Abstract
Background
Extraovarian Brenner tumors (EOBTs) are extremely rare and can be observed incidentally in both female and male patients, raising concerns regarding the origin of Brenner tumors.
Case presentation
A 53-year-old postmenopausal woman presented with a nodular lesion in the left side of the corpus uteri, which was found at a routine health check. Macroscopically, the lesion appeared as a solid nodule with a yellowish-gray cut surface, approximately 6 cm in greatest diameter. Microscopically, the lesion consisted of well-defined epithelial nests and spindled stromal cells. Parenchymal cells expressed CK7, GATA3, CK5/6, 34βE12, and p63. A single layer of cavity-lined cells with umbrella-like shape showed apical Uroplakin III positivity. Stromal cells were positive for SMA, ER, and PR. The final diagnosis was EOBT and the patient was followed for 2 months with no recurrence.
Conclusions
We report here the third case of EOBTs in the uterus. The combination of morphologic and immunohistochemical results supported the involvement of urothelial metaplasia in the development of EOBTs. The similarities between EOBTs and Walthard nests made Müllerian epithelium an attractive candidate as the cellular origin. Changes of tissue structure or sex hormones imbalance may lead to the translocation of Müllerian remnants to distant organs, explaining the pathogenesis of EOBTs.
Similar content being viewed by others
Background
Brenner tumors (BTs) are relatively rare and account for approximately 5% of benign ovarian epithelial tumors [1]. It has been recognized that BTs are of benign nature, their most intriguing aspect lies in their histogenesis. The histological features of BTs suggest several cellular origins, including ovarian celomic epithelium, Walthard nests (WNs), mesothelium, Müllerian, and Wolffian cell.
Except for the ovary, it has been reported that BTs may also involve extraovarian tissues. Extraovarian BTs (EOBTs) are extremely rare and mainly occur around the female reproductive system such as the uterus [2, 3], vagina [4,5,6,7,8], broad ligament [9,10,11,12], and omentum [13]. Another intriguing clinical observation is that EOBTs were observed occasionally in the testis [14,15,16,17] or epididymis [18], further evoking concerns regarding their origins and pathogenesis. However, substantial evidence is limited because of the rarity of EOBTs.
In this study, we report the third case of the uterus BT found beneath the serosa of the left corpus uteri. Immunohistochemistry was used to explore the phenotype of its epithelium and stroma.
Case presentation
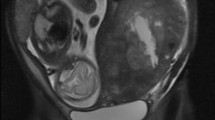
A 53-year-old female patient, gravida 1, para 1, with menopause at age 50, presented to the Department of Gynecology and Obstetrics with a 2-year history of a nodule in the left corpus uteri. The asymptomatic lesion was incidentally detected by ultrasound 2 years ago at a routine health check and had slowly increased in size (Fig. 1a). The sonographic test showed an oval and hypoechoic mass measured 5.8 x 5.7 x 4.6 cm (Fig. 1b). The clinical impression of a leiomyoma was made. Except for an oophorocystectomy of the left ovary 11 years ago for which pathological evaluation revealed a serous cystadenoma, her medical history was silent. Laboratory studies were within normal limits. Biopsy via fractional curettage and colposcope excluded endometrial or cervical-derived tumor. At laparotomy, a nodular neoplasm, protruding into the peritoneal cavity, was located beneath the serosa of the left side of the corpus and completely removed. The patient was followed up for 2 months with no recurrence.
Histopathology
On gross pathological examination, the mass measured 6 x 6 x 5 cm and exhibited a firm and fibrous texture on sectioning. The cut surface was solid and yellowish-gray in color (Fig. 2a). Microscopically, tumor epithelial cells were arranged in oval or cord-like well-defined nests, most of which were surrounded by hyalinized stromal cells (Fig. 2b). Central cavities containing hyaline material were seen in some nests (Fig. 2c). Of note is that a minority of nests consisted of large round cavities that compressed the lining cells resembling WNs (Fig. 2d). Epithelial cells displayed the morphologic continuum from short spindled shapes at the margin of the nests to the umbrella cell-like pattern at the center (Fig. 2c). They contained moderate clear to eosinophilic cytoplasm and oval nuclei with longitudinal grooves (Fig. 2b insert). No intercellular junctions or keratinization were seen. The leading edge of the tumor was examined carefully to explore the direct extension of mesothelium into tumor stroma. Tissue sections showed no evidence of mesothelium-derived cells in the tumor.
Histopathological features of EOBTs. A nodule lesion with yellowish-gray cut surface (a). The well-defined epithelial nests consisted of urothelium-like cells with clear to eosinophilic cytoplasm and longitudinal nuclear grooves (b). Cavities in epithelial islands were lined by a single layer of umbrella-like cell (c). Some cell nests resembled their counterparts in WNs (d). Original magnification x 400
Immunohistochemistry
Immunohistochemistry (IHC) was performed on serial sections to further confirm the nature of the tumor and explore its pathogenesis. BTs and urothelium-derived tumors commonly show highly overlapping phenotypic features. The primary antibody panel, consisting of cytokeratin (CK) 7, CK20, GATA3, CK5/6, 34βE12, p63, PAX8, p53, p16 and cyclinD1, was used to gain more insights into its nature and whether BT involves the transitional-cells metaplasia. Consistent with previous studies, epithelial cells expressed strong to diffuse reactivity for CK7 and negativity for CK20 (Fig. 3a, b). GATA3 belongs to a zinc finger transcription factor family and plays an essential role in the development of Müllerian duct [19]. It always serves as an important marker to distinguish breast or urinary-derived diseases from their mimics. A strong nuclear immunoreaction was observed for GATA3 in the majority of the epithelial cells (Fig. 3c).
CK5/6 are high-molecular-weight cytokeratins, which decorate basal-type cells in normal urothelium [20]. In this case, epithelial cells displayed a graded intensity for CK5/6 and 34βE12 (Fig. 3d, e). The proportion of 34βE12 positive cells was higher than that of CK5/6 positive cells. P63, an essential factor for the development of urothelial and squamous cells, marks basal and intermediate urothelial cells [20]. Consistent with previous studies, more than 80% of epithelial cells showed nuclear positivity for p63 antibody (Fig. 3f).
An interesting observation is that the single layer of cavity-lined cells did not express p63 and CK5/6. However, Uroplakin III revealed a linear apical positivity in a few of these cells (Fig. 4a). Mesothelium markers such as WT1 and calretinin were negative in cell nests (data not shown). The combination of morphologic features and desmin positivity confirmed that spindled stromal cells were smooth muscle cells (Fig. 4b). Like normal counterparts in the corpus uteri, these tumor stromal cells, rather than epithelial cells, expressed estrogen receptor (ER) and progesterone receptor (PR) (Fig. 4c and d). Both epithelial and stromal cells were negative for PAX8, p53, and p16 (Fig. 4e-g). Epithelium showed weak positivity for cyclinD1 (Fig. 4h).
The immunohistochemical results of epithelial and stromal cells. A few of cavity-lined cells exhibited Uroplakin III positivity (a). Desmin immunopositivity confirmed smooth muscle nature of stromal cells (b). Stromal cells expressed ER (c) and PR (d). Tumor cells were negative for PAX8, p53, and p16 (e-g). The epithelium showed weak positivity for cyclinD1 (h). Original magnification x 200 (b, e-h) and x 400 (a, c, d)
Discussion
Despite accumulating evidence, the histogenesis of EOBTs remains unsettled. We report the third case of the uterine EOBT and provide preliminary morphologic and immunophenotypic data to further support Müllerian epithelium as an attractive candidate. The clinical and immunohistochemical features of published EOBTs cases from 1947 and our present case were summarized in the Table 1. Studies to date have demonstrated that the fallopian tubes, corpus uteri, and cervix develop from Müllerian ducts. Diffuse activity of epithelial nests for GATA3 supports a Müllerian origin [19]. However, these epithelial cells show negativity for PAX8, a transcription factor involved in the development of Müllerian organs [8, 13, 21]. PAX8 expression can be detected in a small percentage of epithelial cells in WNs. Although the pathogenesis of WNs is largely unknown, it has been suggested that WNs and BTs have analogous origins because of morphologic and immunophenotypic similarities [21]. This view is further supported by the clinical observation that WNs were found in up to 50% of patients with BTs [22]. The epithelial cells of WNs may serve as progenitors of the parenchymal cells of BTs. With regard to the fact that BTs can occur in non- Müllerian-derived tissues, we propose that EOBTs may derive directly from Müllerian remnants or from WNs through a seeding pattern. CK5/6 and p63-expressing basal-like cells located at the edge of EOBTs nests may represent immature cells. Consistent with previous studies, the positivity of Uroplakin III was restricted to the minority of cavity-lining cells, reflecting urothelial metaplasia process [23,24,25]. The graded intensity of CK5/6 and p63 further supports this possibility.
Accumulating evidence subdivides Brenner tumor into benign, borderline, and malignant types [26, 27]. The nature of EOBT is largely unknown. Morphological features of published EOBT cases, including this case, qualify this tumor more as benign BT. However, this concept has been challenged by the molecular signatures. The epithelium expressed p63 and cyclinD1, rather than p53 and p16, reflecting its borderline propensity [13, 26]. Further studies will be required to elucidate the biological details of EOBT.
EOBTs also can be observed in vagina [4,5,6,7,8]. However, the histogenesis of the vagina is under controversy. Previous morphologic evidence supports the traditional concept that the cranial part (upper third) of the vagina originates from the Müllerian duct and the caudal part (lower two-thirds) arises from urogenital sinus. This hypothesis has not yet addressed a critical issue of how the columnar Müllerian epithelium converts to the squamous cell. Genetic studies have raised an intriguing possibility that the whole vagina is derived from the Müllerian duct based on evidence obtained from transgenic models. Knockout of Wnts family members such as Wnt4, 5a, and 9b, which are essential for the formation of Müllerian ducts, resulted in the absence of both the Müllerian ducts and vagina [28,29,30]. Downregulation of Pax-2, a urogenital transcription factor expressed in the Wolffian and Müllerian ducts, led to the lack of mesonephric and paramesonephric systems, rather than the bladder and urethra [31]. Hoxa 13, a critical gene during the development of the caudal Müllerian duct, is highly expressed in the cervix and vagina of newborn rodents [32]. Hoxa 13 can activate promoter activity and upregulate the expression of bone morphogenetic protein 4 (BMP4), which, in turn, enhances p63 level and induces squamous cell conversion [33].
Müllerian remnants have also been detected in the appendix testis and loose connective tissue between the epididymis and testis [34]. Most of reported BT cases in male patients were located in these sites [14,15,16,17,18]. Thus, it is theoretically possible for EOBTs to occur directly in the whole vagina, uterus, fallopian tube, and paratestis. More substantial evidence is needed to further support this view because of the extreme rarity of EOBTs.
Previous studies have failed to reach a conclusion about the role of stromal cells in development of BTs. As the majority of BTs occur in ovary, it is of great interest to explore the link between hormones and stromal cells. Immunohistochemical results showed that the spindled cells were positive for ER and PR in the present study. Although normal smooth muscle cells of the corpus uteri express these receptors in the setting of physical status, stromal cells of vaginal EOBTs also exhibit ER and PR positivity (Table 1), indicating the involvement of sex hormone-related signaling pathways [7]. Like their ovarian counterparts, EOBTs are usually found in postmenopausal women, who have low levels of circulating estrogen and progesterone. However, androgen production does not suffer from menopause because of sparing of the stromal compartment, suggesting that ageing and sex hormones disorder may be risk factors for BTs. Alterations of the microenvironment may trigger the pathogenesis of EOBTs or WNs.
One hypothesis emerges in elucidating how BTs occurs in non-Müllerian-derived ovary. When the fimbria keeps close to the ovary, Müllerian cells seed and implant on the surface of the ovary to form inclusion cysts, which may develop BTs [35]. The mechanisms of how EOBTs happen in tissues far from Müllerian-derived organs are largely unclear. Up to 57% (4/7) EOBTs patients with available history underwent abdominal surgeries (Table 1). We propose that abdominal surgery or laparotomy, which leads to the alteration of tissue structure, can promote the translocation of Müllerian epithelium to distant sites.
Conclusion
EOBTs are extremely rare and can be found incidentally in both male and female patients. We report the third case of uterine EOBTs with morphologic and immunophenotypic analyses. The well-defined epithelium islands consisted of urothelium-like cells, which exhibited morphologic continuum from basal- to umbrella-like pattern. The reactivity for CK7, GATA3, CK5/6, 34βE12, and Uroplakin III further supported the possibility that these tumor cells were undergoing urothelial metaplasia. The combination of patient’s age at diagnosis (most > 50 years) and ER/PR positivity in stromal cells suggests the involvement of sex hormones imbalance. Overlapping features of WNs and EOBTs implies a common pathogenesis from Müllerian epithelium. Changes of environment such as inflammation and surgery may promote the translocation of Müllerian remnants to distant tissues. Further studies are needed to determine the underlying molecular mechanisms.
Availability of data and materials
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Abbreviations
- BTs:
-
Brenner tumors
- CK:
-
Cytokeratin
- EOBTs:
-
Extraovarian BTs
- ER:
-
Estrogen receptor
- PR:
-
Progesterone receptor
- WNs:
-
Walthard nests
References
Koonings PP, Campbell K, Mishell DR Jr, Grimes DA. Relative frequency of primary ovarian neoplasms: a 10-year review. Obstet Gynecol. 1989;74(6):921–6.
Arhelger RB, Bocian JJ. Brenner tumor of the uterus. Cancer. 1976;38(4):1741–3. https://doi.org/10.1002/1097-0142(197610)38:4<1741::aid-cncr2820380448>3.0.co;2-f.
Angeles-Angeles A, Gutierrez-Villalobos LI, Lome-Maldonado C, Jimenez-Moreno A. Polypoid Brenner tumor of the uterus. Int J Gynecol Pathol. 2002;21(1):86–7.
Chen KT. Brenner tumor of the vagina. Diagn Gynecol Obstet. 1981;3(3):255–8.
Rashid AM, Fox H. Brenner tumour of the vagina. J Clin Pathol. 1995;48(7):678–9. https://doi.org/10.1136/jcp.48.7.678.
Ben-Izhak O, Munichor M, Malkin L, Kerner H. Brenner tumor of the vagina. Int J Gynecol Pathol. 1998;17(1):79–82.
Shaco-Levy R, Benharroch D. Vaginal brenner tumor. Int J Gynecol Pathol. 2013;32(2):238–41. https://doi.org/10.1097/PGP.0b013e3182590ae8.
Park S, Cho MS. Vaginal Brenner tumor with literature review: does this tumour originate from Walthard nests? Malays J Pathol. 2017;39(1):89–93.
Pschera H, Wikstrom B. Extraovarian Brenner tumor coexisting with serous cystadenoma. Case report. Gynecol Obstet Invest. 1991;31(3):185–7. https://doi.org/10.1159/000293153.
Hampton HL, Huffman HT, Meeks GR. Extraovarian Brenner tumor. Obstet Gynecol. 1992;79(5 ( Pt 2)):844–6.
Robinson TG. Extra-ovarian Brenner tumour. J Obstet Gynaecol Br Emp. 1950;57(6):890–1.
Wagner I, Bettendorf U. Extraovarian Brenner tumor. Case report and review. Arch Gynecol. 1980;229(3):191–6.
Hwang CS, Lee CH, Lee SJ, Kim YG, Kim A, Park DY, et al. A peculiar case report of extraovarian Brenner tumor arising in the omentum. World J Surg Oncol. 2017;15(1):72. https://doi.org/10.1186/s12957-017-1135-2.
Vechinski TO, Jaeschke WH, Vermund H. Testicular tumors. An analysis of 112 consecutive cases. Am J Roentgenol Radium Therapy, Nucl Med. 1965;95(2):494–514.
Ross L. Paratesticular Brenner-like tumor. Cancer. 1968;21(4):722–6. https://doi.org/10.1002/1097-0142(196804)21:4<722::aid-cncr2820210425>3.0.co;2-l.
Goldman RL. A brenner tumor of the testis. Cancer. 1970;26(4):853–6. https://doi.org/10.1002/1097-0142(197010)26:4<853::aid-cncr2820260419>3.0.co;2-e.
Nogales FF Jr, Matilla A, Ortega I, Alvarez T. Mixed Brenner and adenomatoid tumor of the testis: an ultrastructural study and histogenetic considerations. Cancer. 1979;43(2):539–43. https://doi.org/10.1002/1097-0142(197902)43:2<539::aid-cncr2820430220>3.0.co;2-e.
Hartz PH. Occurrence of Walthard cell rests or Brenner-like epithelium in the serosa of the epididymis. Am J Clin Pathol. 1947;17(8):654–6. https://doi.org/10.1093/ajcp/17.8.654.
Grote D, Souabni A, Busslinger M, Bouchard M. Pax 2/8-regulated Gata 3 expression is necessary for morphogenesis and guidance of the nephric duct in the developing kidney. Development. 2006;133(1):53–61. https://doi.org/10.1242/dev.02184.
Kaufmann O, Fietze E, Mengs J, Dietel M. Value of p63 and cytokeratin 5/6 as immunohistochemical markers for the differential diagnosis of poorly differentiated and undifferentiated carcinomas. Am J Clin Pathol. 2001;116(6):823–30. https://doi.org/10.1309/21TW-2NDG-JRK4-PFJX.
Roma AA, Masand RP. Ovarian Brenner tumors and Walthard nests: a histologic and immunohistochemical study. Hum Pathol. 2014;45(12):2417–22. https://doi.org/10.1016/j.humpath.2014.08.003.
Seidman JD, Khedmati F. Exploring the histogenesis of ovarian mucinous and transitional cell (Brenner) neoplasms and their relationship with Walthard cell nests: a study of 120 tumors. Arch Pathol Lab Med. 2008;132(11):1753–60. https://doi.org/10.1043/1543-2165-132.11.1753.
Ogawa K, Johansson SL, Cohen SM. Immunohistochemical analysis of uroplakins, urothelial specific proteins, in ovarian Brenner tumors, normal tissues, and benign and neoplastic lesions of the female genital tract. Am J Pathol. 1999;155(4):1047–50. https://doi.org/10.1016/S0002-9440(10)65206-6.
Logani S, Oliva E, Amin MB, Folpe AL, Cohen C, Young RH. Immunoprofile of ovarian tumors with putative transitional cell (urothelial) differentiation using novel urothelial markers: histogenetic and diagnostic implications. Am J Surg Pathol. 2003;27(11):1434–41. https://doi.org/10.1097/00000478-200311000-00005.
Riedel I, Czernobilsky B, Lifschitz-Mercer B, Roth LM, Wu XR, Sun TT, et al. Brenner tumors but not transitional cell carcinomas of the ovary show urothelial differentiation: immunohistochemical staining of urothelial markers, including cytokeratins and uroplakins. Virchows Arch. 2001;438(2):181–91. https://doi.org/10.1007/s004280000315.
Zheng R, Heller DS. Borderline Brenner tumor: a review of the literature. Arch Pathol Lab Med. 2019;143(10):1278–80. https://doi.org/10.5858/arpa.2018-0285-RS.
Meinhold-Heerlein I, Fotopoulou C, Harter P, Kurzeder C, Mustea A, Wimberger P, et al. The new WHO classification of ovarian, fallopian tube, and primary peritoneal cancer and its clinical implications. Arch Gynecol Obstet. 2016;293(4):695–700. https://doi.org/10.1007/s00404-016-4035-8.
Vainio S, Heikkila M, Kispert A, Chin N, McMahon AP. Female development in mammals is regulated by Wnt-4 signalling. Nature. 1999;397(6718):405–9. https://doi.org/10.1038/17068.
Mericskay M, Kitajewski J, Sassoon D. Wnt5a is required for proper epithelial-mesenchymal interactions in the uterus. Development. 2004;131(9):2061–72. https://doi.org/10.1242/dev.01090.
Carroll TJ, Park JS, Hayashi S, Majumdar A, McMahon AP. Wnt9b plays a central role in the regulation of mesenchymal to epithelial transitions underlying organogenesis of the mammalian urogenital system. Dev Cell. 2005;9(2):283–92. https://doi.org/10.1016/j.devcel.2005.05.016.
Torres M, Gomez-Pardo E, Dressler GR, Gruss P. Pax-2 controls multiple steps of urogenital development. Development. 1995;121(12):4057–65.
Taylor HS, Vanden Heuvel GB, Igarashi P. A conserved Hox axis in the mouse and human female reproductive system: late establishment and persistent adult expression of the Hoxa cluster genes. Biol Reprod. 1997;57(6):1338–45. https://doi.org/10.1095/biolreprod57.6.1338.
Kurita T, Mills AA, Cunha GR. Roles of p63 in the diethylstilbestrol-induced cervicovaginal adenosis. Development. 2004;131(7):1639–49. https://doi.org/10.1242/dev.01038.
Sundarasivarao D. The Mullerian vestiges and benign epithelial tumours of the epididymis. J Pathol Bacteriol. 1953;66(2):417–32.
Kurman RJ, Shih IM. Molecular pathogenesis and extraovarian origin of epithelial ovarian cancer--shifting the paradigm. Hum Pathol. 2011;42(7):918–31. https://doi.org/10.1016/j.humpath.2011.03.003.
Acknowledgements
We gratefully acknowledge Dr. Jia-Chen Xiao at Shanghai Jiaotong University for proof-reading the discussion.
Funding
This study was supported by grants from the National Natural Science Foundation of China (No. 81770624 and 81860490), the Science Foundation of Jiangxi Province for Distinguished Young Scholars, China (No. 20192BCB23025), and Foundation of Health and Family Planning Commission of Jiangxi Province (No. 20171098).
Author information
Authors and Affiliations
Contributions
HRY, DYJ, and ZL performed immunohistochemistry and drafted the manuscript. ZHH, ZXW, JN and ZL carried pathological analyses. ZJ, HM, LXQ, XQJ, and PXY collected and analyzed clinical data. DH conceived of this study and drafted the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Patient provided informed consent; the present report were approved by the Ethics Committee of the Fourth Affiliated Hospital of Nanchang University.
Consent for publication
Written informed consent for publication of the clinical details and/or clinical images was obtained from the patient. A copy of the consent form is available for review by the Editor of this journal.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Hu, RY., Deng, YJ., Zhu, HH. et al. Extraovarian Brenner tumor in the uterus: a case report and review of literature. Diagn Pathol 15, 22 (2020). https://doi.org/10.1186/s13000-019-0906-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13000-019-0906-1