Abstract
Background
Complement 5a receptor (C5aR) was demonstrated a receptor of complement 5a (C5a) which is involved in many inflammatory diseases. The functional responses attributed to C5a results from its interaction with its receptors C5aR, which stimulates food intake, plays a role in increasing the inflammatory response in adipose tissue as well as the cardiovascular and neural systems. However, There are unknown associations between the SNPs of C5aR1 gene and coronary artery disease (CAD).
Methods
We examined the role of the tagging single nucleotide polymorphisms (SNPs) of C5aR1 gene for CAD using a case–control design, and determined the prevalence of C5aR1 genotypes in 505 CAD patients and 469 age and sex-matched healthy control subjects of Han population.
Results
The rs10853784 was found to be associated with CAD in dominant model (CC vs TT + CT, P = 0.004). The difference remained statistically significant after multivariate adjustment (OR = 1.430, 95% CI: 1.087 ~ 1.882, P = 0.011). There was no significant difference in genotype distributions of rs4577202 and rs7250152 between CAD patients and control subjects. The frequency of the haplotype (A-T-C) was significantly higher in the CAD patients than in the controls (P = 0.035), and the haplotype (A-C-T) was significantly lower in the CAD patients than in the control subjects in Chinese Han population (P = 0.002).
Conclusion
The results of this study indicate that rs10853784 of C5aR1 gene are associated with CAD in Han population of China, and A-C-T haplotypes may be protective genetic marker and the A-T-C may be risk genetic marker for CAD in Chinese Han population.
Virtual slides
The virtual slide(s) for this article can be found here: http://www.diagnosticpathology.diagnomx.eu/vs/2054871241495194.
Similar content being viewed by others
Background
The role of the immune system and inflammatory pathways in the development of atherosclerotic disease is well established [1]. Recent evidences claimed that inflammation might represent the pathophysiological link between obesity and metabolic syndrome (MetS) [2]. Since adipose tissue is now considered an endocrine organ, affecting metabolism and vascular function. The understanding of these pathways is crucial from a pathophysiological point of coronary artery disease (CAD) risk stratification and to the identification of possible therapeutic targets. Disorders of lipoprotein metabolism such as elevated levels of triglyceride (TG), Lipoprotein (a) [Lp(a)] as well as increased fasting blood glucose are considered to be important risk factors in the pathogenesis of atherosclerosis and CAD [3-6]. CAD is a chronic inflammatory disease. There is evidence on the role of inflammation in all stages of the atherosclerotic disease process [7,8]. Genetic studies have revealed many causal or susceptible inflammatory loci associated with CAD [9,10].
C5a is a multifunctional protein, which promotes the recruitment and activation of neutrophils and monocytes [3]. Pathological conditions such as sepsis and various immunoinflammatory disorders are accompanied by increases in circulating C5a [11-13]. C5a interacts primarily with its two receptors, the classic proinflammatory C5a receptor (C5aR; CD88) and relative newly identified C5a receptore like 2 (C5L2). C5aR is a member of the rhodopsin family of G protein-coupled receptors (GPCR) which is expressed at varying levels in different immune and non-immune cells [14-17]. A recent research demonstrated that the C5aR Knockout(KO) mice have decreased fat mass, glucose clearance and insulin tolerance [18]. The C5a-C5aR pathway has been targeted for pharmacological therapy for treatment of sepsis, cardiovascular diseases, and autoimmune disorders [19,20]. C5L2, which resembles C5aR (58% homology) [12] was demonstrated to be a functional receptor of acylation-stimulating protein (ASP), increased transport of glucose and esterification of fatty acids, leading to a net accumulation of TG stores influence the body’s susceptibility to CAD [21-23].
In previous studies [24-26], we identified C5L2 gene which is associated with CAD and T2DM in Chinese Han and Uygur population. The activity of C5L2 may influence the individual’s susceptibility to CAD. Accordingly, we screened for tagging SNPs of the C5aR1 gene and assessed the association between the genotypes of this gene and CAD in Chinese Han population.
Methods
Ethical approval of the study protocol
Written informed consent was obtained from all participants. An approval of ethics committee by the Ethics Committee of the First Affiliated Hospital of Xinjiang Medical University (Xinjiang, China) was obtained. It was conducted in accordance with the guidelines of the Helsinki Declaration.
Subjects
Participants diagnosed with CAD were recruited at the First Teaching Hospital of Xinjiang Medical University from 2007 to 2010. The detailed diagnostic and selection criteria have been previously described [27,28]. Briefly, CAD was defined as the presence of at least one significant coronary artery stenosis of ≥50% luminal diameter on coronary angiography. Patients were excluded if they had congenital hypercoagulable status with proven disease-limiting life expectancy or had abused cocaine. The healthy participants were selected from the cardiovascular risk survey (CRS). The CRS has been described previously [29]. This study consists of 14,618 subjects and is a multiple-ethnic, community-based, cross-sectional study designed to investigate the prevalence, incidence, and risk factors for cardiovascular diseases in the Han, Uygur, and Kazakh population in Xinjiang (west China) between June 2007 and March 2010. These control individuals did not have a history of CAD, electrocardiographic signs of CAD, regional wall motion abnormalities, and relevant valvular abnormalities in echocardiograms [30].
In this study, 505 patients with CAD and 469 healthy control participants were enrolled. All study participants were Han Chinese ethnicity and were from the same geographic area in Xinjiang. Demographic data and subject characteristics, including hypertension, diabetes mellitus, smoking, alcohol consumption, and serum cholesterol, were collected for all study participants. Diabetes, hypertension, hyperlipidemia, smoking, and alcohol consumption were defined as described previously [27-29].
Biochemical analysis
Serum concentrations of total cholesterol (TC), TG, glucose, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), apolipoprotein B (Apo B), LP (a) were measured using standard methods in the Central Laboratory of First Affiliated Hospital of Xinjiang Medical University as described previously [27-29].
Sample DNA extraction
Blood samples were collected from all participants using a standard venipuncture technique and EDTA-containing tubes. DNA was extracted from the peripheral blood leukocytes using a whole blood genome extraction kit (Beijing Bioteke Corporation, Beijing, China. (http://bioteke.technew.cn) as previously described [27,29].
Genotyping of C5aR1 gene
The genotyping for the C5aR1 gene was performed by using ABI 7900 Real Time PCR System (Applied Biosystems). Amplification was performed in 6ul of volume, including 1ul of DNA, 3ul of Master Mix, 0.05ul of Probe(40X), MiliQ add to 6ul. Real time PCR was performed according to the protocol as follows: After an initial holding step of Pre-heated 95°C for 10 min, samples were cycled 20 times at 95°C for 10s, at 65°C-55°C for 15 s (Each loop drops by 0.5°C), at 60°C for15s. Then the samples were cycled 25 times at 95°C for 10s, at 60°C for 30s, pre/post read (Starting from 60°C at 0.06°C/s was slowly warmed to 61°C, and collecting the fluorescence value). Cooling (cool at 40°C to maintain).
Statistical analyses
Statistical analysis was performed using the SPSS version 17.0 software (SPSS, Chicago, IL, USA), Data are expressed as the mean ± standard deviation (SD). The significance of differences was evaluated using the t-test for continuous variables and the χ2 test for non-continuous variables. The differences between CAD patients and control subjects were assessed by independent-sample t-test. Categorical variables were analyzed as frequencies. Allele and genotype frequencies among CAD cases and controls were compared with values predicted by using the Chi-square test. Hardy-Weinberg equilibrium was assessed by chi-square analysis. Multivariate analysis was performed using a logistic regression analysis for independent variables that were related to the presence or absence of CAD. A value of P < 0.05 was considered significant.
Results
Characteristics of participants
Table 1 shows demographic and clinical characteristics of 974 study subjects. CAD patients (n = 505) and healthy control subjects (n = 469), the following variables were significantly different between the two groups: diabetes and drinking; the serum concentration of glucose, apo B and LP(a) (all P < 0.05). There was no significant difference in the following variables between CAD patients and control subjects: hypertation; smoking, the body mass index (BMI); serum concentration of TG, HDL, and LDL, age, and sex (all P > 0.05).
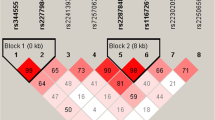
Distribution of the SNPs for C5aR1 gene in CAD patients and controls
Table 2 shows distribution of genotypes and alleles of SNPs for C5aR1 gene. The genotypes distributions for each of the SNPs were in good agreement with the predicted Hardy-Weinberg equilibrium values (data not shown). There was no significant difference between CAD and control subjects for rs4577201 (SNP1) and rs7250152 (SNP2) (all P > 0.05). Distribution of rs10853784 (SNP3) genotypes, dominant model (CC vs TT + CT), additive model (CT vs CC + TT) and allele frequency showed significant difference between CAD and control subjects (P = 0.016, P = 0.004, P = 0.021 and P = 0.004, respectively). There was no significant difference between CAD and control subjects for recessive model (TT vs CC + CT, P = 0.191).
Table 3 shows multivariable logistic regression analysis combining genotypes with following variables: plasma concentration of lipoprotein(a), incidence of diabetes, drinking and smoking which were the major confounding factors for CAD. After multivariate adjustment, rs10853784 remain significantly associated with CAD in dominant model (CC vs CT + TT) (OR = 1.430, 95% confidence interval [CI]:1.087 ~ 1.882, P = 0.011).
Haplotypes of the C5aR1 Gene Associated with CAD
In the haplotype-based case–control analysis, haplotypes were established through the use of different combinations of the SNPs (Table 4). The overall distribution of the haplotypes established by SNP1-SNP2-SNP3 was significantly different between the CAD patients and the control subjects. The frequency of the A-C-T haplotype established by SNP1-SNP2-SNP3 in these two groups was also significantly lower for the CAD patients as compared to the control subjects (OR = 0.714, 95% CI = 0.577-0.883, P = 0.002). Moreover, the frequency of the A-T-C haplotype established by SNP1-SNP2-SNP3 in these two groups was also significantly higher for the CAD patients compared to the control subjects (OR = 1.213, 95% CI = 1.014-1.452, P = 0.035).
Discussion
Factors such as diabetes, hypertension, inflammation and hyperlipidemia have been reported to influence the pathogenesis of CAD. Like hyperlipemia and diabetes, CAD is thought to be a multifactorial disease. Hence, much attention has been focused on the association of gene polymorphisms with CAD.
The C5aR and C5L2, was identified in 1991 [31,32] and 2000 [33], respectively. Both genes are localized to the same region of chromosome 19, q13.33 and encoded in a two exon structure, with the 5′-untranslated region and initiating codon in the first exon, and the remainder of the coding sequence and the 3′-untranslatedregion in the second [34]. This is typical of the members of the chemoattractant receptor family. In the promoter region of C5aR, an SNP at position-245 (T/C) has been discovered [35] and the coding region. C5aR has two non-synonymous SNPs at 4G/A (Asp/Asn at amino-acid position 2) and 859G/T (Asn/Lys at position 278) and two synonymous SNPs: 72 T/C and 727G/A [36]. To date, no SNPs have been identified in the coding region of the C5aR1 gene association with human disease. C5aR, which binds C5a, is involved in many inflammatory diseases [37]. One previous study showed that C5a stimulates food intake [38], the C5aR KO mice demonstrated decreased body weight and fat storage [22]. Studies earlier showed the higher levels of C5a was associated with late lumen loss of drug-eluting stents [39,40]. Early atherosclerotic lesions express C5aR, which may indicate that inflammatory cells are initially recruited into the damaged vessels through C5aR [41]. Other research reported that C5a concentration was more elevated in hypertensive individuals than in healthy people. C5aR signaling pathway on blood monocytes/macrophages plays a pathological role in angiotensin II (Ang II)-induced cardiac inflammation and remodeling. Complement proteins such as complement C3 [42,43], C5L2 [44,45] and C3aR [46] have all been implicated in lipid and glucose metabolism. C5L2 is similar to C5aR in structure, C5L2 was associated with hyperlipidemia and diabetes, and the C5L2 genetic variant was associated with CAD and this association is not modified by the concentration of TG and glucose.
In our study, we found that polymorphisms of C5aR1 were associated with CAD in a Han population. There was significant difference in genotype distribution of rs10853784 between CAD patients and control subjects. For the rs10853784 genotypes, dominant model (CC vs TT + CT), additive model (CT vs CC + TT) and allele frequency showed significant difference between CAD and control subjects. After multivariate adjustment of confounding factors the significant difference was retained.
For genes with multiple susceptibilities, an analysis based on haplotypes has advantages over an analysis based on individual SNPs, particularly when the linkage disequilibria between the SNPs is weak [47]. Our study is the first haplotype-based case–control study to investigate the association between the human C5aR1 gene and CAD in the Chinese Han population. In our study, we succeeded in identifying two haplotypes (A-C-T and A-T-C) of SNP1-SNP2-SNP3 in Chinese Han population. Based on the haplotype and logistic regression analyses, we believe that the haplotype (A-C-T) is a protective factor for CAD (OR = 0.714, P = 0.002), and A-T-C is an risk factor for CAD (OR = 1.213, P = 0.035) in Chinese Han population.
Conclusion
In conclusion, this is the first time that correlations between the human C5aR1 gene and CAD have been examined in the Chinese population. The present data indicate that C5aR1gene is associated with CAD in Han population of China. This result may broaden the knowledge of genetic variants and disease-association studies.
Study limitation
The present study was limited by the relatively small sample size. This may have led to weak statistical significance and wide CIs when estimating ORs.
References
The U.S.National Heart Lung and Blood Institute (NHLBI). National Heart, Lung, and Blood Institute Twin Study. ClinicalTrials gov. 2005; Web site. http://clinicaltrials.gov/ct2/show/record/NCT00005124. Accessed March 29, 2013.
Pattrick M, Luckett J, Yue L, Stover C. Dual role of complement in adipose tissue. Mol Immunol. 2009;46(5):755–60.
Arsenault BJ, Lemieux I, Després JP, Wareham NJ, Kastelein JJ, Khaw KT, et al. The hypertriglyceridemic-waist phenotype and the risk of coronary artery disease: results from the EPIC-Norfolk prospective population study. CMAJ. 2010;182(13):1427–32.
Raz I. Relationship between blood glucose control and improved cardiovascular outcome after stent implantation in diabetic patients. Cardiology. 2010;116(1):48–50.
Goswami B, Rajappa M, Singh B, Ray PC, Kumar S, Mallika V. Inflammation and dyslipidaemia: a possible interplay between established risk factors in North Indian males with coronary artery disease. Cardiovasc J Afr. 2010;21:103–8.
Rajappa M, Sridhar MG, Balachander J, Sethuraman KR, Rajendiran KS. Lipoprotein ratios as surrogate markers for insulin resistance in South Indians with normoglycemic nondiabetic acute coronary syndrome. ISRN Endocrinol. 2014;2014:981524.
Libby P. Inflammation in atherosclerosis. Arterioscler Thromb Vasc Biol. 2012;32(9):2045–51.
Ross R. Atherosclerosis—an inflammatory disease. N Engl J Med. 1999;340:115–26.
McPherson R, Davies RW. Inflammation and coronary artery disease: insights from genetic studies. Can J Cardiol. 2012;28(6):662–6.
Shanker J, Kakkar VV. Implications of genetic polymorphisms in inflammation-induced atherosclerosis. Open Cardiovasc Med J. 2010;4:30–7.
Basen-Engquist K, Chang M. Obesity and cancer risk: recent review and evidence. Curr Oncol Rep. 2011;13(1):71–6.
Fonseca MI, Ager RR, Chu SH, Yazan O, Sanderson SD, LaFerla FM, et al. Treatment with a C5aRantagonist decreases pathology and enhances behavioral performance in murine models of Alzheimer’s disease. J Immunol. 2009;183(2):1375–83.
Rittirsch D, Flierl MA, Ward PA. Harmful molecular mechanisms in sepsis. Nat Rev Immunol. 2008;8(10):776–87.
Rabiet MJ, Huet E, Boulay F. The N-formyl peptide receptors and the anaphylatoxin C5a receptors: an overview. Biochimie. 2007;89(9):1089–106.
Bamberg CE, Mackay CR, Lee H, Zahra D, Jackson J, Lim YS, et al. The C5a receptor (C5aR) C5L2 is a modulator of C5aR-mediated signal transduction. J Biol Chem. 2010;285(10):7633–44.
Monk PN, Scola AM, Madala P, Fairlie DP. Function, structure and therapeutic potential of complement C5a receptors. Br J Pharmacol. 2007;152(4):429–48.
Ricklin D, Hajishengallis G, Yang K, Lambris JD. Complement: a key system for immune surveillance and homeostasis. Nat Immunol. 2010;11(9):785–97.
Roy C, Gupta A, Fisette A, Lapointe M, Poursharifi P, Richard D, et al. C5a receptor deficiency alters energy utilization and fat storage. PLoS One. 2013;8(5), e6531.
Sarma JV, Ward PA. New developments inC5a receptor signaling. Cell Health Cytoskelet. 2012;1(4):73–82.
Woodruff TM, Nandakumar KS, Tedesco F. Inhibiting theC5-C5areceptor axis. Mol Immunol. 2011;48(14):1631–42.
Cui W, Simaan M, Laporte S, Lodge R, Cianflone K. C5a- and ASP mediated C5L2 activation, endocytosis and recycling are lost in S323I-C5L2 mutation. Mol Immunol. 2009;46(15):3086–98.
MacLaren R, Kalant D, Cianflone K. The ASP receptor C5L2 is regulated by metabolic hormones associated with insulin resistance. Biochem Cell Biol. 2007;85(1):11–21.
Kalant D, MacLaren R, Cui W, Samanta R, Monk PN, Laporte SA, et al. C5L2 is a functional receptor for acylation stimulating protein. J Biol Chem. 2005;280(25):23936–44.
Zheng YY, Xie X, Ma YT, Yang YN, Fu ZY, Li XM, et al. Relationship between a novel polymorphism of the C5L2 gene and coronary artery disease. PLoS One. 2011;6(6), e20984.
Zheng YY, Xie X, Ma YT, Yang YN, Fu ZY, Li XM, et al. A novel polymorphism (901G > a) of C5L2 gene is associated with coronary artery disease in Chinese Han and Uyghur population. Lipids Health Dis. 2013;12:139.
Zheng YY, Xie X, Ma YT, Yang YN, Fu ZY, Li XM, et al. Relationship between type 2 diabetes mellitus and a novel polymorphism C698T in C5L2 in the Chinese Han population. Endocrine. 2012;41(2):296–301.
Xie X, Ma YT, Fu ZY, Yang YN, Ma X, Chen BD, et al. Haplotype analysis of the CYP8A1 gene associated with myocardial infarction. Clin Appl Thromb Hem. 2009;15(5):574–80.
Xie X, Ma YT, Fu ZY, Yang YN, Ma X, Chen BD, et al. Association of polymorphisms of PTGS2 and CYP8A1 with myocardial infarction. Clin Chem Lab Med. 2009;47(3):347–52.
Xie X, Ma YT, Yang YN, Fu ZY, Li XM, Huang D, et al. Polymorphisms in the SAA1/2 gene are associated with carotid intima media thickness in healthy Han Chinese subjects: the cardiovascular risk survey. PLoS One. 2010;5(11), e13997.
Subcommittee G. World Health Organization-International Society of Hypertension Guidelines for the Management of Hypertension. J Hypertens. 1999;17:151–83.
Boulay F, Mery L, Tardif M, Brouchon L, Vignais P. Expression cloning of a receptor for C5a anaphylatoxin on differentiated HL-60 cells. Biochemistry. 1991;30(12):2993–9.
Gerard NP, Gerard C. The chemotactic receptor for human C5a anaphylatoxin. Nature. 1991;349(6310):614–7.
Ohno M, Hirata T, Enomoto M, Araki T, Ishimaru H, Takahashi TA. A putative chemoattractant receptor, C5L2, is expressed in granulocyte and immature dendritic cells, but not in mature dendritic cells. Mol Immunol. 2000;37(8):407–12.
Gerard NP, Bao L, Xiao-Ping H, Eddy Jr RL, Shows TB, Gerard C. Human chemotaxis receptor genes cluster at 19q13.3-13.4. characterization of the human C5a receptor gene. Biochemistry. 1993;32:1243–50.
Barnes KC, Caraballo L, Muñoz M, Zambelli-Weiner A, Ehrlich E, Burki M, et al. novel promoter polymorphism in the gene encoding complement component 5 receptor 1 on chromosome 19q13.3 is not associated with asthma and atopy in three independent populations. Clin Exp Allergy. 2004;34(5):736–44.
Birney E, Andrews D, Caccamo M, Chen Y, Clarke L, Coates G, et al. Ensembl 2006. Nucleic Acids Res. 2006;34:D556–61.
Klos A, Tenner AJ, Johswich KO, Ager RR, Reis ES, Köhl J. The role of the anaphylatoxins in health and disease. Mol Immunol. 2009;46(14):2753–66.
Williams CA, Schupf N, Hugli TE. AnaphylatoxinC5a modulation of an alpha-adrenergic receptor system in the rat hypothalamus. J Neuroimmunol. 1985;9(1–2):29–40.
Speidl WS, Kastl SP, Hutter R, Katsaros KM, Kaun C, Bauriedel G, et al. The complement component C5a is present in human coronary lesions in vivo and induces the expression of MMP-1 and MMP-9 in human macrophages in vitro. FASEB J. 2011;25(1):35–44.
Speidl WS, Katsaros KM, Kastl SP, Zorn G, Huber K, Maurer G, et al. Coronary late lumen loss of drug eluting stents is associated with increased serum levels of the complement components C3a and C5a. Atherosclerosis. 2010;208(1):285–9.
Vijayan S, Asare Y, Grommes J, Soehnlein O, Lutgens E, Shagdarsuren G, et al. High expression of C5L2 correlates with high proinflammatory cytokine expression in advanced human atherosclerotic plaques. Am J Pathol. 2014;184(7):2123–33.
Del Conde I, Crúz MA, Zhang H, López JA, Afshar-Kharghan V. Platelet activation leads to activation and propagation of the complement system. J Exp Med. 2005;201(6):871–9.
Iyer A, Woodruff TM, Wu MC, Stylianou C, Reid RC, Fairlie DP, et al. Inhibition of inflammation and fibrosis by a complement C5a receptor antagonist in DOCA-salt hypertensive rats. J Cardiovasc Pharmacol. 2011;58(5):479–86.
Wu J, Wu YQ, Ricklin D, Janssen BJ, Lambris JD, Gros P. Structure of complement fragment C3b-factor H and implications for host protection by complement regulators. Nat Immunol. 2009;10(7):728–33.
Noris M, Remuzzi G. Overview of complement activation and regulation. Semin Nephrol. 2013;33(6):479–92.
Ma F, Li Y, Jia L, Han Y, Cheng J, Li H, et al. Macrophagestimulated cardiac fibroblast production of IL-6 is essential for TGFβ/Smad activation and cardiac fibrosis induced by angiotensin II. PLoS One. 2012;7(5), e35144.
Morris RW, Kaplan NL. On the advantage of haplotype analysis in the presence of multiple disease susceptibility alleles. Genet Epidemiol. 2002;23(3):221–33.
Acknowledgements
This work was supported financially by the National Natural Science Fund of China (81360022) and the Xinjiang Key Laboratory of Cardiovascular Disease Research open topic (XJDX0903-2013-01).
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
Conceived and designed the experiments: YYZ, XX, YTM. Performed the experiments: YYZ, XX, and BDC. Analyzed the data: XML, FL, and ZYF. Contributed reagents/materials/analysis tools: XM, XML, ZYF, FL, SP, and AD. Wrote the paper: YYZ, XX, YTM, and Y-NY. All authors read and approved the final manuscript.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Zheng, YY., Xie, X., Ma, YT. et al. Association of C5aR1genetic polymorphisms with coronary artery disease in a Han population in Xinjiang, China. Diagn Pathol 10, 33 (2015). https://doi.org/10.1186/s13000-015-0261-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13000-015-0261-9