutation (Exon 17) on the functional defects of MDR3 and PFIC3.
Availability of data and materials
The datasets used and/or analyzed in this study are available from the corresponding author on reasonable request.
Abbreviations
- PFIC:
-
Progressive familial intrahepatic cholestasis
- MDR3:
-
Multidrug-resistant protein 3
- γ-GT:
-
γ-glutamyl transpeptidase
- ICP:
-
Intrahepatic cholestasis of pregnancy
References
Bull LN, Thompson RJ. Progressive familial intrahepatic cholestasis. Clin Liver Dis. 2018;22(4):657–69.
Verkade HJ, Bezerra JA, Davenport M, Schreiber RA, Mieli-Vergani G, Hulscher JB, et al. Biliary atresia and other cholestatic childhood diseases: advances and future challenges. J Hepatol. 2016;65(3):631–42.
Srivastava A. Progressive familial intrahepatic cholestasis. J Clin Exp Hepatol. 2014;4(1):25–36.
Jacquemin E, De Vree JM, Cresteil D, Sokal EM, Sturm E, Dumont M, et al. The wide spectrum of multidrug resistance 3 deficiency: from neonatal cholestasis to cirrhosis of adulthood. Gastroenterology. 2001;120(6):1448–58.
Wang Z, Yang F, Wang J, Ma X. A novel mutation of ABCB4 in progressive familial intrahepatic cholestasis 3: like mother, Like Daughter. J Clin Gastroenterol. 2016;50(4):353–4.
Sun HZ, Shi H, Zhang SC, Shen XZ. Novel mutation in a Chinese patient with progressive familial intrahepatic cholestasis type 3. World J Gastroenterol. 2015;21(2):699–703.
Fang LJ, Wang XH, Knisely AS, Yu H, Lu Y, Liu LY, et al. Chinese children with chronic intrahepatic cholestasis and high gamma-glutamyl transpeptidase: clinical features and association with ABCB4 mutations. J Pediatr Gastroenterol Nutr. 2012;55(2):150–6.
Crawford AR, Smith AJ, Hatch VC, Oude Elferink RP, Borst P, Crawford JM. Hepatic secretion of phospholipid vesicles in the mouse critically depends on mdr2 or MDR3 P-glycoprotein expression. Visualization by electron microscopy. J Clin Invest. 1997;100(10):2562–7.
Oude Elferink RP, Paulusma CC. Function and pathophysiological importance of ABCB4 (MDR3 P-glycoprotein). Pflugers Arch. 2007;453(5):601–10.
Jacquemin E. Progressive familial intrahepatic cholestasis. Clin Res Hepatol Gastroenterol. 2012;36(Suppl 1):S26–35.
Supek F, Minana B, Valcarcel J, Gabaldon T, Lehner B. Synonymous mutations frequently act as driver mutations in human cancers. Cell. 2014;156:1324–35.
Kamimura K, Abe H, Kamimura N, Yamaguchi M, Mamizu M, Ogi K, et al. Successful management of severe intrahepatic cholestasis of pregnancy: report of a first Japanese case. BMC Gastroenterol. 2014;14:160.
Acknowledgments
The authors thank the patient and her relatives for agreeing to report her case and for providing a detailed medical history.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
Manuscript preparation: ZPW, SYZ; Data acquisition: ZPW, SYZ; Study concepts and design: LLZ, ML; Corresponding author: ML. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the ethics committee of The first affiliated hospital of Nanchang University, Nanchang China (No. 201920). Written informed consent was obtained from the patient for the storage of samples and data, follow-up contact, and further use of samples and data for research purposes.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wu, Z., Zhang, S., Zhang, L. et al. Novel ABCB4 mutation in a Chinese female patient with progressive familial intrahepatic cholestasis type 3: a case report. Diagn Pathol 15, 39 (2020). https://doi.org/10.1186/s13000-020-00955-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13000-020-00955-7