Abstract
Background
Crystalline silica (cSiO2) is a mineral found in rocks; workers from the construction or denim industries are particularly exposed to cSiO2 through inhalation. cSiO2 inhalation increases the risk of silicosis and systemic autoimmune diseases. Inhaled cSiO2 microparticles can reach the alveoli where they induce inflammation, cell death, auto-immunity and fibrosis but the specific molecular pathways involved in these cSiO2 effects remain unclear. This systematic review aims to provide a comprehensive state of the art on omic approaches and exposure models used to study the effects of inhaled cSiO2 in mice and rats and to highlight key results from omic data in rodents also validated in human.
Methods
The protocol of systematic review follows PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines. Eligible articles were identified in PubMed, Embase and Web of Science. The search strategy included original articles published after 1990 and written in English which included mouse or rat models exposed to cSiO2 and utilized omic approaches to identify pathways modulated by cSiO2. Data were extracted and quality assessment was based on the SYRCLE’s Risk of Bias tool for animal studies.
Results
Rats and male rodents were the more used models while female rodents and autoimmune prone models were less studied. Exposure of animals were both acute and chronic and the timing of outcome measurement through omics approaches were homogeneously distributed. Transcriptomic techniques were more commonly performed while proteomic, metabolomic and single-cell omic methods were less utilized. Immunity and inflammation were the main domains modified by cSiO2 exposure in lungs of mice and rats. Less than 20% of the results obtained in rodents were finally verified in humans.
Conclusion
Omic technics offer new insights on the effects of cSiO2 exposure in mice and rats although the majority of data still need to be validated in humans. Autoimmune prone model should be better characterised and systemic effects of cSiO2 need to be further studied to better understand cSiO2-induced autoimmunity. Single-cell omics should be performed to inform on pathological processes induced by cSiO2 exposure.
Similar content being viewed by others
Background
Crystalline silica (cSiO2) is a major component of rocks such as granite or sand which is found in materials used in the building industries such as cement or kitchen worktops. Workers from the construction or denim production industries as well as miners are particularly exposed to cSiO2 through inhalation [1]. Inhalation of cSiO2 increases the risk of respiratory disorders such as silicosis, but also increases the risk of systemic autoimmune diseases such as Systemic Sclerosis (SSc), Systemic Lupus Erythematosus (SLE) or Rheumatoid Arthritis (RA) [2,3,4,5,6].
Inhaled cSiO2 microparticles enter into the respiratory tract and can reach pulmonary alveoli [7]. The phagocytosis of cSiO2 particles by alveolar macrophages can induce cytotoxicity and macrophage cell death [8, 9] and can also initiate inflammatory responses and fibrosis through NLRP3 inflammasome activation [10]. Moreover, cSiO2-induced lung cell death is responsible for self-dsDNA release, STING-mediated sensing, IFN response and inflammation [11]. This process can be favoured by the impairment of efferocytosis capacities of macrophages exposed to cSiO2 [12]. cSiO2 is also known to induce systemic auto-immunity [13] but pathophysiological mechanisms involved in these effects remain unclear.
Omic methods are high-throughput technologies increasingly used in human and animal studies since the 1990s. They notably explore genomic, transcriptomic, proteomic or metabolomic data without a priori. They allow a better understanding of the overall biological processes and pathways involved in many disorders [14, 15] or in response to xenobiotic exposure including pollutants such as diesel exhaust particles [16]. Several studies using omic approaches have explored pathways involved in cSiO2 toxicity in mouse and rat models, but a comprehensive overview of these results is still lacking. Access to biological samples from patients exposed to cSiO2 is limited (limited access to bronchoalveolar lavages or lung biopsy) but rodent models can reflect cSiO2 exposure in human. Therefore, mechanisms identified in rodent models could also be relevant for humans [17]. A better identification of processes and pathways underlying cSiO2 toxicity in mouse or rat models may help design new therapeutic targets for cSiO2-related diseases in humans.
This systematic literature review (SLR) aimed at 1) providing a comprehensive state of the art on exposure methods and omic approaches used to study the effects of inhaled cSiO2 in mice and rats and 2) identify key results from omic data involved in cSiO2-related disorders and highlight those validated in human.
Methods and analysis
The report for this SLR was designed in accordance with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines [18]. This protocol was registered in February 2022 on the International prospective register of systematic reviews Prospero (ID n°CRD42022299944) before research started.
Search strategy
The search strategy aimed at selecting original studies mentioning cSiO2, mouse or rat models and at least one omic method, as summarised in the PECO form (see Additional file 1).
PubMed (Medline), Embase and Web of Science were selected for the identification of eligible titles and abstracts published before January 3, 2022. A specific search term strategy was designed for each database (see Additional file 2). Search equations were designed with the help of librarians. To test the relevance of our search strategies, eleven milestone articles considered as mandatory for our research question were selected a priori, based our knowledge from the field [19,20,21,22,23,24,25,26,27,28,29]. All eleven milestone articles were retrieved in each database confirming the relevance of the selected search terms. The first publications using omic techniques was published in the 90's, therefore only articles published after 1990 were explored. As this SLR focused on cSiO2, titles and abstracts mentioning nanoparticles of silica were excluded. Only rat or mouse experimentations were kept, others in vivo or in vitro models were excluded. Omic methods selected for this systematic review were genomics, transcriptomics, proteomics or metabolomics. Selected omic methods included RNA-seq, iTRAQ, Nanostring, microarray or mass spectrometry at the bulk or single-cell levels.
Study selection
Title and abstract screening was performed using Rayyan software (https://www.rayyan.ai/). Two reviewers (AL & LM) independently screened all titles and abstracts, after publication and validation of the SLR protocol on Prospero. There were two screening phases for article selection. The first one consisted in screening titles and abstracts and the second selection consisted in screening selected full texts to include only relevant manuscripts for data extraction. A third reviewer (VL) resolved disagreements between reviewers for abstract and full text screening.
Title and abstract screening
Title and abstract screening aimed at pre-selecting articles that included mouse or rat models, silica exposure and the use of at least one omic method. Studies mentioning silica gel or column, silica-coated beads, silica nanoparticles or micro/nanospheres, mesoporous silica, silica spicules or bleomycin exposure were excluded. Studies without available English abstract were also excluded.
Full text screening
Inclusion and exclusion criteria for final article selection are summarized in Table 1. Briefly, only studies on mouse or rat models exposed to cSiO2 by inhalation were included. All strain, sex and age of mice and rats could be included except genetically modified animals such as knockout mice or rats because they were considered too different from the physiological conditions, and such results could not be applied to human physiology. NZBWF1 and NZM2410 were included because of their spontaneous genetic background for autoimmunity, similarly to what could be observed in human. All doses and frequency of exposure to cSiO2 were included. Only studies using omic approaches were finally kept for data extraction. Outcomes based on omic techniques were kept, including -but not limited to- genomic, transcriptomic, proteomic or metabolomic analyses; gene or protein expression profiles; miRNA-expression profiling/levels; RNA-seq; NanoString nCounter; iTRAQ; single-cell; microarray analyses; SAGE-seq; LC–MS. Outcomes assessed through other technological approaches than omic methods were excluded.
Assessment of methodological quality
The quality of animal experiments was evaluated following the SYRCLE’s Risk of Bias (RoB) tool for animal studies [30]. This tool uses ten items to evaluate experimental bias and ten related questions. The response options are: “1 = yes” indicating that the study follows criteria of evaluation and it is free of bias; “0 = no” indicating the presence of bias in the study; “Un = unclear” indicating the absence of mention about these criteria in the study, with subsequent unclear risk of bias. The evaluation of quality assessment was performed by LM and checked by AL and VL.
Data extraction
Data extraction was performed by LM and checked by AL and VL following a data extraction template that was adapted throughout the process. This template included the following items: 1. article characteristics including title, author, publication year; 2. Animal model including species, strain, age, sex, number of animals per group, control group considered as reference; 3. cSiO2 characteristics including size, purity and exposure including exposure route, dosage, frequency; 4. omic methods used, organ studied and outcomes including timing of measurement. Main biological processes, cellular components, molecular functions, pathways, networks and markers (gene, protein, miRNA) modulated by cSiO2 were extracted. Data were expressed in the format provided in the articles in accordance with existing databases for pathways, biological processes, networks and markers (Gene Ontology (GO), Kyoto encyclopaedia of genes and genomes (KEGG), etc.) and sub-classified depending on the assessment time: acute (≤ 1 week), sub-acute (2–11 weeks) and long-term effects (≥ 12 weeks) after cSiO2 exposure.
Data from a first set of 10% articles was also separately extracted by AL. Data independently retrieved by AL and LM were compared to ensure consistency and data extraction strategy was adapted in case of discrepancies. Once data extraction was completed by LM, accuracy of extracted data was checked by AL and VL for all articles.
Results
Studies included
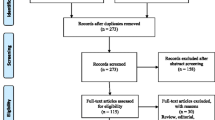
The flow chart of included studies is provided in Fig. 1. 607 relevant studies were identified through database searching. Among these studies, 439 were selected for title and abstract screening and 62 for full-text screening. There were 94.5% (N = 413/439) and 90.3% (N = 56/62) of agreement between AL and LM for title and abstract screening; and for full-text screening respectively. Based on full text evaluation, 41 studies were finally selected for data extraction [19,20,21,22,23,24,25,26,27,28,29, 31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59].
Evaluation of experimental quality
The assessment of studies risk of bias is presented in additional file (see Additional file 3). All studies were free of selective outcome reporting (Q9) and 41.5% (N = 17) of the studies indicated an allocation sequence adequately generated (Q1) and similar animal groups at baseline (Q2). However, only 19.5% (N = 8) and 12.2% (N = 5) mentioned random outcome assessment (Q6) and blinding outcome assessor (Q7) respectively. Few studies mentioned random housing (Q4) and incomplete outcome data (Q8) (2.4% (N = 1) and 4.9% (N = 2) respectively). Any studies indicated suitable allocation concealment (Q3) and blinding caregivers or investigators (Q5).
Rat and mouse characteristics
The characteristics of animal models used in included studies are described in Table 2. Rats were more frequently used than mice (N = 23 (56%)). The majority of animals was males (N = 30 (66.7%)) and their age at the beginning of experiment was comprised between 6 to 10 weeks (N = 25 (59.6%)). Some studies did not mention the sex (N = 2 (4.4%)) and the age (N = 1 (2.4%)) of animals. Few studies (N = 6 (14.6%)) used mouse strains prone for autoimmune diseases such as lupus-prone NZBWF1 and NZM2410. The number of animals per experimental group was commonly between 6 and 10 (N = 20 (46.5%)). However, the number of animals specifically studied with omic methods was lower than 5 in 38.6% (N = 17) of the studies and 31.8% (N = 14) of studies did not mention this number (N = 14 (31.8%)).
Crystalline silica exposure
Characteristics of cSiO2 as well as the timing, dose and frequency of exposure are shown in Table 3. Half of the studies (N = 22 (53.6%)) exposed mice or rats to Min-U-Sil-5 silica, measuring 1.5–2 µm with a purity higher than 99% [60]. Other types of cSiO2 with same size and purity were used in several studies such as the silica from Sigma Aldrich mainly used on rat models (N = 10 (24.4%)) and Forsman Scientific only used on mouse models (N = 3 (7.3%)). Only one study exposed rats to DQ12, a silica with average size of 2.2 µm and purity of 87% [60]. Among the types of exposures, intratracheal instillation and inhalation chamber were the most chosen ones (N = 15 (36.6%) and N = 14 (34.1%) respectively), although inhalation chambers were only used on rats. Other methods included intranasal instillation (N = 8 (19.5%)) and oropharyngeal instillation (N = 4 (9.8%)) which were used only on mice.
Half of studies exposed mice and rats to acute cSiO2 exposure (N = 23 (53.5%)) and the other half to chronic cSiO2-exposure (N = 20 (46.6%)). Mice were mainly exposed acutely to cSiO2 (N = 14 (32.6%)) while rats were mostly exposed chronically (N = 14 (32.6%)). Among studies using acute exposure (N = 23), the majority of animals were exposed to a single dose of cSiO2 (N = 22 (51.2%)) and the dosage was mainly lower than 5 mg for mice (N = 9 (20.9%)) and higher than 21 mg for rats (N = 7 (16.3%)).
Among chronic exposure, the daily cSiO2 exposure concerned only rat models. Twenty percent of the studies (N = 9) used chronic exposure on rats 6 h/day with dosage mainly comprised between 2 to 15 mg/m3 (N = 9) during several days (minimum of 5 days) or weeks (maximum of 12 weeks). The timing of outcome measurement was mostly comprised between 2 and 11 weeks for mice (N = 16 (25%)) and higher than 12 weeks for rats (N = 14 (21.9%)).
Omic methods and organ studied
The summary of omic methods and organs or fluids used in studies is presented in additional file (see Additional file 4). The omic methods were more commonly applied to lung samples but blood, serum, plasma, kidney and spleen were also evaluated in some experiments. Sub-acute effects of cSiO2 exposure were more represented through omic methods (N = 30) than acute effects (N = 10) or long-term effects (N = 24), considering that one single study could use multiple omic approaches at the same time. All types of omics are provided in Table 4. This table highlights the diversity of techniques, particularly in transcriptomic and proteomic analyses, which can limit comparability among studies. Transcriptomic studies represented 76% (N = 35) of all omic methods used whereas proteomics represented 19.6% (N = 9) and metabolomics 4.3% (N = 2). mRNA microarray and mRNA-sequencing were the most commonly used transcriptomics methods (34.8% (N = 16) and 10.9% (N = 5) respectively). Among proteomic studies, mass spectrometry (N = 5 (10,9%)) and protein microarray (N = 4 (8,7%)) were mainly used.
As biological function is carried by proteins, we checked whether a change in mRNA expression was also related to a change at the protein level (Table 5). The number of transcriptomic studies for which mRNA results were validated at protein level represented 39.3% (N = 11) and the main techniques used were western blotting (14.3%, N = 4), ELISA/multiplex assays (21.4%, N = 6) and immunohistology (17.9%, N = 5).
Effects of crystalline silica as assessed by omic approaches in mouse
Lung was the most studied organ in rat and mouse models (N = 21/23 and N = 17/18 respectively) (see Additional file 5). Outcomes studied through omic methods after cSiO2 exposure at several time points in the lungs are shown in Fig. 2. Biological processes, pathways, networks and mRNA expression were the most frequently reported outcomes.
Heatmap representing the main domains of biological processes, pathways and networks found in omic approaches is presented in Fig. 3. Biological processes comprised mostly cellular and immunity domains while pathways and networks mostly included immunity and inflammation. The other domains were diseases, IFN response, transcription factor, ECM production, stress and cell death. Cellular response was the third most represented sub-domain in which movement, response to stimulus, adhesion, communication and growth/proliferation terms were mainly found (Additional file 6). Detailed characterisation of “Immunity” and “inflammation” in lungs or at systemic levels (serum, plasma, and spleen) is provided in Fig. 4A and B respectively. Several terms were common to the pulmonary and systemic levels such as those related to cytokines, notably IL-10, TNF, IL-6, IL-17 and IL-1 terms. In the lungs, several “immunity” and “inflammation” terms were linked to immune cell recruitment and activation such as cell activation, movement/adhesion, antigen presentation, aggregation, communication, cytokines, chemokines, B-cell, T-cell and macrophage functions. “Complement” term retrieved in the lungs could be linked to “phagocytosis” as complement-induced phagocytosis is a major innate immune mechanism. At systemic level (i.e. mostly blood and serum) in context of long-term cSiO2 exposure, adaptive immune response was frequently reported with the terms related to T- and B-cell function, antibody and antigen presentation.
Heatmap representing the main domains of biological processes, pathways and networks found in included studies. Heatmap is expressed as the percentage of biological processes, pathways and networks of each domain mentioned in studies among the total number of biological processes (N = 157), pathways (N = 210) and networks (N = 42) retrieved in all included studies
Heatmap representing the main immunity (A) and inflammation (B) sub-domains found in the lungs or at systemic level (serum, plasma, spleen) in included studies. Heatmap is expressed as the percentage of immunity (A) and inflammation (B) sub-domains of all biological processes, pathways and networks mentioned in studies among the total number of different immune responses or inflammation terms in lungs (N = 123 and N = 22 respectively) and at systemic level (N = 72 and N = 24 respectively) retrieved in all included studies
Validation of omic data in humans
Omic results obtained from animal studies provide data on gene and protein expression during exposure to cSiO2, however, these results need to be confirmed in humans to demonstrate their relevance for patients. Among the 41 included studies, only 17% (N = 7) validated their results from rodent in human samples (Table 6). The majority of these studies validated their results in seric or lung samples from patients with silicosis (N = 4). However, three studies validated their data in patients with idiopathic pulmonary fibrosis (IPF) which was less relevant to demonstrate the impact of cSiO2 exposure in human.
Pang et al. [22] and Shichino et al. [24] consistently showed changes in lipid metabolism in response to cSiO2 exposure in mice and confirmed these results in the lungs of patients with silicosis and/or IPF (Table 6). The arachidonic acid (AA) pathway was found activated and prostaglandin D2 (PGD2) and thromboxane A2 (TXA2) were upregulated in cSiO2-exposed mice and silicosis human lungs [22]. In addition, Srebf1, a transcription factor involved in the regulation of lipid metabolism, was downregulated in cSiO2-exposed mouse and the lungs of patients with IPF [24]. Gremlin1, an antagonist of bone morphogenetic protein (BMP), was found upregulated independently both in cSiO2-exposed mice and in the lungs of patients with IPF [45, 56], demonstrated the importance of this inhibitor as a key pro-fibrotic factor.
None of the omic studies exploring the systemic effects of cSiO2 in rodents validated their results in humans. This lack of data identifies a gap in the literature, with a need to fully validate systemic effects of cSiO2 and related systemic pathways.
Discussion
This review provides a comprehensive state of the art on exposure methods and omic approaches used to study the effects of inhaled cSiO2 in mice and rats. Our work highlighted that male gender and rat models were more frequently used than females and mice and only a limited number of studies used mouse strains prone to autoimmunity, although it is a well endorsed effect of cSiO2 in human. Both acute and chronic exposure were explored in rats and mice and acute, sub-acute and long-term effects being mainly observed in the lungs. Transcriptomic approaches were more commonly performed, only a few studies used proteomic, metabolomic or single-cell omic approaches. Biological processes, pathways and networks were the most commonly reported outcomes in omic analyses. Among these outcomes, immunity and inflammation were the top two domains that were reported as impacted by exposure to cSiO2 in the lungs. Interestingly, only a few studies validate their transcriptomic results at the protein level in rodents and the number of translational studies was less than 20%, with only a few studies validating their results in humans.
Experimental quality
The evaluation of experimental quality performed following SYRCLE’s risk of bias tool showed that studies quality was not sufficient in the majority of studies using omic approaches. Indeed, many criteria were insufficiently reported or lacking such as suitable allocation concealment, random housing, blinding caregivers or investigators and incomplete outcome. Experimental quality was not linked to the type of omic approaches. Improving experimental quality will be important in the future to allow a proper comparison of animal studies and to generalise their findings to clinical studies in humans.
Species, sex and age of rodent models
The majority of animals used to study the effects of cSiO2 through omic methods were males. While transcriptomics, proteomics, genomics and single-cell omics were performed in males, only transcriptomic techniques were explored in female models. This focus on males in omic studies is relevant for human health as men are more represented among workers exposed to cSiO2 [1]. However, cSiO2 is also known to induce systemic autoimmunity which is over represented in women [61]. Using both male and female models in omic approaches is important considering the potential differential effects of cSiO2 on both genders [62]. As cSiO2 is able to promote autoimmunity [6], more studies should evaluate the effects of cSiO2 through omic approaches on mouse strains prone to autoimmunity to better understand the mechanisms involved in cSiO2-related autoimmune diseases.
The majority of experiments included young animals with a maximal age of sixteen weeks at the beginning of experiment. This could be considered as selection and experimental bias, considering that cSiO2 effects on old-age models could especially be relevant as a role of senescent cells has recently been suggested in silica-induced pulmonary fibrosis [63].
Types of crystalline silica
Quartz is the most abundant form of cSiO2 in nature and is used as a raw material in several industrial and building processes. Several quartzes were used in experimental studies such as Min-U-Sil or DQ12. Min-U-Sil 5 is composed of 99% of quartz while DQ12 is a quartz sand composed of 87% of cSiO2 and 13% of amorphous silica and kaolinite, their particle size is less than 5 µm [60]. A large number of studies included in this SLR exposed mice and rats to Min-U-Sil5 or to other types of silica with the same characteristics (Sigma Aldrich, Forsman Scientific). However, the global effects of exposure to artificial stone, containing silica and resins, remains to be explored whereas an epidemic of silicosis was recently observed in artificial stone producer countries [64].
Dosage and frequency of cSiO2 exposure
Both acute and chronic exposure to cSiO2 were evaluated through omic techniques in rats and mice. Chronic exposure is relevant to translate the effects of cSiO2 in mice and rats to human workers but acute exposure can be representative of acute silica hazards such as silicoproteinosis [65]. Based on the limitation established for workers exposure, it is determined that 8.28 mg of inhaled cSiO2 in mice corresponds to an exposure of human workers during 40 years [4]. Among acute exposure, the most commonly used dosages in omic studies were less than 5 mg per exposure for mice corresponding to less than one half of human lifetime exposure. Dosage of cSiO2 for rat acute exposure were mostly higher than 21 mg, which is consistent with the higher weight of the rats. Chronic exposures carried-out in most omic studies consisted in exposing rats to 2 to 15 mg/m3 of cSiO2 6 h per day during several days or weeks that is more representative of human workers chronic exposure. Transcriptomic and proteomic techniques were well distributed in the different dosage and frequency of exposure.
Timing of outcome assessment after cSiO2 exposure is crucial to identify relevant effects. Outcome measurements in omic studies mainly concerned sub-acute effects of cSiO2 but chronic effects were also well studied with transcriptomic and proteomic approaches. Silicosis is induced by cSiO2 exposure and is characterised by chronic inflammation and fibrosis [66]. As the onset of symptoms classically occurs at a later stage of the disease, sub-acute and long-term effects of cSiO2 are especially relevant to study such late outcomes. Moreover, long-term effects allow the exploration of autoimmune features such as cSiO2-related autoantibody production [13].
Omic methods
Omic approaches such as genomics, transcriptomics, metabolomics or proteomics provide a global perspective from large dataset in organisms and contribute to the understanding of mechanisms involved in human diseases. There are various technologies to study omics but, separately, they cannot explore the entire complexity of organisms and each of these techniques has their strengths and limitations.
Transcriptomic studies were the most omic approaches used in included studies and microarray analysis was more used than RNA-seq. Microarray allows the identification of differentially expressed genes using chip with thousands of short single-stranded DNA sequences, therefore there is an a priori knowledge of the sequences and their number is limited. On the other hand, RNA-seq allows sequencing whole transcript of cells or tissues without quantitative limitations and a priori. Studies using RNA-seq were published after 2018 while those using microarray were published earlier corresponding to the development and wider use of RNA-seq characterised by a higher sensitivity and specificity as well as broader data sets with higher comprehensiveness.
Although differentially expressed genes allows a better understanding of the mechanisms involved, it is essential to focus on the proteins encoded by these differentially expressed mRNAs as proteins are the actual actors of the considered physiological and pathological processes. Despite such important considerations, only less than a half of transcriptomic studies validated their results at the protein level. Moreover, proteomic approaches were not widely used in included studies and only a few studies used mass spectrometry techniques and microarrays to identify differentially expressed proteins. Mass spectrometry enables identification and quantification of whole differentially expressed proteins without targeted strategy while protein microarrays require target proteins. Autoantibodies microarray development could be of great interest to profile circulating autoantibodies in autoimmune diseases [67] and, systematically apply such technics in studies assessing the effects of cSiO2 on adaptive immunity may help decipher the key processes involved in cSiO2-related autoimmunity.
Transcriptomics and proteomics provide data on differentially expressed genes and proteins respectively but do not provide direct results regarding cells involved in the process at stake. For this reason, single-cell omics have been developed and single-cell transcriptomics (single-cell RNA-seq), proteomics (mass cytometry CyTOF) or spatial omics (Hyperion) are increasingly used [68]. However, single-cell techniques were rarely found in included studies, only one study published in 2021 used single-cell RNA-seq [25]. We may expect that the high dimensional single cell analysis at the protein level might be more used to identify which cell types can drive cSiO2 effects. Multi-omic approaches combining transcriptomics, proteomics and metabolomics in a same study and/or protocol may also help improve the overall understanding of the mechanisms involved in the physiopathology of cSiO2 and foster the design of new therapeutic targets [14].
cSiO2 exposure effects
In included studies, omic approaches were mainly carried out on lungs and more rarely on blood, plasma, serum, spleen and kidney. However this focus on lungs may lead to a gap of knowledge regarding the systemic effects of silica dust [69]. Biological processes, pathways, networks and mRNA expression were the most studied omic outcomes in response to cSiO2 exposure in rodents. Among them, immunity- and inflammation-related outcomes were the two most frequent domains reported both at the lung and systemic level, suggesting a similar and global response to cSiO2 exposure. Indeed, a wide range of cytokines were retrieved in response to cSiO2. The identification of IL-1β is consistent with the well-described inflammasome (NLRP3) activation induced in response to cSiO2, along with the involvement of TLR pathway resulting in IL-1β cytokine release [70]. Moreover, the cSiO2-induced IL-17 pathways identified in included studies was previously reported [71]. This interleukin is known to be increase in autoimmune disorders [72] and could be implicated in cSiO2-induced autoimmunity [13]. Innate and adaptive immunity both play a role in the response to cSiO2 exposure. Indeed, terms related to cell activation, functions and recruitment were mainly found at pulmonary and systemic levels, which is consistent with the known recruitment and activation of inflammatory cells in response to cSiO2. Phagocytosis were also retrieved in lungs, consistently with existing data regarding the response to cSiO2 exposure [9]. Identification of the complement term was in accordance with its role in silicosis and inflammation [73]. The adaptive immunity-related terms such as antibody and T- and B-cell function were especially identified as long-term effects and at systemic levels, that is in coherence with the time of an autoimmune response to cSiO2 [13, 17]. All these results from omics studies on rodents enable us to appreciate the overall effects of cSiO2 exposure in rodents, which are now well described in the literature. Therefore, it would be interesting to investigate the mechanisms involved in its effects and to carry out translational studies to gain a better understanding of the effects of crystalline silica on human pathologies.
Translation of omic results from rodents to humans
Validating results obtained in murine models into humans is an important step in determining whether the results from animal studies are relevant to patients. Among included studies, less than 20% validated in humans the omic results obtained in rodents. More translational studies are thus needed. Indeed, rodent models are required to explore the effects of cSiO2 since doses and frequencies of exposure can be controlled, making it easier to obtain reproducible and comparable results among studies. However, confirming these results in humans by studying organs, peripheral blood mononuclear cells or serum is still mandatory. Regarding systemic effects of cSiO2, none of the included studied validate their results in human. This could be explained by the lack of available human samples, suggesting that fostering the implementation of biorepositories (PBMC, serum and/or plasma biobank) is an important unmet need. Beyond biorepositories, public transcriptome data in humans are available through open access and could enable in silico comparisons with animal results.
Validated results in patients with silicosis or IPF revealed changes in lipid metabolism in response to cSiO2 exposure in two independent studies [22, 24]. The AA pathway was found reprogrammed, with an up-regulation of PGD2 and TXA2, two inflammatory mediators potentially involved in silicosis-related fibrogenesis. Such effects may rely on Srebf1, a transcription factor involved in the regulation of AA pathways, that was found downregulated in one of the included study [74, 75]. Moreover, transcriptome network analyses shown that Srebf1 was connected with some pro-fibrotic gene such as Gremlin1 [24], that was shown upregulated in two independent studies first in cSiO2-exposed mice (long-term effect) and then in lung of patients with IPF [45, 56]. The overexpression of this protein, as an antagonist of BMP, can imbalance the BMP and TGF-ꞵ pathways, leading to fibrosis [76]. Gremlin1 was also upregulated in response to asbestos in the lungs of exposed-mice and to coal dust inhalation in the serum of patient with coal worker’s pneumoconiosis [77, 78]. It was also retrieved at high levels in the serum of SSc patients with interstitial lung disease (ILD), an autoimmune disease for which exposure to cSiO2 is a risk factor [79]. Therefore, Gremlin1 could be use as biomarker of asbestos, coal or cSiO2 dust exposure.
Perspectives for omic methods to study cSiO2 effects
This comprehensive overview on cSiO2 effects highlighted gaps in the literature. 1/ cSiO2 effects on male mouse or rat models are well studied through omic approaches but analysis on female models only focused on transcriptomic approaches and need to be extended. 2/ Min-U-Sil silica is more commonly cSiO2 type used in omic studies. The comparison between Min-U-sil, DQ12 or other type of cSiO2 exposure through omic methods may help identify key differences in the biological impact of different types of silica. 3/ Transcriptomic studies need to be better validated at protein level and/or in tissues, since proteins are the actual players of the biological response. 4/ Systemic effects of cSiO2 should be further studied and omic techniques should be performed on other samples than lungs such as whole blood, kidney or spleen; notably to explore the autoimmune effects of silica [17]. Moreover, only a few omic analyses have been performed on autoimmune disease prone models exposed to cSiO2 despite the lack of understanding of cSiO2-induced autoimmunity. 5/ The use of single-cell transcriptomic and proteomic analyses that provide data on each cell type should also be fostered as they allow the identification of specific cell types involved in cSiO2-related pathological processes. 6/ Among included studies using omic approaches, only a few studies have compared omic results from rodents to omic data from human cohorts exposed to cSiO2 although such translational approaches are crucial to confirm that biological processes, pathways or networks identified in rodents are also relevant in humans. Translational studies are therefore still needed.
Strengths and limitations
Strengths of the proposal: This SLR is the first study providing a comprehensive and systematic overview of studies exploring the effects of inhaled cSiO2 in the mouse or rat models using omic approaches. Our SLR follows the PRISMA recommendations for SLRs [18]. We used three different databases for article selection providing a comprehensive analysis of the literature on the subject. Moreover, the protocol of this systematic review was published on Prospero prior to the beginning of abstract screening. Combining transcriptomic and proteomic results provide an unprecedented overview of the effects of inhaled cSiO2 in mouse and rat models. This review includes studies using different time points of outcome measurement after the last cSiO2 exposure, at both pulmonary and systemic levels.
Limitation of the proposal: we used an a priori definition of omic approaches that is not endorsed or validated. The heterogeneity of the protocols used in the studies (dosage, frequency, duration, cSiO2 type and methods of exposure) can be considered a limitation as it may preclude a direct comparison of the obtained results. Results may also vary depending on the omic techniques, the platform where the omic approaches are performed and statistical analyses of the data. In addition, we did not specifically explore the impact of the dose or frequency of cSiO2 exposure. In our work, we only explored the main biological processes, pathways and networks identified in the studies, some pathways are therefore not retained although they may have a role in cSiO2 effects. By focusing on studies using omic techniques, this review may not include results obtained from others techniques and therefore all the mechanisms modulated by cSiO2 could not be identified although such aim was beyond the scope of this study.
Conclusion
In this SLR review, providing an overview of cSiO2 effects in mice and rats, omic techniques were more commonly carried out on lungs and analysis of the systemic effects of cSiO2 were neglected. Perform further omic analysis on autoimmune prone mouse models and on female models may help identify mechanisms involved in cSiO2-induced autoimmunity. Proteomics and single-cell analysis are still lacking to identify the main actors of the pathological processes induced by cSiO2 and validation in humans are only performed in less than 20% of the available studies. Current validated results from independent omic studies in rodent translated to humans, supported the impact of cSiO2 on lipid metabolism (AA pathways) and the role of Gremlin1 as a TGF-ꞵ regulator in cSiO2-related fibrogenesis. Identifying and validating new prominent pathways could help design and evaluate relevant therapeutic approaches for lung and systemic effects of cSiO2.
Availability of data and materials
Not applicable.
Abbreviations
- AA:
-
Arachidonic pathway
- BMP:
-
Bone morphogenic protein
- cSiO2 :
-
Crystalline silicon dioxide
- CyTOF:
-
Cytometry by time of flight
- DNA:
-
Deoxyribonucleic acid
- ECM:
-
Extracellular matrix
- ELISA:
-
Enzyme-Linked Immunosorbent Assay
- GO:
-
Gene ontology
- IL:
-
Interleukin
- IFN:
-
Interferon
- lncRNA:
-
Long non-coding RNA
- IPF:
-
Idiopathic pulmonary fibrosis
- iTRAQ:
-
Isobaric tags for relative and absolute quantitation
- KEGG:
-
Kyoto encyclopaedia of genes and genomes
- LC-MS:
-
Liquid chromatography-mass spectrometry
- MALDI-TOF-MS PMF:
-
Matrix-assisted laser desorption/ionization-time of flight-mass spectrometry-peptide mass fingerprint
- Mesh:
-
Medical subject headings
- miRNA:
-
Micro RNA
- mRNA:
-
Messenger
- ND:
-
Not determined
- NF-kB:
-
Nuclear factor-kappa B
- NGS:
-
Next-generation sequencing
- NLRP3:
-
NOD-like receptor family, pyrin domain containing 3
- NMR:
-
Nuclear magnetic resonance
- NZBWF1:
-
New Zealand Black and New Zealand White F1
- NZM2410:
-
New Zealand Mixed 21410
- PBMC:
-
Peripheral blood mononuclear cell
- PECO:
-
Population, Exposure, Comparator, and Outcome
- PGD2:
-
Prostaglandin D2
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- qPCR:
-
Quantitative polymerase chain reaction
- RA:
-
Rheumatoid arthritis
- RNA:
-
Ribonucleic acid
- RNA-seq:
-
RNA sequencing
- RoB:
-
Risk of Bias
- SAGE-seq:
-
Serial analysis of gene expression sequencing
- SLE:
-
Systemic lupus erythematosus
- SLR:
-
Systematic Literature Review
- Srebf1:
-
Sterol regulatory element binding transcription factor 1
- SSc:
-
Systemic sclerosis
- SSH-cDNA:
-
Suppression subtractive hybridization complementary DNA
- STING:
-
Stimulator of Interferon genes
- SYRCLE:
-
Systematic Review Center for Laboratory Animal Experimentation
- TGF-ꞵ:
-
Transforming growth factor beta
- TLR:
-
Toll-like receptors
- TMT LC-MS:
-
Tandem mass tag liquid chromatography-mass spectrometry
- TNF:
-
Tumor necrosis factor
- TOF-MS:
-
Time of flight mass spectrometry
- TXA:
-
Thromboxane A2
References
Barnes H, Goh NSL, Leong TL, Hoy R. Silica-associated lung disease: An old-world exposure in modern industries. Respirology. 2019;24:1165–75.
Miller FW, Alfredsson L, Costenbader KH, Kamen DL, Nelson L, Norris JM, et al. Epidemiology of Environmental Exposures and Human Autoimmune Diseases: Findings from a National Institute of Environmental Health Sciences Expert Panel Workshop. J Autoimmun. 2012;39:259–71.
Marie I, Menard J-F, Duval-Modeste A-B, Joly P, Dominique S, Bravard P, et al. Association of occupational exposure with features of systemic sclerosis. J Am Acad Dermatol. 2015;72:456–64.
Bates MA, Brandenberger C, Langohr II, Kumagai K, Lock AL, Harkema JR, et al. Silica-triggered autoimmunity in lupus-prone mice blocked by docosahexaenoic acid consumption. PLoS ONE. 2016;11:e0160622.
De Decker E, Vanthuyne M, Blockmans D, Houssiau F, Lenaerts J, Westhovens R, et al. High prevalence of occupational exposure to solvents or silica in male systemic sclerosis patients: a Belgian cohort analysis. Clin Rheumatol. 2018;37:1977–82.
Boudigaard SH, Schlünssen V, Vestergaard JM, Søndergaard K, Torén K, Peters S, et al. Occupational exposure to respirable crystalline silica and risk of autoimmune rheumatic diseases: a nationwide cohort study. Int J Epidemiol. 2021;50:1213–26.
Migliaccio CT, Hamilton RF, Holian A. Increase in a distinct pulmonary macrophage subset possessing an antigen-presenting cell phenotype and in vitro APC activity following silica exposure. Toxicol Appl Pharmacol. 2005;205:168–76.
Honarpisheh M, Foresto-Neto O, Desai J, Steiger S, Gómez LA, Popper B, et al. Phagocytosis of environmental or metabolic crystalline particles induces cytotoxicity by triggering necroptosis across a broad range of particle size and shape. Sci Rep. 2017;7:15523.
Gilberti RM, Joshi GN, Knecht DA. The Phagocytosis of Crystalline Silica Particles by Macrophages. Am J Respir Cell Mol Biol. 2008;39:619–27.
Cassel SL, Eisenbarth SC, Iyer SS, Sadler JJ, Colegio OR, Tephly LA, et al. The Nalp3 inflammasome is essential for the development of silicosis. Proc Natl Acad Sci USA. 2008;105:9035–40.
Benmerzoug S, Rose S, Bounab B, Gosset D, Duneau L, Chenuet P, et al. STING-dependent sensing of self-DNA drives silica-induced lung inflammation. Nat Commun. 2018;9:5226.
Lescoat A, Ballerie A, Lelong M, Augagneur Y, Morzadec C, Jouneau S, et al. Crystalline silica impairs efferocytosis abilities of human and mouse macrophages: implication for silica-associated systemic sclerosis. Front Immunol. 2020;11:219.
Mayeux JM, Escalante GM, Christy JM, Pawar RD, Kono DH, Pollard KM. Silicosis and silica-induced autoimmunity in the diversity outbred mouse. Front Immunol. 2018;9:874.
Sun YV, Hu Y-J. Integrative analysis of multi-omics data for discovery and functional studies of complex human diseases. Adv Genet. 2016;93:147–90.
Karczewski KJ, Snyder MP. Integrative omics for health and disease. Nat Rev Genet. 2018;19:299–310.
Lecureur V. Comparative study on gene expression profile in rat lung after repeated exposure to diesel and biodiesel exhausts upstream and downstream of a particle filter. Environ Pollut. 2020;11.
Janssen LMF, Ghosh M, Lemaire F, Michael Pollard K, Hoet PHM. Exposure to silicates and systemic autoimmune-related outcomes in rodents: a systematic review. Part Fibre Toxicol. 2022;19:4.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, The PRISMA, et al. statement: an updated guideline for reporting systematic reviews. BMJ. 2020;2021: n71.
Bates MA, Benninghoff AD, Gilley KN, Holian A, Harkema JR, Pestka JJ. Mapping of dynamic transcriptome changes associated with silica-triggered autoimmune pathogenesis in the lupus-prone NZBWF1 mouse. Front Immunol. 2019;10:632.
Cai W, Zhang B, Li T, Jin F, Li Y, Xu H, et al. Transcriptomic analysis identifies upregulation of secreted phosphoprotein 1 in silicotic rats. Exp Ther Med. 2021;21:579.
Chen J, Yao Y, Su X, Shi Y, Song X, Xie L, et al. Comparative RNA-Seq transcriptome analysis on silica induced pulmonary inflammation and fibrosis in mice silicosis model. J Appl Toxicol JAT. 2018;38:773–82.
Pang J, Qi X, Luo Y, Li X, Shu T, Li B, et al. Multi-omics study of silicosis reveals the potential therapeutic targets PGD(2) and TXA(2). Theranostics. 2021;11:2381–94.
Sellamuthu R, Umbright C, Roberts JR, Chapman R, Young S-H, Richardson D, et al. Transcriptomics analysis of lungs and peripheral blood of crystalline silica-exposed rats. Inhal Toxicol. 2012;24:570–9.
Shichino S, Ueha S, Hashimoto S, Otsuji M, Abe J, Tsukui T, et al. Transcriptome network analysis identifies protective role of the LXR/SREBP-1c axis in murine pulmonary fibrosis. JCI Insight. 2019;4.
Song M-Y, Wang J-X, Sun Y-L, Han Z-F, Zhou Y-T, Liu Y, et al. Tetrandrine alleviates silicosis by inhibiting canonical and non-canonical NLRP3 inflammasome activation in lung macrophages. Acta Pharmacol Sin. 2021;
Souma K, Shichino S, Hashimoto S, Ueha S, Tsukui T, Nakajima T, et al. Lung fibroblasts express a miR-19a-19b-20a sub-cluster to suppress TGF-β-associated fibroblast activation in murine pulmonary fibrosis. Sci Rep. 2018;8:16642.
Zhao H, Jiang Z, Lv R, Li X, Xing Y, Gao Y, et al. Transcriptome profile analysis reveals a silica-induced immune response and fibrosis in a silicosis rat model. Toxicol Lett. 2020;333:42–8.
Zhu Y, Yao J, Duan Y, Xu H, Cheng Q, Gao X, et al. Protein Expression Profile in Rat Silicosis Model Reveals Upregulation of PTPN2 and Its Inhibitory Effect on Epithelial-Mesenchymal Transition by Dephosphorylation of STAT3. Int J Mol Sci. 2020;21.
Benninghoff AD, Bates MA, Chauhan PS, Wierenga KA, Gilley KN, Holian A, et al. Docosahexaenoic acid consumption impedes early interferon- and chemokine-related gene expression while suppressing silica-triggered flaring of murine lupus. Front Immunol. 2019;10:2851.
Hooijmans CR, Rovers MM, de Vries RB, Leenaars M, Ritskes-Hoitinga M, Langendam MW. SYRCLE’s risk of bias tool for animal studies. BMC Med Res Methodol. 2014;14:43.
Beamer CA, Migliaccio CT, Jessop F, Trapkus M, Yuan D, Holian A. Innate immune processes are sufficient for driving silicosis in mice. J Leukoc Biol. 2010;88:547–57.
Bo C, Geng X, Zhang J, Sai L, Zhang Y, Yu G, et al. Comparative proteomic analysis of silica-induced pulmonary fibrosis in rats based on tandem mass tag (TMT) quantitation technology. PLoS ONE. 2020;15:e0241310.
Brown JM, Schwanke CM, Pershouse MA, Pfau JC, Holian A. Effects of rottlerin on silica-exacerbated systemic autoimmune disease in New Zealand mixed mice. Am J Physiol Lung Cell Mol Physiol. 2005;289:L990-998.
Cai W, Xu H, Zhang B, Gao X, Li S, Wei Z, et al. Differential expression of lncRNAs during silicosis and the role of LOC103691771 in myofibroblast differentiation induced by TGF-β1. Biomed Pharmacother Biomed Pharmacother. 2020;125:109980.
Cao Z-J, Liu Y, Zhang Z, Yang P-R, Li Z-G, Song M-Y, et al. Pirfenidone ameliorates silica-induced lung inflammation and fibrosis in mice by inhibiting the secretion of interleukin-17A. Acta Pharmacol Sin [Internet]. 2021; Available from: https://www.embase.com/search/results?subaction=viewrecord&id=L2013292006&from=export
Chauhan PS, Wagner JG, Benninghoff AD, Lewandowski RP, Favor OK, Wierenga KA, et al. Rapid induction of pulmonary inflammation, autoimmune gene expression, and ectopic lymphoid neogenesis following acute silica exposure in lupus-prone mice. Front Immunol. 2021;12:635138.
Dorman DC, Mokashi V, Wagner DJ, Olabisi AO, Wong BA, Moss OR, et al. Biological responses in rats exposed to cigarette smoke and Middle East sand (dust). Inhal Toxicol. 2012;24:109–24.
Ellinger-Ziegelbauer H, Pauluhn J. Pulmonary toxicity of multi-walled carbon nanotubes (Baytubes) relative to alpha-quartz following a single 6h inhalation exposure of rats and a 3 months post-exposure period. Toxicology. 2009;266:16–29.
Faxuan W, Qin Z, Dinglun Z, Tao Z, Xiaohui R, Liqiang Z, et al. Altered microRNAs expression profiling in experimental silicosis rats. J Toxicol Sci. 2012;37:1207–15.
Gao X, Xu H, Xu D, Li S, Wei Z, Li S, et al. MiR-411-3p alleviates Silica-induced pulmonary fibrosis by regulating Smurf2/TGF-β signaling. Exp Cell Res. 2020;388:111878.
Hu JZ, Rommereim DN, Minard KR, Woodstock A, Harrer BJ, Wind RA, et al. Metabolomics in lung inflammation: a high-resolution 1H NMR study of mice exposedto silica dust. Toxicol Mech Methods. 2008;18:385–98.
Ji X, Wu B, Fan J, Han R, Luo C, Wang T, et al. The anti-fibrotic effects and mechanisms of microRNA-486-5p in pulmonary fibrosis. Sci Rep. 2015;5:14131.
Jin Z, Liu B, Feng D, Chen C, Li X, Hu Y, et al. Identification of differentially expressed genes in rat silicosis model by suppression subtractive hybridization analysis. ACTA Biochim Biophys Sin. 2008;40:740–6.
Kim YM, Chung SI, Lee SY. Roles of plasma proteins in the formation of silicotic nodules in rats. Toxicol Lett. 2005;158:1–9.
Koli K, Sutinen E, Ronty M, Rantakari P, Fortino V, Pulkkinen V, et al. Gremlin-1 overexpression in mouse lung reduces silica-induced lymphocyte recruitment - a link to idiopathic pulmonary fibrosis through negative correlation with CXCL10 chemokine. PLOS ONE. 2016;11.
Langley RJ, Mishra NC, Peña-Philippides JC, Rice BJ, Seagrave J-C, Singh SP, et al. Fibrogenic and redox-related but not proinflammatory genes are upregulated in Lewis rat model of chronic silicosis. J Toxicol Environ Health A. 2011;74:1261–79.
Pestka JJ, Akbari P, Wierenga KA, Bates MA, Gilley KN, Wagner JG, et al. Omega-3 Polyunsaturated Fatty Acid Intervention Against Established Autoimmunity in a Murine Model of Toxicant-Triggered Lupus. Front Immunol [Internet]. 2021;12. Available from: https://www.embase.com/search/results?subaction=viewrecord&id=L634823233&from=export
Rajasinghe L, Li Q, Zhu C, Yan M, Chauhan P, Wierenga K, et al. Omega-3 fatty acid intake suppresses induction of diverse autoantibody repertoire by crystalline silica in lupus-prone mice. Autoimmunity. 2020;53:415–33.
Sager T, Roberts J, Umbright C, Barger M, Kashon M, Fedan J, et al. Biological effects of inhaled hydraulic fracturing sand dust. V. Pulmonary inflammatory, cytotoxic and oxidant effects. Toxicol Appl Pharmacol. 2020;408.
Sai L, Yu G, Bo C, Zhang Y, Du Z, Li C, et al. Profiling long non-coding RNA changes in silica-induced pulmonary fibrosis in rat. Toxicol Lett. 2019;310:7–13.
Sai L, Qi X, Yu G, Zhang J, Zheng Y, Jia Q, et al. Dynamic assessing silica particle-induced pulmonary fibrosis and associated regulation of long non-coding RNA expression in Wistar rats. GENES Environ. 2021;43.
Sellamuthu R, Umbright C, Li S, Kashon M, Joseph P. Mechanisms of crystalline silica-induced pulmonary toxicity revealed by global gene expression profiling. Inhal Toxicol. 2011;23:927–37.
Sellamuthu R, Umbright C, Roberts JR, Chapman R, Young S-H, Richardson D, et al. Blood gene expression profiling detects silica exposure and toxicity. Toxicol Sci Off J Soc Toxicol. 2011;122:253–64.
Sellamuthu R, Umbright C, Roberts J, Cumpston A, McKinney W, Chen B, et al. Molecular insights into the progression of crystalline silica-induced pulmonary toxicity in rats. J Appl Toxicol. 2013;33:301–12.
Thakur SA, Beamer CA, Migliaccio CT, Holian A. Critical role of MARCO in crystalline silica-induced pulmonary inflammation. Toxicol Sci Off J Soc Toxicol. 2009;108:462–71.
Shichino S, Abe J, Ueha S, Otsuji M, Tsukui T, Kosugi-Kanaya M, et al. Reduced supply of monocyte-derived macrophages leads to a transition from nodular to diffuse lesions and tissue cell activation in silica-induced pulmonary fibrosis in mice. Am J Pathol. 2015;185:2923–38.
Umbright C, Sellamuthu R, Roberts JR, Young S-H, Richardson D, Schwegler-Berry D, et al. Pulmonary toxicity and global gene expression changes in response to sub-chronic inhalation exposure to crystalline silica in rats. J Toxicol Environ Health A. 2017;80:1349–68.
Wiethoff AJ, Reed KL, Webb TR, Warheit DB. Assessing the role of neutrophil apoptosis in the resolution of particle-induced pulmonary inflammation. Inhal Toxicol. 2003;15:1231–46.
Xiaojun W, Yan L, Hong X, Xianghong Z, Shifeng L, Dingjie X, et al. Acetylated α-Tubulin Regulated by N-Acetyl-Seryl-Aspartyl-Lysyl-Proline(Ac-SDKP) Exerts the Anti-fibrotic Effect in Rat Lung Fibrosis Induced by Silica. Sci Rep. 2016;6:32257.
Barosova H, Karakocak BB, Septiadi D, Petri-Fink A, Stone V, Rothen-Rutishauser B. An in vitro lung system to assess the proinflammatory hazard of carbon nanotube aerosols. Int J Mol Sci. 2020;21:5335.
Ngo ST, Steyn FJ, McCombe PA. Gender differences in autoimmune disease. Front Neuroendocrinol. 2014;35:347–69.
Ray JL, Holian A. Sex differences in the inflammatory immune response to multi-walled carbon nanotubes and crystalline silica. Inhal Toxicol. 2019;31:285–97.
Mukherjee A, Epperly MW, Fisher R, Hou W, Shields D, Wang H, et al. Silica induced lung fibrosis is associated with senescence, Fgr, and recruitment of bone marrow monocyte/macrophages. In Vivo. 2021;35:3053–66.
Shtraichman O, Blanc PD, Ollech JE, Fridel L, Fuks L, Fireman E, et al. Outbreak of autoimmune disease in silicosis linked to artificial stone. Occup Med Oxf Engl. 2015;65:444–50.
Kefeli M, Akpolat I, Zeren H, Atici AG, Dumortier P, Honma K, et al. Clinical, histopathological and mineralogical analysis findings of an unusual case of pneumoconiosis. Turk J Pathol. 2012;28:184.
Leung CC, Yu ITS, Chen W. Silicosis. The Lancet. 2012;379:2008–18.
Ren J, Wang H, Wei C, Yang X, Yu X. Development of a protein microarray for profiling circulating autoantibodies in human diseases. PROTEOMICS Clin Appl. 2022;2100132.
Liu J, Qu S, Zhang T, Gao Y, Shi H, Song K, et al. Applications of single-cell omics in tumor immunology. Front Immunol. 2021;12:697412.
Zhao Y, Hao C, Li M, Qu Y, Guo Y, Deng X, et al. PD-1/PD-L1 inhibitor ameliorates silica-induced pulmonary fibrosis by maintaining systemic immune homeostasis. Biomed Pharmacother. 2022;148:112768.
Dostert C, Pétrilli V, Van Bruggen R, Steele C, Mossman BT, Tschopp J. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science. 2008;320:674–7.
Song L, Weng D, Dai W, Tang W, Chen S, Li C, et al. Th17 can regulate silica-induced lung inflammation through an IL-1b-dependent mechanism. 2014;18.
Yasuda K, Takeuchi Y, Hirota K. The pathogenicity of Th17 cells in autoimmune diseases. Semin Immunopathol. 2019;41:283–97.
Callis AH, Sohnle PG, Mandel GS, Mandel NS. The role of complement in experimental silicosis. Environ Res. 1986;40:301–12.
Murakami M, Naraba H, Tanioka T, Semmyo N, Nakatani Y, Kojima F, et al. Regulation of prostaglandin E2 biosynthesis by inducible membrane-associated prostaglandin E2 synthase that acts in concert with cyclooxygenase-2. J Biol Chem. 2000;275:32783–92.
Bae S, Kim M-K, Kim HS, Moon Y-A. Arachidonic acid induces ER stress and apoptosis in HT-29 human colon cancer cells. Anim Cells Syst. 24:260–6.
O’Reilly S. Gremlin: a complex molecule regulating wound healing and fibrosis. O.
Myllärniemi M, Lindholm P, Ryynänen MJ, Kliment CR, Salmenkivi K, Keski-Oja J, et al. Gremlin-mediated decrease in bone morphogenetic protein signaling promotes pulmonary fibrosis. 2008;177.
Hou Z, Zhang X, Gao Y, Geng J, Jiang Y, Dai H, et al. Serum osteopontin, KL-6, and Syndecan-4 as potential biomarkers in the diagnosis of coal workers’ pneumoconiosis: a case–control study. Pharmacogenomics Pers Med.
O’Reilly S. Circulating Gremlin-1 is elevated in systemic sclerosis patients. J Scleroderma Relat Disord. 2021;6:286–9.
Acknowledgements
The authors thank Xavier Chard-Hutchinson and Damien Belvèze, Librarians at the University Library of Rennes 1 for giving us advice on the creation of our research equations and on the software to use.
Funding
None.
Author information
Authors and Affiliations
Contributions
The study was elaborated by AL, VL and LM. Title, abstract and full-text screening was conducted by AL and LM and disagreements between AL and LM was resolved by VL. The experimental quality evaluation and data extraction was performed by LM and checked by AL and VL. The manuscript was written by LM and reviewed by VL and AL, the final manuscript was approved by all authors.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1
. Table S1. The PECO (Population, Exposure, Comparator, Outcome).
Additional file 2.
Table S2. Search terms used in databases.
Additional file 3.
Table S3. Assessment of studies risk of bias using SYRCLE’s risk of bias tool.
Additional file 4.
Table S4. Summary of omics methods and organs used in studies
Additional file 5.
Fig. S1. Crystalline silica exposure outcomes studied in rat and mouse organs
Additional file 6.
Fig. S2. Heatmap representing the main cellular responses sub-domains found in the lungs or at systemic level (serum, plasma, spleen) in included studies. Heatmap is expressed as the percentage of cellular response sub-domains of all biological processes, pathways and networks mentioned in studies among. the total number of different cellular response terms in lungs (N = 61) and at systemic level (N = 2) retrieved in all included studies.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Morin, L., Lecureur, V. & Lescoat, A. Results from omic approaches in rat or mouse models exposed to inhaled crystalline silica: a systematic review. Part Fibre Toxicol 21, 10 (2024). https://doi.org/10.1186/s12989-024-00573-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12989-024-00573-x