Abstract
The gastrointestinal tract is a complex interface between the external environment and the immune system. Its ability to control uptake across the mucosa and to protect the body from damage of harmful substances from the lumen is defined as the intestinal barrier function (IBF). The IBF involves four elements: the intestinal microbiota, the mucus layer, the epithelium and the immune system. Its dysfunction is linked with human diseases including inflammatory, metabolic, infectious, autoimmune and neurologic disorders. Most of these diseases are complex and involve genetic, psychological and environmental factors. Over the past 10 years, many genetic polymorphisms predisposing to inflammatory bowel disease (IBD) have been identified. Yet, it is now clear that they are insufficient to explain the onset of these chronic diseases. Although it has been evidenced that some environmental factors such as cigarette smoking or carbohydrate intake are associated with IBD, other environmental factors also present potential health risks such as ingestion of food additives introduced in the human diet, including those composed of mineral particles, by altering the four elements of the intestinal barrier function. The aim of this review is to provide a critical opinion on the potential of TiO2 particles, especially when used as a food additive, to alter the four elements of the intestinal barrier function, and consequently to evaluate if this additive would likely play a role in the development and/or exacerbation of IBD.
Similar content being viewed by others
Background
The gastrointestinal tract is a complex interface between the external environment, internal tissues and the immune system, establishing a dynamic barrier that enables the absorption of dietary nutrients and the exclusion of potentially hazardous substances, including pathogenic bacteria and toxic chemical agents, from the intestinal lumen. The ability to control their uptake across the mucosa and to protect from these harmful substances present within the lumen is defined as the intestinal barrier function (IBF). It includes several lines of defence, the first one being the lumen itself, where commensal bacteria contribute to the protection against pathogens (i.e. harmful biological agents) by producing anti-microbial substances and by competing with them for access to nutrients, thereby limiting their colonisation [1]. The second line of defence is the mucus layer, which first constitutes a tight physical protection of the epithelium against all harmful substances that may transit in the gut lumen, and second is rich in secreted IgA and antimicrobial peptides (AMP), thereby preventing the access of bacteria to the epithelium [2]. The third barrier is the monolayer of intestinal epithelial cells that further refrains toxic molecules and enteric pathogens from reaching internal tissues [3]. This constitutes the last line of defence before the mucosal immune system, which constitutes the ultimate barrier against pathogens [1]. Many human diseases including inflammatory, metabolic, infectious, neurologic, and cardiovascular disorders are linked to deficiency of the IBF [4].
Crohn’s disease (CD) and ulcerative colitis (UC), the two main subtypes of inflammatory bowel diseases (IBD), are chronic relapsing inflammatory disorders of the gastrointestinal tract. CD can be distinguished from UC in that the inflammation associated with CD is transmural and often discontinuous [5]. IBD may be complicated by occlusions and perforations of the intestine. Patients suffering from IBD have a high risk of developing colitis-associated colorectal cancer (CAC) and have high mortality from these diseases [6, 7]. Indeed, chronic inflammation in the digestive tract results in particular pathological fibrosis that will affect the crypts. More importantly, in the majority of patients who did not show any signs of IBD pathogenesis prior to CRC onset, tumor-associated inflammation is evident in clinical samples and has been shown to drive cancer development in the gut, suggesting a fundamental role for inflammation in both CAC and sporadic colorectal cancer (CRC) development [8]. Current estimations indicate that 6.8 million people live with IBD worldwide [9]. The highest prevalence of IBD is in Europe and North America, but these countries show stabilized or decreased incidence of these diseases while their incidence is increasing in Africa, Asia, Eastern Europe and South America [10]. IBD is currently thought to be linked to environmental factors associated with the occidental way of life, which are currently unknown. Although cigarette smoking [11] or carbohydrate intake [12] are associated with CD, other environmental factors such as ingestion of food additives may present potential health risks by altering the IBF and then favouring IBD [13, 14]. Among them, the food additive E171, composed of titanium dioxide particles, has been shown to contain up to 55% of nanosized particles (TiO2-NPs) and is among the most commonly used mineral particle-based food additive in consumer products [15,16,17,18]. As reported in the present review, TiO2 has already been described to alter the IBF and to translocate in the internal body (for instance, see [19] where the absorption of TiO2 is demonstrated and [20, 21] where TiO2 is observed in the liver of rats exposed via oral administration). For this reason, this food additive is a good candidate as an environmental factor that could be implicated in IBD and CAC.
The aim of this review is to compile the published data that show how TiO2 food additive may impact the four elements of the IBF, in order to shed some light and to provide a critical opinion on the mechanisms by which it could be implicated in IBD and CAC. It is not intended to evaluate the risk associated with the ingestion of this additive, which has been already documented [22]. This review is based on a literature survey up to January 2021, using the databases Pubmed, IsiWeb and Scopus, which were searched with the keywords TiO2, titanium dioxide, E171, intestine, gut, inflammation, immunity, inflammatory bowel disease, mucus, microbiota, toxicity. The included publications were chosen thanks to quality criteria including minimal description of the origin and physico-chemical characteristics of the used TiO2 particles, good laboratory practices and correct description of the employed methods, particularly in the in vivo studies, conclusions matching the presented results and presence of appropriate controls.
Description of the four compartments of the intestinal barrier function
Commensal bacteria
The human gut contains around 1014 bacteria and constitutes the largest microbial community associated with human body [23]. The gut microbiota acts as a metabolic organ, through breakdown of indigestible dietary carbohydrates and proteins, with generation of fermentation end-products, vitamins, and ion absorption [24, 25]. It contributes to the barrier effect by constituting an obstacle to pathogen invasion. The microbiota also enhances the resistance to pathogens by both direct and indirect mechanisms of action including production of antimicrobial substances such as bacteriocins, pH modification and competition for nutrients, immune or epithelial cell-mediated mechanisms [1]. For example, B. thetaiotaomicron consumes carbohydrates used by C. rodentium, which contributes to the competitive exclusion of the pathogen. B. thetaiotaomicron also enhances the production by epithelial cells of the peptidoglycan-binding C-type lectin regenerating islet-derived protein IIIγ (REGIIIγ), which is an antimicrobial peptide that kills Gram+ bacteria [26].
The mucus layer
The mucus layer overlying the epithelium promotes the elimination of gut content, providing the second line of defence against injury caused by ingested food, particles and microorganisms [27]. This layer is present all along the GIT, from the stomach to the rectum, and is thicker in the stomach and colon than in the small intestine [2]. It is classically divided into a dense sterile inner layer, which is firmly attached to the epithelium, and a removable outer layer that is colonized by anaerobic commensal bacteria [2]. In rodents, the mucus layer in the small intestine is not attached to the epithelium and only shows one layer [28]. The major components of the mucus layer are glycoproteins termed mucins, which are secreted by goblet cells and form a gel at the surface of epithelial cells [29]. In addition to mucins, goblet cells also synthesize trefoil factors, resistin-like molecule β, and Fc-γ binding protein [30]. Paneth cells also contribute to this barrier function by secreting substances contained in secretory granules, including antimicrobial substances such as lysozyme, phospholipase A2, α-defensins and the REGIIIγ [31]. Finally enterocytes produce β-defensins and REGIIIα [32, 33]. All these mucins and AMP compose the mucus wall and act synergistically to maintain the homeostasis of the mucus layer [29, 34].
The epithelial monolayer
The epithelial monolayer is a semi-permeable barrier that allows the passage of water and nutrients from the gut lumen to the underlying tissues. Passage occurs via the paracellular or the transcellular route. The paracellular route, through tight junctions (TJs) that link adjacent cells, makes possible the strictly controlled passage of water, solutes and ions and is determined by the size of TJ pores with a maximum opening reaching 120 Å, i.e., 12 nm [35]. Four transmembrane families of proteins contribute to TJ formation, which are occludin, claudin, junctional adhesion molecule and tricellulin [35]. The intracellular domains of these transmembrane proteins interact with cytosolic scaffolding proteins, i.e. zonula occludens proteins, which in turn anchor the transmembrane proteins to the perijunctional actomyosin meshwork [35]. The circumferential contraction of the actinomyosin ring is regulated by the myosin light chain kinase [35, 36]. Other mechanisms like endocytosis of TJ proteins, regulation of TJ genes expression and epithelial cell apoptosis regulate the paracellular route. If TJs are not disrupted, they strictly regulate the passage of molecules and therefore the passage of nanoparticles with size > 120 Å, i.e., 12 nm, would be hindered.
A vast literature shows that particles larger than the TJ pore size would rather cross the gut epithelial layer by using the transcellular route, i.e. by passing through gut cells by transcytosis (for instance, see [36]). Although epithelial and dendritic cells are able to capture antigens and microbes from the gut lumen, full transcellular transport is mainly ascribed to microfold (M)-cells, which are part of the follicle associated epithelium (FAE) overlying isolated lymphoid follicles (ILF) or Peyer’s patches (PP) [37]. The transcellular route across M-cells and other cells exhibits two classical pathways depending on the size of the substance. Proteins and bacterial products can be taken up by endocytosis [38]. Larger entities, including bacteria, are captured via macropinocytosis or phagocytosis [38]. After crossing the cell, these particles are released on the other side of M-cells at the vicinity of immune cells that populate PPs. These two passage routes across the epithelium are closely controlled so that the intestinal homeostasis is maintained. In case of epithelial barrier disruption, enhanced passage of substances, including particles and microorganisms could lead to chronic inflammation [35].
The intestinal immune system
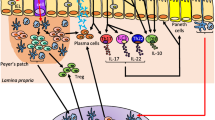
This section gives a brief overview of the intestinal immune system, which is highly complex. Some excellent reviews describe its components and functioning, the authors suggest the reader to refer to the reviews cited in the text for more details. Two main sites of immune response have been described, i.e., the inductive site (immune response initiation – antigen encountering) and the effector site. Gut-associated lymphoid tissue (GALT), including PPs and ILFs, represent the inductive compartment for B and T cells. After encountering antigens in the GALT, B and T cells leave the effector site, transit through the lymph, enter the circulation and join the effector site of the intestine, that is, the mucosal epithelia and the lamina propria. Intestinal lymphocytes are continuously exposed to food and microbial antigens via antigen presenting cells (APC) including dendritic cells (DCs). DCs present in GALT and lamina propria have a high tolerogenic potential and a break of this tolerance triggers inflammation leading to IBD or cancer (for review, see [39,40,41]. These lymphocytes help to maintain the integrity of the intestinal barrier and immune homeostasis. Owing to their proximity to luminal antigens, they have a dual function: regulatory function (i.e., maintaining tolerance toward food antigens and commensal microbiota) and effector function (i.e., prevention of pathogenic invasion) (for review, see [42]). Innate lymphoid cells (ILCs) are also present in the lamina propria, those lymphocytes do not express the type of diversified antigen receptors expressed on T cells and B cells. ILCs are tissue-resident cells participating in tissue homeostasis (for review, see [43]). Intraepithelial lymphocytes (IELs) are also an important contributor to intestinal homeostasis. They reside within the intestinal epithelial layer and are involved in intestinal repair and defence against infections [44]. In IBD, alterations of these four elements constituting the IBF have been described.
Absorption, distribution and elimination of TiO2 particles upon ingestion and associated toxicity
Upon request of the European Food Safety Authority (EFSA), a group of experts reviewed the nanomaterials that are currently used in agriculture, feed and food [45]. The food industry uses nanomaterials either in packaging or for nano-encapsulation of bioactive compounds such as vitamins. In addition, synthetic amorphous silica, titanium dioxide, silver and iron oxide are used as food additives for their anti-caking property or as food colorant [45]. Titanium dioxide food additive (E171) is a powder composed of particles with an average primary grain size higher than 100 nm. It also contains a significant amount of nanosized particles, which may arise from the production process. Depending on the analysed powder, this nanosized fraction is reported to be up to 36% [17], 35% [18], 55% [15] or 10–15% [16]. Thus, E171 is not a nanomaterial with respect to the current European recommendation for a definition of nanomaterials [46]. Its use in food products was authorized in 1969 by the JECFA (Joint FAE/WHO Expert Committee on Food Additives) with an acceptable daily intake “not specified” (report: NMRS 46/TRS 445-JECFA 13/12). This authorization relies on a literature survey that proved that E171 was non-toxic and not absorbed in the gastro-intestinal tract (GIT). In March 2021, the EFSA panel on Food Additives and Flavourings re-evaluated the safety of E171 when used as food additive, based on recent literature including a study dedicated to the analysis of E171 reproductive and developmental toxicity. The Panel concluded on potential immunotoxicity and inflammation with E171, as well as potential neurotoxicity and potential induction of aberrant crypt foci in the intestine. Moreover, a concern for genotoxicity could not be ruled out. Consequently, the Panel concluded that “E171 can no longer be considered as safe when used as a food additive” [47].
Since it is used as a white food colorant, the largest quantity of TiO2 is found in candies, sweets and pastries, with 0.01–1 mg TiO2 per unit [17]. In sugar-coated chewing gums, the nanoparticulate fraction of TiO2 reaches 27.7–43.7 and 95% of TiO2-NPs are swallowed upon chewing [48]. Based on consumer intake data, human exposure has been estimated at 1–2 mg and 0.2–0.7 mg TiO2/kg/day for US children under 10 and other US consumers, respectively [17]. More recent estimation concludes that daily intake of TiO2 for Dutch population is rather 0.66–0.70 mg/kg/day for children under 7, 0.16–0.18 mg/kg/day for consumers between 7 and 70, and 0.05–0.07 for adults over 70, with lifelong intake of TiO2 estimated at 0.19 mg/kg/day [49]. Lastly, the EFSA panel on food additives and nutrient sources estimates that daily intake of TiO2 reaches 0.4 mg/kg bw per day for infants and the elderly and 10.4 mg/kg bw per day for children, for the maximum level exposure assessment scenario, at the mean, which is the highest estimated values reported in their publication [50]. Based on the latter estimated values, and taking into consideration the existing guidance for conversion of doses between animals and humans such as the Nair and Jacob practice guide or the FDA’s guidelines, it can considered that a realistic in vivo exposure concentration would be up to 50–60 mg/kg b.w./day [51]. In vitro, exposing 2D-cells to 25 μg/cm2 (of cell culture surface) would represent ~ 10,000-fold the daily ingested dose by a human adult [52], therefore a realistic exposure concentration would be as low as 2.5 ng/cm2, i.e., ~ 5 ng/mL in classical 2D cell culture conditions. In such in vitro mechanistic studies, unrealistically high concentrations are often used in order to determine the effect and no-effect level of NMs [53]. Although it is recommended to use realistic concentrations and long-term exposure in vitro, such high concentrations can be considered as worst case scenarios and are acceptable as long as they are interpreted with caution and as they do not exceed the concentration at which NMs agglomerate or interfere with the readout of the test, i.e., generally 1–100 μg/mL [53].
Due to continuous exposure via ingestion, TiO2 might be distributed in intestinal tissues and in internal organs if absorbed through the gastrointestinal tract. Indeed, early reports describe the presence of so-called “pigmented cells” in human PPs. These cells contain some dark granules, which were analysed as being composed of Ti, Si and Al [54]. The authors propose that these pigments derive from the ingestion of synthetic food additive (Ti from TiO2), aluminosilicates (Al, Si) and mixed environmental silicates (Si). In addition to this local accumulation in immune cells, a recent study on human volunteers shows significant intestinal absorption of Ti after exposure via ingestion of two capsules containing 100 mg of food-grade TiO2 (which would represent 1–1.5 mg/kg b.w.) [19]. Ti is detected in the bloodstream of the volunteers as soon as 2 h after ingestion, and reflectance microscopy shows that Ti is in the physical form of a particle, not as ions that would have been released from the particles [19]. Moreover, the kinetics of appearance of Ti in the bloodstream suggests two routes of intestinal absorption, first in the proximal part of the small intestine, then in the distal part of the ileum, possibly through PPs [19]. In contrast, Jones et al. report insignificant absorption of TiO2 in human volunteers orally exposed to a single dose of 5 mg/kg b.w. of TiO2 micro and nanoparticles (15 nm, 100 nm and < 5 μm) dispersed in water [55]. However, in the Jones study the baseline level of Ti in the bloodstream is high, which impairs the detection of small amounts of Ti that would have been absorbed [55]. Finally, in 2017, Ruiz et al. show from a cohort study that the titanium level in the blood of human patients having active UC disease is higher than that of healthy donors [56].
Several in vivo studies in rodents report TiO2 absorption and toxicity when administered per os, although other studies report the opposite. When focusing on studies performed with E171 or TiO2 particles with physico-chemical properties that are close to those of E171, the literature is scarce. In 2015, three studies published in the same article clearly demonstrate absence of toxicity of TiO2 with physico-chemical characteristics close to those of food-grade TiO2. These three studies are performed according to the Organisation for Economic Co-operation and Development (OECD) guidelines for i) 90 day subchronic toxicity, ii) 28 days repeated dose toxicity and iii) acute oral toxicity [57]. In addition, negligible absorption, lack of distribution and an overall excretion in the feces is observed in rats exposed to E171 incorporated in food pellets at 30 mg/kg b.w./day for 7 days in a study conducted following the OECD TG417 [58]. Oral gavage of E171 at 10, 100 or 1000 mg/kg b.w./day for 90 days (OECD TG408) leads to accumulation of Ti only in the colon [59]. Conversely, some non-OECD guideline studies show the absorption and distribution of E171 in the liver of rats exposed for 7 days to E171 at 10 mg/kg b.w./day by intragastric gavage [20] and in mouse exposed by dripping some E171 into the mouth of the mouse at 5 mg/kg b.w./day, 3 days per week for 3 weeks [21].
In addition to this literature, some studies performed with TiO2 particles with properties differing from those of E171 report either no or significant absorption of TiO2 and in some of them some toxicity. Absorption of TiO2-NPs is reported for 25 or 80 nm NPs when administered to mice at a single dose of 5 g/kg b.w., which is an unrealistically high dose, with signs of toxicity in the liver, kidneys and brain as well as altered serum biochemical parameters [60]. The result of this study should be interpreted with caution due to the excessively high dose administered to the animals. Similarly, oral exposure of mice with 16 nm rutile or 20 nm anatase TiO2-NPs at the dose of 100 mg/kg/day for 28 days, which can also be considered as a high dose as compared to estimated human exposure, causes Ti distribution in the spleen, lung, and kidney without causing any significant effects on organ histology [61]. Jani et al. report in 1994 that 500 nm TiO2-NPs are absorbed in rats and accumulate in GALT, lungs and peritoneal tissues after oral administration at 12.5 mg TiO2/kg b.w. per day for 10 days. No Ti is detected in the liver and spleen [62]. More recently, a systematic study performed with five benchmark TiO2 particles from the JRC repository, differing in their physico-chemical characteristics, shows that none of these particles are significantly absorbed by rats after 5 consecutive days of oral exposure at the realistic concentration of 2.3 mg/animal per day (i.e., 7–12 mg/kg b.w. depending on the weight of the animal). No distribution to the liver, spleen and mesenteric lymph nodes is observed, except in a small number of animals [63]. This study also shows that when exposed by intravenous injection, TiO2 distributes mainly to the liver, spleen and lung. No TiO2 is detected in the urine and feces during the 90 days post-exposure, proving that its elimination is negligible [63]. This suggests that even if TiO2 absorption through the GI tract is mild, lifelong cumulative absorption combined to low elimination would result in potential accumulation of TiO2 in the tissues. Noteworthy, data from this study have been considered as the most accurate toxicokinetics data, and have been used for risk assessment of TiO2 oral exposure [22]. In contrast, while no TiO2-NP is detected in the liver, kidneys, spleen and brain of rats exposed by repeated gavage to 520, 1041 or 2083 mg/kg b.w./day for 13 weeks (OECD TG409) of 26 nm-diameter TiO2-NPs, TiO2 is significantly eliminated in the faeces [64]. The doses administered to rats in the latter study are unrealistic and higher than the dose in the study by Geraets et al. [63], which can explain the stronger elimination. Regarding TiO2 toxicity, while no significant distribution of TiO2 to the blood, liver, kidney and spleen is reported by Wang et al. in rats exposed to 10–200 mg/kg b.w. of 75 nm TiO2-NPs for 30 days, significant damage is observed both in young and adult animals [65]. In young rats, this repeated oral administration induces liver oedema and heart damage as well as cell activation in the stomach, but only at doses > 50 mg/kg b.w., which are higher than the estimated human exposure [65]. In adult rats, it causes damage to the liver, kidneys and compromises intestinal permeability, also at the highest doses, showing age-dependent impact of TiO2 [65]. Conversely, no systemic toxicity of P25 TiO2-NPs (21 nm in diameter, mix anatase/rutile) is observed in rats exposed for 28 or 90 days (OECD TG407 and 408) at 250, 500 or 1000 mg/kg b.w./day by gavage [66]. Finally, Ruiz et al. show dose-dependent distribution of TiO2 in the spleen of mice having dextran sodium sulfate (DSS)-induced colitis, when orally exposed to 30–50 nm rutile TiO2-NPs repeatedly for 7 days, at 50 or 500 mg/kg b.w./day [56]. This shows that the colitis caused by DSS facilitates absorption of TiO2 nanoparticles, which was expected as colitis perturbs intestinal permeability. Finally, a series of articles from the Fashui Hong team reports impact of TiO2 nanoparticles on diverse organs after oral administration, but these articles are not considered in this review because of flaws in the presentation and statistical analysis of the data, as has already been pointed out on a series of articles from the same team related to the pulmonary impact of similar TiO2 nanoparticles [67]. In these articles the reported standard errors are exactly 5% of the reported mean, rather than the actual standard errors.
Overall, it can be considered that TiO2 absorption is mild and its elimination in the faeces is slow, in realistic exposure conditions. Taking these conclusions into consideration, risk assessment for oral exposure to TiO2 concludes that a potential risk exists for liver, ovaries and testes [22]. Such an evaluation, including TiO2-NPs kinetics, indicates a possible effect on liver, spleen, ovaries and testes [22].
Impact of TiO2 on the intestinal barrier function
Early reports suggest that ingestion of food additives composed of mineral particles such as TiO2 may participate in the aetiology and pathogenesis of IBD [13]. Current studies suggest that TiO2 particles alter all four elements of the IBF. Therefore, they may promote the development and/or the aggravation of IBD and the associated CRC.
Alteration of the intestinal microbiota by TiO2 particles
The antibacterial properties of metals such as silver and zinc are known for centuries and this has been exploited in modern medicine for infection control. For example, silver nitrate was one of the first substances suggested for the management of dental cavities [68]. In addition, metal-containing nanomaterials arise in several chemical forms, including solid nanoparticles of metal or metal oxides like Ag or TiO2-NPs as well as composite materials with layers of distinct metals. Some of these nanoparticles are used as antibacterial agents [69] and there is now interest to use them for controlling bacterial infections [70]. The precise mechanisms for bacterial toxicity of these NPs is still being elucidated, but the current paradigms include free metal ion toxicity arising from the dissolution of metals from the surface of the NPs (e.g., Ag+ from Ag NPs) [71] or oxidative stress via the generation of reactive oxygen species (ROS) on the surface of some NPs.
It is unlikely that TiO2 nanoparticles, which are considered to be poorly soluble [72], can release sufficient quantities of Ti ion to confer them antibacterial properties. TiO2 is known as an antimicrobial agent because of its photocatalytic properties, which lead to the release of ROS when TiO2 is irradiated with UV light [73]. This property is currently used in many applications such as water treatment [74], but cannot explain the potential dysbiosis caused by TiO2 since no UV light reaches the GIT. Still, it is also demonstrated that TiO2 can generate some free radicals in the dark [75]. Such free radicals can then synergistically act by attacking polyunsaturated phospholipids in bacteria and damage their DNA [76, 77], which eventually may cause bacterial cell death. In the context of the GIT, such damage to bacteria could alter the intestinal microbiota.
Generally, the impact of TiO2 on the microbiota is reported to be mild (Table 1). Considering the E171 food additive, Radziwill-Bienkowska et al. show mild impact of E171 on a panel of Gram+/Gram− bacterial strains chosen to be representative of gut microbiota [78]. E171 is internalized in 7% of E. coli cells, with minor consequences on bacterial viability [78]. According to Dudefroi et al., E171 does not affect bacterial gas production and only mildly impact bacterial fatty acid profiles in a defined human gut bacterial community omposed of 33 different strains (microbial ecosystem therapeutic 1, MET-1) [79]. DNA profiles and phylogenetic distributions confirm limited impact on the bacterial community, with a modest decrease of Bacteroides ovatus in favour to Clostridium cocleatum [79]. In a human colon model reactor, after 5 days of exposure at 36 mg/L (corresponding to oral exposure to ~ 0.3–0.7 mg/kg), both E171 and 21 nm TiO2-NPs hinder the natural shift in microbial composition that is observed in the control condition, favouring Firmicutes phylum over Proteobacteria [80]. The authors’ hypothesis is that this natural shift is probably due to the shift from a vegetarian diet (host) to a diet with high levels of animal proteins and fats (colon medium) [80]. After exposure to 21 nm TiO2-NPs, the amount of Firmicutes and Bacteroidetes increases, with Firmicutes being approximately as abundant as Proteobacteria [80]. Particles with diameter 122 nm, supposed to represent food grade TiO2 particles, trigger a slight decrease of Proteobacteria as well as a minor increase of Firmicutes and Bacteroidetes, but overall do not affect the initial ratio of these phyla. TiO2-NPs also decrease colonic pH, with 122 nm particles having a higher impact than 21 nm NPs [80]. The authors hypothesize that it could be due to a direct interaction between TiO2-NPs and bacteria, and/or binding of microbial nutrients at the particle surface [80]. More recently, a comprehensive study reports the impact of E171 or TiO2-NPs (33 nm in diameter) on the microbiota and the colon inflammatory status in non-obese and obese mice [81]. Exposure for 8 weeks to 0.1 wt% TiO2 incorporated into the feed leads to microbiota dysbiosis, characterized by increased abundance of Firmicutes and decreased abundance of Bacteroidetes phyla, i.e. the same trend as observed in IBD patients, together with reduced abundance of Bifidobacterium and Lactobacillus genera [81]. This dysbiosis concurs with decreased cecal levels of short-chain fatty acids (SCFAs), which has also been reported to be associated with IBD. Histological observation shows typical signs of inflammation in the colon, both in directly exposed animals and in control animals that have received fecal transplant from TiO2-exposed mice, suggesting direct impact of the microbiota on the intestinal inflammatory status [81]. Overall, these effects are more intense in obese mice compared to non-obese mice, and E171 shows mild impact while TiO2-NPs exert the strongest effect [81].
Other studies also report mild impact on the microbiota of TiO2 nanoparticles with physico-chemical properties that differ from E171. Chen et al. find no disturbance of gut microbiota in mice exposed by oral gavage to 19 nm TiO2-NPs, at 2.5 mg/kg/day for 7 days [82]. In faeces of both control and TiO2-NP exposed mice, the Bacteroidetes phylum is preponderant, followed by Firmicutes then Proteobacteria and exposure to TiO2-NPs does not affect their ratio [82]. Moreover, anatase or rutile TiO2-NPs, orally administrated to mice for 28 days at 100 mg/kg b.w./day, do not alter gut microbiota diversity but modifies its composition [61]. Rutile NPs have a more pronounced impact than anatase NPs [61]. The most altered phylum is Proteobacteria, which is significantly increased by rutile but not by anatase. At the genus level, the abundance of Prevotella is decreased by both TiO2-NPs, while the abundance of Rhodococcus is enriched by rutile NPs, and the abundance of Bacteroides is increased by anatase NPs [61]. Finally, food grade TiO2 minimally affects the composition of the microbiota in mice exposed in the drinking water, but it alters bacterial metabolite release and enhances biofilm formation from commensal bacteria, in vitro [83].
Overall, although some articles show that TiO2 may alter gut microbiota, other articles report the opposite. Therefore, this impact of TiO2 on gut microbiota is still a matter of debate. In vivo, human microbiome has been characterised, and its alleged composition varies among authors. However, Firmicutes and Bacteroidetes phyla are always shown to be predominant with a similar abundance, with Proteobacteria and Actinobacteria being far less present but still more abundant than all remaining phyla [84,85,86]. Dysbiosis has been linked to some intestinal pathologies, the most severe one being Clostridium difficile infection, but still alteration of the microbiota concurs with intestinal disorders such as IBD, irritable bowel disease and CRC [87]. For this reason any disturbance of the intestinal microbiota by TiO2 food additive could contribute to the aggravation of the symptoms of IBD and CRC in predisposed individuals.
Impact on the epithelial monolayer
Studies assessing the impact of TiO2 on intestinal epithelial cells are summarized in Table 2. In vivo, exposure of mice to E171 at 50 mg/kg b.w./day for 4 weeks reduces the length of colonic crypts but not the total length of the colon [83]. Tight junction protein 1 (zonula occludens type 1, ZO-1) expression in the colon is not affected when mice are exposed to 10 or 50 mg/kg b.w./day of E171 for 4 weeks, which suggests no alteration of intestinal permeability [83]. Decreased crypt length is also observed in mice exposed for 16 weeks to 5 mg/kg b.w./day of E171, provided in the drinking water when the mice are fed with either a regular fat diet or a high-fat diet [88]. Conversely, Talamini et al. do not observe any structural and histological modifications in the intestine of mice exposed by dripping ~ 2 mg/kg b.w./day of E171 in the mouth of the mice, 3 days per week for 3 weeks [21]. The mode of administration of E171 could explain such apparently discrepant results.
Although in vivo experiments are considered to be more informative than in vitro experiments on established cell lines, which are often derived from cancers, in vitro experiments might be useful to decrypt the mode of action of toxic substances such as nanomaterials. In vitro, E171 causes disruption of microvilli organization in Caco-2BBe1 cells exposed to concentrations of E171 as low as 100 ng/cm2 (i.e. 350 ng/mL) [89]. This disruption could be due to sedimentation of particles on the microvilli, which appears limp and fewer in number in exposed cells. When exposing Caco-2BBe1 cells to E171 using an inverted configuration, in which particles cannot sediment on top of the cell layer, disruption of microvilli is also observed [89]. Therefore, exposure to TiO2 affects the morphology of intestinal epithelium. Regarding the impact on intestinal epithelium of non-food-grade TiO2 nanoparticles, impact on intestinal villi and intestinal permeability is also reported. Li et al. show that daily gavage of mice for 28 days (100 mg/kg/day) with 16 nm rutile NPs but not with 21 nm anatase NPs results in longer intestinal villi and irregular arrangement of villus epithelial cells [61]. The ratio of villi height/crypt depth is modified in mice exposed for 1 or 3 months to anatase NPs or rutile microparticles via their feed (1% w./w.) and the level of ZO-1 and occludin is increased in the ileum after 1 month of exposure [90]. In vivo and ex vivo, agglomerates of 12 nm anatase TiO2-NPs alter the paracellular permeability of the ileum and colon epithelia of mice after a single oral gavage at 12.5 mg/kg b.w [52]. Gene expression of ZO-1 and -2, claudin 2 and 3 and occludin are down-regulated in the ileum, which is attributed by the authors to tight junction remodelling that may occur due to epithelial integrity alteration [52]. In the same study, in vitro exposure of a monoculture of Caco-2 cells (regular epithelium) and a co-culture of Caco-2 and HT29-MTX cells (mucus-secreting regular epithelium) to 50 μg/mL of these TiO2-NPs does not alter the paracellular permeability of the epithelia, nor transport through P-glycoprotein [52]. Moreover, acute exposure of Caco-2 cells to 50 μg/mL of anatase (12 nm) or rutile (20 nm) TiO2-NPs induces early upregulation of a battery of efflux pumps and nutrient transporters, suggesting that it might affect the efflux of toxic substances and nutrient absorption [91]. Moreover, another in vitro study demonstrates that, in addition to altering tight junctions, acute exposure for 4 h and repeated daily exposure for 5 days of Caco-2/HT29-MTX to 30 nm TiO2-NPs reduces absorptive cell microvilli, which are involved in nutrient absorption. This concurs with alteration of nutrient transport (specifically Fe and Zn) as well as fatty acid uptake [92]. The concentration used in this study are 106, 108 and 1010 particles/cm2 (acute exposure), or three times these concentrations (chronic exposure).
Overall, all these data highlight the fact that the protective and absorptive functions of intestinal epithelial cell can be impaired by TiO2 exposure.
Impact of TiO2 on the mucus layer
Concerning the impact of TiO2 on the mucus and AMP secretions from epithelial cells including goblet cells, Paneth cells and enterocytes, the literature is scarce (Table 3). HT29-MTX cells, developed by Lesuffleur et al. [93], are an ideal model to study the interaction of NPs with the intestinal mucus, in vitro. The mucus is organized as islands on top of these cells, and these islands of mucus can trap E171 [94]. In vitro, repeated exposure of Caco-2/HT29-MTX cells for 21 days to 10, 50 or 100 μg/mL of E171 or 21 nm anatase/rutile TiO2-NPs causes mucus secretion to increase, while mucin gene expression remains unchanged [95]. In vivo, Medina-Reyes et al. show hyperplasia and hypertrophy of goblet cells and increased mucin gene expression in mice exposed for 16 weeks to 5 mg/kg b.w. of E171 in the drinking water and fed with either regular diet or high fat diet [88]. Inversely, intragastric administration of E171 to mice, both wild type and with chemically induced colitis-associated cancer, at 5 mg TiO2/kg b.w./day, 5 days a week during 10 weeks, decreases the number of goblet cells in the distal colon [96]. This would rather suggest that TiO2 reduces the capacity of mucus secretion in exposed animals. Moreover, Talbot et al. show no modification of cecal short-chain fatty acid (SCFA) profiles, which play a role in mucin synthesis and mucus characteristics, and of gut mucin O-glycosylation patterns in rats orally exposed to E171 or 21 nm anatase/rutile TiO2-NPs, which suggests that these particles show no significant effect on mucus quantity and quality [94]. In the latter study, rats have been exposed to 0.1 mg E171/kg b.w./day for 60 days or 10 mg E171/kg b.w./day for 7 days. While the concentration of E171 to which animals are exposed within the three latter studies are comparable and realistic, the exposure time varies from 7 days to 16 weeks. Their results point to the time-dependence of E171 impact on mucus secretion. They suggest that mucus secretion would not be affected at short exposure times (7 or 60 days) [94], it would then be reduced after 10 weeks of exposure [96] and finally increased after 16 weeks of exposure [88]. Still, such an interpretation should be treated with caution, as exposure patterns and E171 preparation procedure vary from one study to another. It is accepted that the procedure of TiO2 dispersion plays a pivotal role on its agglomeration state and biocorona; for instance TiO2-NPs agglomerate due to high ionic strength and when submitted to simulated digestion [97, 98], while E171 strongly adsorb some mucins on its surface, which leads to reduced agglomeration [99]. Thus, exposing animals via intragastric gavage or via drinking water would result in different biocorona on the particles and different agglomeration states, which could explain these apparently discrepant results.
To put into perspective these recent studies, an abundant literature demonstrates the impact of TiO2-NPs on the mucus secretion from the respiratory system and on the development of the pulmonary diseases like asthma, chronic obstructive pulmonary disease or cystic fibrosis. Analysis of this literature is reported as Supplementary materials and suggests the implication of calcium signalling in the induction of mucus secretion triggered by TiO2 [100].
Impact on the intestinal immune system
A paradigm of TiO2 toxicity, especially via inhalation, is that it causes inflammation [101]. Several studies have also reported that TiO2 would induce an inflammatory profile in the intestine when ingested (Table 4). In rats orally exposed for 7 days to 10 mg/kg b.w./day of E171, increased DC (CD103 + MHCII+) population and decreased helper T cells (Th) (CD4 + CD25+) and regulatory T cells (Treg) (CD4 + CD25 + Foxp3+) populations were observed in PPs [20]. This observation of immune cells population shift is completed by functional in vitro experiments through TcR stimulation of isolated cells from PP and spleen. Interestingly, E171 decreases CD3/CD28-induced IFNγ at the intestinal level but increases its secretion at the systemic level. Higher IL-17 secretion is also observed in the spleen of rats exposed to E171 without modification at the intestinal level [20]. Moreover, 100 days of oral exposure to E171 at 10 mg/kg b.w./day lead to decrease of Th and Treg populations in PP [20]. This inflammatory signature could lead to IBD in susceptible individuals. In contrast, Blevins and al. do not report any modification of DCs, nor Th, nor Treg in PPs after 7 or 100 days of exposure to E171 at 400 mg/kg of bw/day, when E171 is incorporated in the feed [102]. However, after 100 days of E171 oral exposure, IL-17 concentration is higher in the colon of exposed animals [102]. So far, the consequences of oral exposure to TiO2 on intestinal ILC, IEL or B cells populations are unknown. Accumulation of TiO2-NPs inside the intestinal cells, especially in PP, might lead to damaging outcomes such as inflammation and has been postulated as being potentially involved in the pathogenesis of IBD [13, 62, 103]. In 2012, Nogueira et al. evidenced that TiO2-NPs induce a pro-inflammatory response in the small intestine by increasing inflammatory cytokine production and T CD4+ cell proliferation [104]. TiO2 induces recruitment of CD4+ T cells in the ileum, as shown via histological analyses, associated with higher secretion of IL-12, IFNγ, TNFα, IL-4, IL-23 and TGF-β [104], which suggests that TiO2 commits immune response toward Th1. Moreover, hypertrophy and hyperplasia of the small intestinal mucosa are also observed in mice exposed to TiO2-NPs. This induction of inflammation in the small intestine is probably linked to the presence of more M-cells in this area (PP and ILF), which are known to be the main pathway of nanoparticle uptake across the gastrointestinal tract [105, 106]. Although there remains some ambiguity surrounding the specific immunomodulatory and cytotoxic effects resulting from in vivo TiO2-NPs exposure, it seems clear that TiO2 has the potential to cause immune cell and tissue damage and dysfunction.
To our knowledge, only these three recent articles report in vivo consequences of TiO2 exposure –only two of them having been conducted with food-grade TiO2- on the intestinal immune system. The difficulty to characterize intestinal immune response could explain this lack of data. Therefore, to complete this section, we include some data related to the consequences of oral exposure to TiO2-NPs with characteristics differing from those of E171 on the systemic immune system, i.e., in the spleen, liver, PBMC and microglia. In vivo, administration of TiO2-NPs has been demonstrated to have multiple immunomodulatory effects, characterized by TiO2-NPs distribution in peripheral lymphoid organs, alterations of immune cell number, viability and function. Although they do not recapitulate the complexity of the local intestinal immune system, simple in vitro or ex vivo models using systemic immune cells could inform on the mechanisms of TiO2-driven immune cell dysfunctions. TiO2-NPs (5–6 nm) administered by intragastric gavage at 2.5, 5 or 10 mg/kg b.w./day for 90 days cause macrophage infiltration in the spleen [107]. Moreover, mice gavage with 2.5, 5 or 10 mg/kg b.w./day of the same 5–6 nm anatase TiO2-NPs for six consecutive months causes distribution of TiO2-NPs in the liver, associated with liver dysfunction, infiltration of inflammatory cells and altered mRNA levels of Th-1 and Th-2 type cytokines, i.e., IL-4, IL-5 and IL-12, as well as their targets IFN-γ, GATA3, GATA4, T-bet, STAt3, STAT6, eotaxin, MCP-1, and MIP-2 [108]. In vitro, treatment with TiO2-NPs of the peripheral tissue equivalent (PTE) or the modular immune in vitro construct (MIMIC) system, which is an immunological construct comprising blood vein endothelial cells (HUVECs) and peripheral blood mononuclear cells (PBMCs), also shows some inflammatory response. Indeed, treatment with TiO2-NPs leads to a dose-dependent elevation of inflammatory cytokines including IL-1α, IL-1β, IL-6, IL-8, IL-10, IFN-γ and TNF-α, as well as upregulated expression of the maturation marker CD83 and the co-stimulatory molecule CD86 on dendritic cells [109]. Notably, 48 h of exposure to TiO2-NPs also results in a 10–20-fold increase of ROS levels in PBMCs [109]. Treatment of RAW 264.7 macrophages with very low concentrations of TiO2-NPs, such as those that might be found in consumer products, induces NF-κB activation, which results in upregulation of pro-inflammatory gene expression [110]. Similarly, TiO2-NPs alter the expression of adhesion molecules and the chemokine receptor type 4 (CXCR4) in PBMCs and trigger an increase in T cell proliferation in response to PHA stimulation at concentrations that are not cytotoxic to the immune cells [111]. The direct exposure of microglia (which is also a tissue specific macrophage-like cell) to TiO2-NPs elevates the iNOS expression associated to an increase of NO release from the microglia [112]. The expression levels of MCP-1, MIP-1α, TNFα, IL-1β and IL-6 are also upregulated, as is the activation of NF-κB [112]. Further demonstrating the direct impact of TiO2-NPs on immune cells, TiO2-NPs induce the expression of NOD-like receptor family, pyrin domain containing 3 (NLRP3) inflammasome by dendritic cells and macrophages [113, 114]. TiO2-NPs activate NLRP3 inflammasome in macrophages via a mechanism that is dependent upon the release of ATP. Namely, the activation of phospholipase C-β has been shown to be necessary for TiO2-NP induced NLRP3 activation and for the resulting IL-1β production. IL-1β production in response to TiO2-NPs is augmented by ATP and ADP hydrolysis, and suppressed by adenosine degradation or selective A2A or A2B receptor inhibition [115].
Although multiple studies indicate that TiO2-NPs induce immune cell activation and the subsequent release of inflammatory factors, TiO2-NP exposure also possesses the potential to cause immune cell death. For example, exposure of dendritic cells to TiO2-NPs induces a dose-dependent decrease of cell viability, with concentrations of TiO2 nanoparticles in the range of 10–100 μM eliciting an approximate 20% decline in viability [109]. Human dermal fibroblasts capable of exhibiting inflammatory responses also show a time and dose-dependent decrease in cell viability upon exposure to TiO2 nanoparticles, however, an increase in ROS levels and expression of several pro-inflammatory genes including the TLRs, genes of the MAP kinase cascade and IL-1β is observed. Notably, several mediators of the NF-κB pathway are activated: ERK1/2 expression is upregulated and p38 is phosphorylated. Interestingly, NF-κB knockdown fibroblasts are resistant to TiO2 nanoparticle-induced cell death, suggesting that the activation of the NF-κB pathway plays a role in TiO2 nanoparticle-induced immune cell death [116]. In conclusion, although they are not strictly derived from experiments involving the intestinal immune system and the E171 food additive, these data underscore the ability of TiO2-NPs to modulate multiple aspects of immune function.
Potential implications of TiO2 particles in chronic intestinal pathologies
Despite much research on this topic, the pathophysiology of IBD and associated CRC are not well understood. The IBD pathogenesis model is based on the dysregulation of the normally controlled immune response to commensal bacteria, which could be precipitated by infection or by defects in the mucosal barrier. This involves infiltration of immune cells producing chemokines and cytokines, which in turn exacerbate the dysfunctional immune response and activate either Th1, Th2 or Th17 cells in the gut mucosa of IBD patients [117]. More precisely, the pathogenesis of IBD occurs via three distinct stages: (i) penetration of luminal contents into underlying tissues which may be facilitated by environmental factors like infection [38], toxic particles or inherent defects in mucosal barrier linked with genetic mutations [118, 119], (ii) impaired clearance of luminal material from the bowel wall [120, 121] and (iii) a compensatory acquired immune response which leads to a chronic inflammatory response and gives rise to characteristic IBD lesions and further on CRC [122].
Implication of TiO2 particles in inflammatory bowel diseases
Various specific and non-specific environmental factors have been associated with the induction and/or exacerbation of disease activity in patients with CD and UC [54, 123]. Among them, some papers have suspected that TiO2 from food may have a detrimental role in the pathogenesis of the IBD (Table 4). The first study to evidence a link between particles and alterations of the immune cell of the GALT is the article of Powell et al., published in 1996 [54]. It shows the presence of microparticles, including TiO2 particles, in the phagolysosomes of macrophages populating the GALT and suggests that the pathogenicity of these microparticles should be further investigated. Indeed, in susceptible individuals, the same intracellular distribution of these three types of particles can cause chronic latent granulomatous inflammation which is one cardinal feature of CD [54]. The same group of researchers has also evidenced that ultrafine particles of TiO2 strongly enhance the secretion of inflammatory cytokines by peripheral blood mononuclear cells and intestinal macrophages from IBD patients [123]. This result demonstrates that dietary particles are not immunologically inert and may be important adjuncts in overcoming normal gut cell hypo-responsiveness to endogenous luminal molecules [123]. As already discussed in the previous sections of this review, the same type of microparticles is observed in children having IBD or not; 42% of the patients show these pigmented cells, with significant difference between healthy children, children with UC and children with CD [124]. Patients with CD show lower number of pigment cells as compared with non-IBD patients and patients with UC [124]. A hypothesis to explain this observation is that these pigmented cells are involved in the inflammatory process in CD patients, and consequently they migrate from their initial site of activation to active sites of the inflammatory response [124]. Therefore, these microparticles may play a role in the inflammatory process of CD [124].
Since these discoveries, some researches have focused on the impacts of these fine and ultrafine particles on IBD development and/or exacerbation. As described in the previous sections of this manuscript, TiO2 may impact the gut microbiota. In addition to the perturbations described in this previous sections, recent studies evidence that TiO2-NPs enhance the development of the infection and/or the immune cells response to LPS. Due to its non-degradability, TiO2 can also interact with intestinal microorganisms or their products and induce or aggravate inflammatory processes. For example, bacterial lipopolysaccharide (LPS), abundant in the gut, avidly binds TiO2-NPs surface, facilitated by calcium-bridging cations and mucosal secretions. This complex induces release of inflammatory cytokines in primary human mononuclear phagocytes [125] or in intestinal explants [123]. Similarly, other studies evidence that 139 nm TiO2-NP exposure, at 80 μg/mL, influences both production and structural architecture of the biofilm synthetized by Listeria monocytogenes [126] and that food-grade TiO2 promotes biofilm formation of E. coli, E. faecalis and mice commensal bacteria, in vitro [83]. Moreover, TiO2-NPs alter bacterial interaction with the intestinal cells [126]. Thus, increased biofilm production due to TiO2 exposure may favour bacterial survival in the environment and its transmission to animal and human hosts. In addition to its role on the gut microbes, including bacteria (pathogenic or not) and viruses, as developed in the previous sections of this review, TiO2-NPs are also able to disrupt the integrity of three others components of the intestinal mucosa: epithelium, mucus layer and the immune system. TiO2-NPs also have a synergistic effect with some bacterial product to aggravate the inflammation [127]. As TiO2-NPs can aggravate intestinal inflammation, it is of interest to investigate their pathogenicity on models mimicking IBD patients, such as in a study by Ruiz et al., which shows that oral administration of TiO2 NPs triggered the aggravation of acute colitis in a dextran sodium sulfate (DSS) mouse model of colitis through the activation of the NLRP3 inflammasome [56]. As already commented, these TiO2 NPs accumulate in a dose-dependent manner in the spleen of these mice, highlighting the fact that ingested TiO2 NPs can cross an altered intestinal barrier and reach the systemic circulation. Those results were corroborated by the fact that, after oral administration of TiO2 NPs, patients with active ulcerative colitis presented increased levels of titanium in their systemic circulation [56].
Implication of TiO2 particles in colorectal cancer
The International Agency for Research on Cancer (IARC), after comprehensively studying its hazardous potential, has rated TiO2 as possibly carcinogenic for humans by inhalation (Group 2B) [128]. This classification, in addition to the fact that TiO2 can disrupt the intestinal barrier and induce intestinal inflammation, calls for studies investigating the impact of titanium on intestinal carcinogenesis. While TiO2 does not appear as carcinogenic for healthy mice, it enhances the formation of intestinal tumours in mice suffering from induced colitis associated cancer (CAC) [96] (Table 4). This aggravated tumour formation is linked to dysplastic changes in the colonic epithelium, but also to a dramatic decrease in goblet cells (mucin-secreting cells) leading to intestinal barrier disruption [96]. Intestinal cancer formation could also be favoured by gene expression alterations caused by TiO2, and in this perspective transcriptomic studies show that food-grade TiO2 affects the response to oxidative stress, the immune response, DNA repair and carcinogenesis [129, 130]. Tumorigenesis could also be linked to ROS production as well as to DNA damage triggered by the interaction of E171 with microtubules or oxidative DNA damage in cells acutely or repeatedly exposed to E171 or TiO2-NPs [15, 131]. In addition, TiO2-NPs have been reported to promote the epithelial to mesenchymal transition (EMT) process in colorectal cancer cells [132]. More precisely, TiO2-NPs activate the transforming growth factor-β (TGF-β)/mitogen-activated protein kinase (MAPK) and wingless (Wnt) pathways that drives the EMT process [132]. Moreover, the colon of rats exposed for 100 days to E171 presents pre-neoplastic lesions, this exposure condition favouring the development of aberrant crypt foci in a chemically induced carcinogenesis model [20]. Nevertheless, when provided to rats in a food matrix, E171 do not produce such effects [102], suggesting that the food matrix would be protective for the intestinal epithelium. Taken together, although some studies support the fact that exposure to TiO2 could promote the development of CRC, additional studies must be conducted to validate or exclude a deleterious impact of these particles on colonic stem cell capacity to derive in carcinogenic cells.
Conclusions and perspectives
In conclusion, these data indicate that TiO2 is able to alter the four compartments of IBF and to induce a low-grade intestinal inflammation associated or not with pre-neoplastic lesions. However, since multiple evidence has shown that the intestinal homeostasis in adults results directly from the perinatal establishment of the IBF, it is of major importance to investigate the impact of TiO2 for the early phases of the intestinal development, i.e., during the embryogenic and lactating periods. Moreover, since IBD is a complex disease involving genetic, psychologic and environmental factors, it is important to check the impact of this material in models exhibiting IBD susceptibility like models mutated in the genes encoding the NOD2, ATG16L1, IRGM or IL-23 receptors. Finally, to demonstrate the likely involvement of these particles in the IBD genesis, it is also crucial to investigate whether exposure to these particles in combination with episodes of stress, which are described to be involved in the IBD, may induce an intestinal inflammation and linked colorectal cancer similar to those observed in IBD, in mice with genetic susceptibility to IBD.
Availability of data and materials
Not applicable.
Abbreviations
- TiO2 :
-
Titanium dioxide
- IBD:
-
Inflammatory bowel disease
- AMP:
-
Antimicrobial peptide
- CAC:
-
Colitis-associated cancer
- CD:
-
Crohn’s disease
- CXCR4:
-
Chemokine receptor type 4
- EFSA:
-
European food safety agency
- FAE:
-
Follicle-associated epithelium
- GALT:
-
Gut-associated lymphoid tissue
- IBF:
-
Intestinal barrier function
- ILF:
-
Isolated lymphoid follicle
- JECFA:
-
Joint FAE/WHO expert committee on food additives
- MAPK:
-
Mitogen-activated protein kinase
- NRLP3:
-
NOD-like receptor family, pyrin domain containing 3
- OECD:
-
Organisation for economic co-coperation and development
- PBMC:
-
Peripheral blood mononuclear cell
- PP:
-
Peyer’s patches
- REGIIIγ:
-
Regenerating islet-derived protein 3
- ROS:
-
Reactive oxygen species
- TGFβ:
-
Transforming growth factor β
- Th:
-
T-helper
- IARC:
-
International agency for research on cancer
- TJ:
-
Tight junction
- SAS:
-
Synthetic amorphous silica
- UC:
-
Ulcerative colitis
- Wnt:
-
Wingless and Int-1
References
Perez-Lopez A, Behnsen J, Nuccio SP, Raffatellu M. Mucosal immunity to pathogenic intestinal bacteria. Nat Rev Immunol. 2016;16(3):135–48. https://doi.org/10.1038/nri.2015.17.
McGuckin MA, Lindén SK, Sutton P, Florin TH. Mucin dynamics and enteric pathogens. Nat Rev Microbiol. 2011;9(4):265–78. https://doi.org/10.1038/nrmicro2538.
Peterson LW, Artis D. Intestinal epithelial cells: regulators of barrier function and immune homeostasis. Nat Rev Immunol. 2014;14(3):141–53. https://doi.org/10.1038/nri3608.
König J, Wells J, Cani PD, García-Ródenas CL, MacDonald T, Mercenier A, et al. Human Intestinal Barrier Function in Health and Disease. Clin Transl Gastroenterol. 2016;7:10–e196. https://doi.org/10.1038/ctg.2016.54.
Podolsky DK. Inflammatory bowel disease. N Engl J Med. 2002;347(6):417–29. https://doi.org/10.1056/NEJMra020831.
Feagins LA, Souza RF, Spechler SJ. Carcinogenesis in IBD: potential targets for the prevention of colorectal cancer. Nat Rev Gastroenterol hepatol. 2009;6(5):297–305. https://doi.org/10.1038/nrgastro.2009.44.
Lakatos PL, Lakatos L. Ulcerative proctitis: a review of pharmacotherapy and management. Expert Opin Pharmacother. 2008;9(5):741–9. https://doi.org/10.1517/14656566.9.5.741.
Grivennikov SI, Karin M. Inflammation and oncogenesis: a vicious connection. Curr Opin Genet Dev. 2010;20(1):65–71. https://doi.org/10.1016/j.gde.2009.11.004.
Collaborators GIBD. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990-2017: a systematic analysis for the global burden of disease study 2017. Lancet Gastroenterol Hepatol. 2020;5(1):17–30. https://doi.org/10.1016/s2468-1253(19)30333-4.
Ng SC, Shi HY, Hamidi N, Underwood FE, Tang W, Benchimol EI, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2017;390(10114):2769–78. https://doi.org/10.1016/s0140-6736(17)32448-0.
Cosnes J, Carbonnel F, Carrat F, Beaugerie L, Cattan S, Gendre J. Effects of current and former cigarette smoking on the clinical course of Crohn's disease. Aliment Pharmacol Ther. 1999;13(11):1403–11; apt630 [pii]. https://doi.org/10.1046/j.1365-2036.1999.00630.x.
Chan SS, Luben R, van Schaik F, Oldenburg B, Bueno-de-Mesquita HB, Hallmans G, et al. Carbohydrate intake in the etiology of Crohn's disease and ulcerative colitis. Inflamm Bowel Dis. 2014;20(11):2013–21. https://doi.org/10.1046/j.1365-2036.1999.00630.x.
Lomer MC, Thompson RP, Powell JJ. Fine and ultrafine particles of the diet: influence on the mucosal immune response and association with Crohn's disease. Proc Nutr Soc. 2002;61(1):123–30; S0029665102000174 [pii]. https://doi.org/10.1079/PNS2001134.
Rogler G, Vavricka S. Exposome in IBD: recent insights in environmental factors that influence the onset and course of IBD. Inflamm Bowel Dis. 2015;21(2):400–8. https://doi.org/10.1097/mib.0000000000000229.
Dorier M, Beal D, Marie-Desvergne C, Dubosson M, Barreau F, Houdeau E, et al. Continuous in vitro exposure of intestinal epithelial cells to E171 food additive causes oxidative stress, inducing oxidation of DNA bases but no endoplasmic reticulum stress. Nanotoxicology. 2017;11(6):751–61. https://doi.org/10.1080/17435390.2017.1349203.
Peters RJ, van Bemmel G, Herrera-Rivera Z, Helsper HP, Marvin HJ, Weigel S, et al. Characterization of titanium dioxide nanoparticles in food products: analytical methods to define nanoparticles. J Agric Food Chem. 2014;62(27):6285–93. https://doi.org/10.1021/jf5011885.
Weir A, Westerhoff P, Fabricius L, Hristovski K, von Goetz N. Titanium dioxide nanoparticles in food and personal care products. Env Sci Technol. 2012;46(4):2242–50. https://doi.org/10.1021/es204168d.
Yang Y, Doudrick K, Bi X, Hristovski K, Herckes P, Westerhoff P, et al. Characterization of food-grade titanium dioxide: the presence of nanosized particles. Env Sci Technol. 2014;48(11):6391–400. https://doi.org/10.1021/es500436x.
Pele LC, Thoree V, Bruggraber SF, Koller D, Thompson RP, Lomer MC, et al. Pharmaceutical/food grade titanium dioxide particles are absorbed into the bloodstream of human volunteers. Part Fibre toxicol. 2015;12(1):26. https://doi.org/10.1186/s12989-015-0101-9.
Bettini S, Boutet-Robinet E, Cartier C, Comera C, Gaultier E, Dupuy J, et al. Food-grade TiO2 impairs intestinal and systemic immune homeostasis, initiates preneoplastic lesions and promotes aberrant crypt development in the rat colon. Sci Rep. 2017;7(1):40373. https://doi.org/10.1038/srep40373.
Talamini L, Gimondi S, Violatto MB, Fiordaliso F, Pedica F, Tran NL, et al. Repeated administration of the food additive E171 to mice results in accumulation in intestine and liver and promotes an inflammatory status. Nanotoxicology. 2019;13(8):1087–101. https://doi.org/10.1080/17435390.2019.1640910.
Heringa MB, Geraets L, van Eijkeren JC, Vandebriel RJ, de Jong WH, Oomen AG. Risk assessment of titanium dioxide nanoparticles via oral exposure, including toxicokinetic considerations. Nanotoxicology. 2016;10(10):1515–25. https://doi.org/10.1080/17435390.2016.1238113.
Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, Sargent M, et al. Diversity of the human intestinal microbial flora. Science. 2005;308(5728):1635–8. https://doi.org/10.1126/science.1110591.
Falony G, Vlachou A, Verbrugghe K, De Vuyst L. Cross-feeding between Bifidobacterium longum BB536 and acetate-converting, butyrate-producing colon bacteria during growth on oligofructose. Appl Environ Microbiol. 2006;72(12):7835–41. https://doi.org/10.1128/AEM.01296-06.
Macfarlane S, Macfarlane GT. Regulation of short-chain fatty acid production. Proc Nutr Soc. 2003;62(1):67–72. https://doi.org/10.1079/PNS2002207.
Vaishnava S, Yamamoto M, Severson KM, Ruhn KA, Yu X, Koren O, et al. The antibacterial lectin RegIIIgamma promotes the spatial segregation of microbiota and host in the intestine. Science. 2011;334(6053):255–8. https://doi.org/10.1126/science.1209791.
Mayer L. Mucosal immunity. Pediatrics. 2003;111(6 Pt 3):1595–600.
Ermund A, Schütte A, Johansson ME, Gustafsson JK, Hansson GC. Studies of mucus in mouse stomach, small intestine, and colon. I. Gastrointestinal mucus layers have different properties depending on location as well as over the Peyer's patches. Am J Physiol Gastrointest Liver Physiol. 2013;305(5):G341–7. https://doi.org/10.1152/ajpgi.00046.2013.
Hollingsworth MA, Swanson BJ. Mucins in cancer: protection and control of the cell surface. Nat Rev Cancer. 2004;4(1):45–60. https://doi.org/10.1038/nrc1251.
Kim YS, Ho SB. Intestinal goblet cells and mucins in health and disease: recent insights and progress. Curr Gastroenterol Rep. 2010;12(5):319–30. https://doi.org/10.1007/s11894-010-0131-2.
Antoni L, Nuding S, Wehkamp J, Stange EF. Intestinal barrier in inflammatory bowel disease. World J Gastroenterol. 2014;20(5):1165–79. https://doi.org/10.3748/wjg.v20.i5.1165.
Natividad JM, Hayes CL, Motta JP, Jury J, Galipeau HJ, Philip V, et al. Differential induction of antimicrobial REGIII by the intestinal microbiota and Bifidobacterium breve NCC2950. Appl Environ Microbiol. 2014;79(24):7745–54. https://doi.org/10.1128/AEM.02470-13.
Wehkamp J, Harder J, Weichenthal M, Mueller O, Herrlinger KR, Fellermann K, et al. Inducible and constitutive beta-defensins are differentially expressed in Crohn's disease and ulcerative colitis. Inflamm Bowel Dis. 2003;9(4):215–23. https://doi.org/10.1097/00054725-200307000-00001.
Makkink MK, Schwerbrock NM, Mahler M, Boshuizen JA, Renes IB, Cornberg M, et al. Fate of goblet cells in experimental colitis. Dig Dis Sci. 2002;47(10):2286–97. https://doi.org/10.1023/A:1020147630032.
Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol. 2009;9(11):799–809. https://doi.org/10.1038/nri2653.
Keita AV, Soderholm JD. The intestinal barrier and its regulation by neuroimmune factors. Neurogastroenterol Motil. 2010;22(7):718–33. https://doi.org/10.1111/j.1365-2982.2010.01498.x.
Keita AV, Soderholm JD. Barrier dysfunction and bacterial uptake in the follicle-associated epithelium of ileal Crohn's disease. Ann N Y Acad Sci. 2012;1258(1):125–34. https://doi.org/10.1111/j.1749-6632.2012.06502.x.
Barreau F, Hugot J. Intestinal barrier dysfunction triggered by invasive bacteria. Curr Opin Microbiol. 2014;17C:91–8; S1369–5274(13)00233–6. https://doi.org/10.1016/j.mib.2013.12.003.
Gaudino SJ, Kumar P. Cross-talk between antigen presenting cells and T cells impacts intestinal homeostasis, bacterial infections, and tumorigenesis. Front Immunol. 2019;10:360. https://doi.org/10.3389/fimmu.2019.00360.
Sun T, Nguyen A, Gommerman JL. Dendritic cell subsets in intestinal immunity and inflammation. J Immunol. 2020;204(5):1075–83. https://doi.org/10.4049/jimmunol.1900710.
Yang ZJ, Wang BY, Wang TT, Wang FF, Guo YX, Hua RX, et al. Functions of dendritic cells and its association with intestinal diseases. Cells. 2021;10(3):3. https://doi.org/10.3390/cells10030583.
Ma H, Tao W, Zhu S. T lymphocytes in the intestinal mucosa: defense and tolerance. Cell Mol Immunol. 2019;16(3):216–24. https://doi.org/10.1038/s41423-019-0208-2.
Rankin L, Groom J, Mielke LA, Seillet C, Belz GT. Diversity, function, and transcriptional regulation of gut innate lymphocytes. Front Immunol. 2013;4:22. https://doi.org/10.3389/fimmu.2013.00022.
Ribot JC, Lopes N, Silva-Santos B. γδ T cells in tissue physiology and surveillance. Nat Rev Immunol. 2021;21(4):221–32. https://doi.org/10.1038/s41577-020-00452-4.
Peters RJB, Bouwmeester H, Gottardo S, Amenta V, Arena M, Brandhoff P, et al. Nanomaterials for products and application in agriculture, feed and food. Trends Food Sci Technol. 2016;54:155–64. https://doi.org/10.1016/j.tifs.2016.06.008.
Commission Recommendation of 18 October 2011 on the definition of nanomaterial (Text with EEA relevance) (2011/696/EU). Official Journal of the European Union. 2011.
Younes M, Aquilina G, Castle L, Engel KH, Fowler P, Frutos Fernandez MJ, et al. Safety assessment of titanium dioxide (E171) as a food additive. EFSA Journal. 2021;19(5):e06585. https://doi.org/10.2903/j.efsa.2021.6585.
Chen XX, Cheng B, Yang YX, Cao A, Liu JH, Du LJ, et al. Characterization and preliminary toxicity assay of nano-titanium dioxide additive in sugar-coated chewing gum. Small. 2013;9(9–10):1765–74. https://doi.org/10.1002/smll.201201506.
Rompelberg C, Heringa MB, van Donkersgoed G, Drijvers J, Roos A, Westenbrink S, et al. Oral intake of added titanium dioxide and its nanofraction from food products, food supplements and toothpaste by the Dutch population. Nanotoxicology. 2016;10(10):1404–14. https://doi.org/10.1080/17435390.2016.1222457.
EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS). Re-evaluation of titanium dioxide (E 171) as a food additive. EFSA Journal. 2016;14(9):4545.
Hashem MM, Abo-El-Sooud K, Abd-Elhakim YM, Badr YA, El-Metwally AE, Bahy-El-Dien A. The long-term oral exposure to titanium dioxide impaired immune functions and triggered cytotoxic and genotoxic impacts in rats. J Trace Elem Med Biol. 2020;60:126473. https://doi.org/10.1016/j.jtemb.2020.126473.
Brun E, Barreau F, Veronesi G, Fayard B, Sorieul S, Chaneac C, et al. Titanium dioxide nanoparticle impact and translocation through ex vivo, in vivo and in vitro gut epithelia. Part Fibre Toxicol. 2014;11(1):13. https://doi.org/10.1186/1743-8977-11-13.
Drasler B, Sayre P, Steinhauser KG, Petri-Fink A, Rothen-Rutishauser B. In vitro approaches to assess the hazard of nanomaterials. Nanoimpact. 2017;8:99–116. https://doi.org/10.1016/j.impact.2017.08.002.
Powell JJ, Ainley CC, Harvey RS, Mason IM, Kendall MD, Sankey EA, et al. Characterisation of inorganic microparticles in pigment cells of human gut associated lymphoid tissue. Gut. 1996;38(3):390–5. https://doi.org/10.1136/gut.38.3.390.
Jones K, Morton J, Smith I, Jurkschat K, Harding AH, Evans G. Human in vivo and in vitro studies on gastrointestinal absorption of titanium dioxide nanoparticles. Toxicol Let. 2015;233(2):95–101. https://doi.org/10.1016/j.toxlet.2014.12.005.
Ruiz PA, Moron B, Becker HM, Lang S, Atrott K, Spalinger MR, et al. Titanium dioxide nanoparticles exacerbate DSS-induced colitis: role of the NLRP3 inflammasome. Gut. 2017;66(7):1216–24. https://doi.org/10.1136/gutjnl-2015-310297.
Warheit DB, Brown SC, Donner EM. Acute and subchronic oral toxicity studies in rats with nanoscale and pigment grade titanium dioxide particles. Food Chemical Toxicol. 2015;84:208–24. https://doi.org/10.1016/j.fct.2015.08.026.
Farrell TP, Magnuson B. Absorption, distribution and excretion of four forms of titanium dioxide pigment in the rat. J Food Sci. 2017;82(8):1985–93. https://doi.org/10.1111/1750-3841.13791.
Han HY, Yang MJ, Yoon C, Lee GH, Kim DW, Kim TW, et al. Toxicity of orally administered food-grade titanium dioxide nanoparticles. J Appl Toxicol. 2020;41(7):1127–47. https://doi.org/10.1002/jat.4099.
Wang J, Zhou G, Chen C, Yu H, Wang T, Ma Y, et al. Acute toxicity and biodistribution of different sized titanium dioxide particles in mice after oral administration. Toxicol Lett. 2007;168(2):176–85; doi: S0378-4274(06)01379-8 [pii]. https://doi.org/10.1016/j.toxlet.2006.12.001.
Li J, Yang S, Lei R, Gu W, Qin Y, Ma S, et al. Oral administration of rutile and anatase TiO2 nanoparticles shifts mouse gut microbiota structure. Nanoscale. 2018;10(16):7736–45. https://doi.org/10.1039/c8nr00386f.
Jani PUMD, Florence AT. Titanium dioxide rutile particle uptake from the rat GI tract and translocation to systemic organs after oral-administration. Int J Pharma. 1994;105(2):157–68. https://doi.org/10.1016/0378-5173(94)90461-8.
Geraets L, Oomen AG, Krystek P, Jacobsen NR, Wallin H, Laurentie M, et al. Tissue distribution and elimination after oral and intravenous administration of different titanium dioxide nanoparticles in rats. Part Fibre Toxicol. 2014;11(1):30. https://doi.org/10.1186/1743-8977-11-30.
Cho WS, Kang BC, Lee JK, Jeong J, Che JH, Seok SH. Comparative absorption, distribution, and excretion of titanium dioxide and zinc oxide nanoparticles after repeated oral administration. Part Fibre Toxicol. 2013;10(1):9. https://doi.org/10.1186/1743-8977-10-9.
Wang Y, Chen Z, Ba T, Pu J, Chen T, Song Y, et al. Susceptibility of young and adult rats to the oral toxicity of titanium dioxide nanoparticles. Small. 2013;9(9–10):1742–52. https://doi.org/10.1002/smll.201201185.
Heo MB, Kwak M, An KS, Kim HJ, Ryu HY, Lee SM, et al. Oral toxicity of titanium dioxide P25 at repeated dose 28-day and 90-day in rats. Part Fibre Toxicol. 2020;17(1):34. https://doi.org/10.1186/s12989-020-00350-6.
Møller P, Wallin H, Cassee FR, Loft S. Does intranasal instillation TiO(2) cause pulmonary tumorigenesis in male mice? Environ Toxicol. 2018;33(11):1095–6. https://doi.org/10.1002/tox.22490.
Hill TJAF. The effect of silver nitrate in the prevention of dental caries. J Dent Res. 1937;16(1):23–8. https://doi.org/10.1177/00220345370160010301.
Li Q, Mahendra S, Lyon DY, Brunet L, Liga MV, Li D, et al. Antimicrobial nanomaterials for water disinfection and microbial control: potential applications and implications. Water Res. 2008;42(18):4591–602; S0043-1354(08)00333-3 [pii]. https://doi.org/10.1016/j.watres.2008.08.015.
Allaker RP. The use of nanoparticles to control oral biofilm formation. J Dent Res. 2010;89(11):1175–86; 0022034510377794 [pii]. https://doi.org/10.1177/0022034510377794.
Kim JS, Kuk E, Yu KN, Kim JH, Park SJ, Lee HJ, et al. Antimicrobial effects of silver nanoparticles. Nanomedicine. 2007;3(1):95–101; S1549-9634(06)00346-7 [pii]. https://doi.org/10.1016/j.nano.2006.12.001.
Sohal IS, Cho YK, O'Fallon KS, Gaines P, Demokritou P, Bello D. Dissolution behavior and biodurability of ingested engineered nanomaterials in the gastrointestinal environment. ACS Nano. 2018;12(8):8115–28. https://doi.org/10.1021/acsnano.8b02978.
Schneider J, Matsuoka M, Takeuchi M, Zhang J, Horiuchi Y, Anpo M, et al. Understanding TiO2 photocatalysis: mechanisms and materials. Chem Rev. 2014;114(19):9919–86. https://doi.org/10.1021/cr5001892.
Laxma Reddy PV, Kavitha B, Kumar Reddy PA, Kim KH. TiO(2)-based photocatalytic disinfection of microbes in aqueous media: a review. Environ Res. 2017;154:296–303. https://doi.org/10.1016/j.envres.2017.01.018.
Fenoglio I, Greco G, Livraghi S, Fubini B. Non-UV-induced radical reactions at the surface of TiO2 nanoparticles that may trigger toxic responses. Chemistry. 2009;15(18):4614–21. https://doi.org/10.1002/chem.200802542.
Wong MS, Chu WC, Sun DS, Huang HS, Chen JH, Tsai PJ, et al. Visible-light-induced bactericidal activity of a nitrogen-doped titanium photocatalyst against human pathogens. Appl Environ Microbiol. 2006;72(9):6111–6; 72/9/6111 [pii]. https://doi.org/10.1128/AEM.02580-05.
Hirakawa K, Mori M, Yoshida M, Oikawa S, Kawanishi S. Photo-irradiated titanium dioxide catalyzes site specific DNA damage via generation of hydrogen peroxide. Free Radic Res. 2004;38(5):439–47. https://doi.org/10.1080/1071576042000206487.
Radziwill-Bienkowska JM, Talbot P, Kamphuis JBJ, Robert V, Cartier C, Fourquaux I, et al. Toxicity of food-grade TiO2 to commensal intestinal and transient food-borne Bacteria: new insights using Nano-SIMS and synchrotron UV fluorescence imaging. Front Microbiol. 2018;9:794. https://doi.org/10.3389/fmicb.2018.00794.
Dudefoi W, Moniz K, Allen-Vercoe E, Ropers MH, Walker VK. Impact of food grade and nano-TiO2 particles on a human intestinal community. Food Chem Toxicol. 2017;106(Pt A):242–9. https://doi.org/10.1016/j.fct.2017.05.050.
Waller TCC, Walker SL. Food and Industrial Grade Titanium Dioxide Impacts Gut Microbiota. Environ Eng Sci. 2017;34(8):34–8.
Cao X, Han Y, Gu M, Du H, Song M, Zhu X, et al. Foodborne Titanium Dioxide Nanoparticles Induce Stronger Adverse Effects in Obese Mice than Non-Obese Mice: Gut Microbiota Dysbiosis, Colonic Inflammation, and Proteome Alterations. Small. 2020;16(36):e2001858. https://doi.org/10.1002/smll.202001858.
Chen HZR, Wang B, Cai C, Zheng L, Wang H, Wang M, et al. The effects of orally administered ag, TiO2 and SiO2 nanoparticles on gut microbiota composition and colitis induction in mice. NanoImpact. 2017;9:8–88. https://doi.org/10.1016/j.impact.2017.07.005.
Lamas B, Martins Breyner N, Houdeau E. Impacts of foodborne inorganic nanoparticles on the gut microbiota-immune axis: potential consequences for host health. Part Fibre Toxicol. 2020;17(1):19. https://doi.org/10.1186/s12989-020-00349-z.
Arumugam M, Raes J, Pelletier E, Le Paslier D, Yamada T, Mende DR, et al. Enterotypes of the human gut microbiome. Nature. 2011;473(7346):174–80. https://doi.org/10.1038/nature09944.
Das B, Ghosh TS, Kedia S, Rampal R, Saxena S, Bag S, et al. Analysis of the Gut Microbiome of Rural and Urban Healthy Indians Living in Sea Level and High Altitude Areas. Sci Rep. 2018;8(1):10104. https://doi.org/10.1038/s41598-018-28550-3.
Human Microbiome Project C. Structure, function and diversity of the healthy human microbiome. Nature. 2012;486(7402):207–14. https://doi.org/10.1038/nature11234.
Tojo R, Suárez A, Clemente MG, de los Reyes-Gavilán CG, Margolles A, Gueimonde M, et al. Intestinal microbiota in health and disease: role of bifidobacteria in gut homeostasis. World J Gastroenterol. 2014;20(41):15163–76. https://doi.org/10.3748/wjg.v20.i41.15163.
Medina-Reyes EI, Delgado-Buenrostro NL, Díaz-Urbina D, Rodríguez-Ibarra C, Déciga-Alcaraz A, González MI, et al. Food-grade titanium dioxide (E171) induces anxiety, adenomas in colon and goblet cells hyperplasia in a regular diet model and microvesicular steatosis in a high fat diet model. Food Chem Toxicol. 2020;146:111786. https://doi.org/10.1016/j.fct.2020.111786.
Faust JJ, Doudrick K, Yang Y, Westerhoff P, Capco DG. Food grade titanium dioxide disrupts intestinal brush border microvilli in vitro independent of sedimentation. Cell Biol Toxicol. 2014;30(3):169–88. https://doi.org/10.1007/s10565-014-9278-1.
Zhang Y, Duan S, Liu Y, Wang Y. The combined effect of food additive titanium dioxide and lipopolysaccharide on mouse intestinal barrier function after chronic exposure of titanium dioxide-contained feedstuffs. Part Fibre Toxicol. 2021;18(1):8. https://doi.org/10.1186/s12989-021-00399-x.
Dorier M, Brun E, Veronesi G, Barreau F, Pernet-Gallay K, Desvergne C, et al. Impact of anatase and rutile titanium dioxide nanoparticles on uptake carriers and efflux pumps in Caco-2 gut epithelial cells. Nanoscale. 2015;7(16):7352–60. https://doi.org/10.1039/c5nr00505a.
Guo Z, Martucci NJ, Moreno-Olivas F, Tako E, Mahler GJ. Titanium dioxide nanoparticle ingestion alters nutrient absorption in an in vitro model of the small intestine. NanoImpact. 2017;5:70–82. https://doi.org/10.1016/j.impact.2017.01.002.
Lesuffleur T, Porchet N, Aubert JP, Swallow D, Gum JR, Kim YS, et al. Differential expression of the human mucin genes MUC1 to MUC5 in relation to growth and differentiation of different mucus-secreting HT-29 cell subpopulations. J Cell Sci. 1993;106(Pt 3):771–83. https://doi.org/10.1242/jcs.106.3.771.
Talbot P, Radziwill-Bienkowska JM, Kamphuis JBJ, Steenkeste K, Bettini S, Robert V, et al. Food-grade TiO2 is trapped by intestinal mucus in vitro but does not impair mucin O-glycosylation and short-chain fatty acid synthesis in vivo: implications for gut barrier protection. J Nanobiotechnol. 2018;16(1):53. https://doi.org/10.1186/s12951-018-0379-5.
Dorier M, Beal D, Tisseyre C, Marie-Desvergne C, Dubosson M, Barreau F, et al. The food additive E171 and titanium dioxide nanoparticles indirectly alter the homeostasis of human intestinal epithelial cells in vitro. Environ-Sci Nano. 2019;6(5):1549–61. https://doi.org/10.1039/c8en01188e.
Urrutia-Ortega IM, Garduno-Balderas LG, Delgado-Buenrostro NL, Freyre-Fonseca V, Flores-Flores JO, Gonzalez-Robles A, et al. Food-grade titanium dioxide exposure exacerbates tumor formation in colitis associated cancer model. Food Chem Toxicol. 2016;93:20–31. https://doi.org/10.1016/j.fct.2016.04.014.
Cao XQ, Zhang T, DeLoid GM, Gaffrey MJ, Weitz KK, Thrall BD, et al. Evaluation of the cytotoxic and cellular proteome impacts of food-grade TiO2 (E171) using simulated gastrointestinal digestions and a tri-culture small intestinal epithelial model. Nanoimpact. 2020;17:100202. https://doi.org/10.1016/j.impact.2019.100202.
Marucco A, Prono M, Beal D, Alasonati E, Fisicaro P, Bergamaschi E, et al. Biotransformation of food-grade and Nanometric TiO(2) in the Oral-gastro-intestinal tract: driving forces and effect on the toxicity toward intestinal epithelial cells. Nanomaterials. 2020;10(11):11. https://doi.org/10.3390/nano10112132.
Zhou H, Pandya JK, Tan Y, Liu J, Peng S, Muriel Mundo JL, et al. Role of mucin in behavior of food-grade TiO2 nanoparticles under simulated Oral conditions. J Agric Food Chem. 2019;67(20):5882–90. https://doi.org/10.1021/acs.jafc.9b01732.
Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003;4(7):517–29. https://doi.org/10.1038/nrm1155.
Shi H, Magaye R, Castranova V, Zhao J. Titanium dioxide nanoparticles: a review of current toxicological data. Part Fibre Toxicol. 2013;10(1):15. https://doi.org/10.1186/1743-8977-10-15.
Blevins LK, Crawford RB, Bach A, Rizzo MD, Zhou J, Henriquez JE, et al. Evaluation of immunologic and intestinal effects in rats administered an E 171-containing diet, a food grade titanium dioxide (TiO2). Food Chem Toxicol. 2019;133:110793. https://doi.org/10.1016/j.fct.2019.110793.
Evans SM, Ashwood P, Warley A, Berisha F, Thompson RP, Powell JJ. The role of dietary microparticles and calcium in apoptosis and interleukin-1beta release of intestinal macrophages. Gastroenterology. 2002;123(5):1543–53; S0016508502002883 [pii]. https://doi.org/10.1053/gast.2002.36554.
Nogueira CM, de Azevedo WM, Dagli ML, Toma SH, Leite AZ, Lordello ML, et al. Titanium dioxide induced inflammation in the small intestine. World J Gastroenterol. 2012;18(34):4729–35. https://doi.org/10.3748/wjg.v18.i34.4729.
Florence AT. The oral absorption of micro- and nanoparticulates: neither exceptional nor unusual. Pharm Res. 1997;14(3):259–66. https://doi.org/10.1023/A:1012029517394.
Li H, Zhao X, Ma Y, Zhai G, Li L, Lou H. Enhancement of gastrointestinal absorption of quercetin by solid lipid nanoparticles. J Control Release. 2009;133(3):238–44; S0168-3659(08)00629-9 [pii]. https://doi.org/10.1016/j.jconrel.2008.10.002.
Sheng L, Wang L, Sang X, Zhao X, Hong J, Cheng S, et al. Nano-sized titanium dioxide-induced splenic toxicity: a biological pathway explored using microarray technology. J Hazard Mater. 2014;278:180–8; S0304-3894(14)00462-2 [pii]. https://doi.org/10.1016/j.jhazmat.2014.06.005.
Hong J, Wang L, Zhao X, Yu X, Sheng L, Xu B, et al. Th2 factors may be involved in TiO(2) NP-induced hepatic inflammation. J Agric Food Chem. 2014;62(28):6871–8. https://doi.org/10.1021/jf501428w.
Schanen BC, Karakoti AS, Seal S, Drake DR 3rd, Warren WL, Self WT. Exposure to titanium dioxide nanomaterials provokes inflammation of an in vitro human immune construct. ACS Nano. 2009;3(9):2523–32. https://doi.org/10.1021/nn900403h.
Giovanni M, Yue J, Zhang L, Xie J, Ong CN, Leong DT. Pro-inflammatory responses of RAW264.7 macrophages when treated with ultralow concentrations of silver, titanium dioxide, and zinc oxide nanoparticles. J Hazard Mater. 2015;297:146–52; S0304-3894(15)00379-9 [pii]. https://doi.org/10.1016/j.jhazmat.2015.04.081.
Lozano-Fernandez T, Ballester-Antxordoki L, Perez-Temprano N, Rojas E, Sanz D, Iglesias-Gaspar M, et al. Potential impact of metal oxide nanoparticles on the immune system: the role of integrins, L-selectin and the chemokine receptor CXCR4. Nanomedicine. 2014;10(6):1301–10; S1549-9634(14)00125-7 [pii]. https://doi.org/10.1016/j.nano.2014.03.007.
Xue Y, Wu J, Sun J. Four types of inorganic nanoparticles stimulate the inflammatory reaction in brain microglia and damage neurons in vitro. Toxicol Lett. 2012;214(2):91–8; S0378-4274(12)01254-4 [pii]. https://doi.org/10.1016/j.toxlet.2012.08.009.
Sun B, Wang X, Ji Z, Li R, Xia T. NLRP3 inflammasome activation induced by engineered nanomaterials. Small. 2012;9(9–10):1595–607. https://doi.org/10.1002/smll.201201962.
Winter M, Beer HD, Hornung V, Kramer U, Schins RP, Forster I. Activation of the inflammasome by amorphous silica and TiO2 nanoparticles in murine dendritic cells. Nanotoxicology. 2011;5(3):326–40. https://doi.org/10.3109/17435390.2010.506957.
Baron L, Gombault A, Fanny M, Villeret B, Savigny F, Guillou N, et al. The NLRP3 inflammasome is activated by nanoparticles through ATP, ADP and adenosine. Cell Death Dis. 2015;6(2):e1629; cddis2014576 [pii]. https://doi.org/10.1038/cddis.2014.576.
Romoser AA, Figueroa DE, Sooresh A, Scribner K, Chen PL, Porter W, et al. Distinct immunomodulatory effects of a panel of nanomaterials in human dermal fibroblasts. Toxicol Lett. 2012;210(3):293–301; S0378-4274(12)00054-9 [pii]. https://doi.org/10.1016/j.toxlet.2012.01.022.
Matricon J, Barnich N, Ardid D. Immunopathogenesis of inflammatory bowel disease. Self Nonself. 2010;1(4):299–309. https://doi.org/10.4161/self.1.4.13560.
Barreau F, Madre C, Meinzer U, Berrebi D, Dussaillant M, Merlin F, et al. Nod2 regulates the host response towards microflora by modulating T cell function and epithelial permeability in mouse Peyer's patches. Gut. 2010;59(2):207–17; gut.2008.171546 [pii]. https://doi.org/10.1136/gut.2008.171546.
Barreau F, Meinzer U, Chareyre F, Berrebi D, Niwa-Kawakita M, Dussaillant M, et al. CARD15/NOD2 is required for Peyer's patches homeostasis in mice. PLoS One. 2007;2(6):e523.
Lapaquette P, Bringer MA, Darfeuille-Michaud A. Defects in autophagy favour adherent-invasive Escherichia coli persistence within macrophages leading to increased pro-inflammatory response. Cell Microbiol. 2012;14(6):791–807. https://doi.org/10.1111/j.1462-5822.2012.01768.x.
Travassos LH, Carneiro LA, Ramjeet M, Hussey S, Kim YG, Magalhaes JG, et al. Nod1 and Nod2 direct autophagy by recruiting ATG16L1 to the plasma membrane at the site of bacterial entry. Nat Immunol. 2010;11(1):55–62; ni.1823 [pii]. https://doi.org/10.1038/ni.1823.
Abraham C, Cho JH. Inflammatory bowel disease. N Engl J Med. 2009;361(21):2066–78. https://doi.org/10.1056/NEJMra0804647.
Powell JJ, Harvey RS, Ashwood P, Wolstencroft R, Gershwin ME, Thompson RP. Immune potentiation of ultrafine dietary particles in normal subjects and patients with inflammatory bowel disease. J Autoimmun. 2000;14(1):99–105. https://doi.org/10.1006/jaut.1999.0342 S0896-8411(99)90342-6 [pii].
Hummel TZ, Kindermann A, Stokkers PC, Benninga MA, ten Kate FJ. Exogenous pigment in Peyer patches of children suspected of having IBD. J Pediatr Gastroenterol Nutr. 2014;58(4):477–80. https://doi.org/10.1097/mpg.0000000000000221.
Ashwood P, Thompson RP, Powell JJ. Fine particles that adsorb lipopolysaccharide via bridging calcium cations may mimic bacterial pathogenicity towards cells. Exp Biol Med. 2007;232(1):107–17 232/1/107 [pii].
Ammendolia MG, Iosi F, De Berardis B, Guccione G, Superti F, Conte MP, et al. Listeria monocytogenes behaviour in presence of non-UV-irradiated titanium dioxide nanoparticles. PLoS One. 2014;9(1):e84986. https://doi.org/10.1371/journal.pone.0084986 PONE-D-13-40481 [pii].
Moon C, Park HJ, Choi YH, Park EM, Castranova V, Kang JL. Pulmonary inflammation after intraperitoneal administration of ultrafine titanium dioxide (TiO2) at rest or in lungs primed with lipopolysaccharide. J Toxicol Environ Health A. 2010;73(5):396–409; 919250852 [pii]. https://doi.org/10.1080/15287390903486543.
Baan R, Straif K, Grosse Y, Secretan B, El Ghissassi F, Cogliano V. Carcinogenicity of carbon black, titanium dioxide, and talc. Lancet Oncol. 2006;7(4):295–6. https://doi.org/10.1016/S1470-2045(06)70651-9.
Proquin H, Jetten MJ, Jonkhout MCM, Garduno-Balderas LG, Briede JJ, de Kok TM, et al. Gene expression profiling in colon of mice exposed to food additive titanium dioxide (E171). Food Chem Toxicol. 2018;111:153–65. https://doi.org/10.1016/j.fct.2017.11.011.
Proquin H, Jetten MJ, Jonkhout MCM, Garduno-Balderas LG, Briede JJ, de Kok TM, et al. Transcriptomics analysis reveals new insights in E171-induced molecular alterations in a mouse model of colon cancer. Sci Rep. 2018;8(1):9738. https://doi.org/10.1038/s41598-018-28063-z.
Proquin H, Rodriguez-Ibarra C, Moonen CG, Urrutia Ortega IM, Briede JJ, de Kok TM, et al. Titanium dioxide food additive (E171) induces ROS formation and genotoxicity: contribution of micro and nano-sized fractions. Mutagenesis. 2017;32(1):139–49. https://doi.org/10.1093/mutage/gew051.
Setyawati MI, Sevencan C, Bay BH, Xie J, Zhang Y, Demokritou P, et al. Nano-TiO2 Drives Epithelial-Mesenchymal Transition in Intestinal Epithelial Cancer Cells. Small. 2018;14(30):e1800922. https://doi.org/10.1002/smll.201800922.
Pinget G, Tan J, Janac B, Kaakoush NO, Angelatos AS, O'Sullivan J, et al. Impact of the food additive titanium dioxide (E171) on gut microbiota-host interaction. Front Nutr. 2019;6:57. https://doi.org/10.3389/fnut.2019.00057.
Jensen DM, Løhr M, Sheykhzade M, Lykkesfeldt J, Wils RS, Loft S, et al. Telomere length and genotoxicity in the lung of rats following intragastric exposure to food-grade titanium dioxide and vegetable carbon particles. Mutagenesis. 2019;34(2):203–14. https://doi.org/10.1093/mutage/gez003.
Hong F, Wu N, Zhou Y, Ji L, Chen T, Wang L. Gastric toxicity involving alterations of gastritis-related protein expression in mice following long-term exposure to nano TiO2. Food Res Int. 2017;95:38–45. https://doi.org/10.1016/j.foodres.2017.02.013.
Acknowledgements
None.
Funding
This work was supported by the French French National Research Agency (ANR) [grant numbers: ANR-16-CE34–0011-01, PAIPITO and ANR-20-CE34–010-03, INPAGE]. It is a contribution to the Labex Serenade [grant number: ANR-11-LABX-0064] funded by the French Government’s “Investissements d’Avenir” ANR program, through the A*MIDEX project [grant number: ANR-11-IDEX-0001-02]. It has been supported by the French Atomic Energy and Alternative Energies Commission (CEA) through the ‘Toxicology’ research program [grant: IMAGINATOX], the French Environment and Energy Management Agency (ADEME), the French Plan Cancer [grant: SiFoodCan grant] and by grants from the Institut National de la Santé et de la Recherche Médicale (INSERM) and the Association François Au Petit (AFA).
Author information
Authors and Affiliations
Contributions
FB and MC are the guarantors of the article. FB, MC, CT, AF and SM performed the bibliographic research, wrote and revised the manuscript. All authors approved the final version of the manuscript and approved its submission.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Barreau, F., Tisseyre, C., Ménard, S. et al. Titanium dioxide particles from the diet: involvement in the genesis of inflammatory bowel diseases and colorectal cancer. Part Fibre Toxicol 18, 26 (2021). https://doi.org/10.1186/s12989-021-00421-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12989-021-00421-2