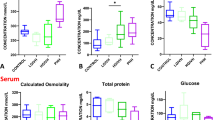
Abstract
Background
Interpretation of cerebrospinal fluid (CSF) studies can be challenging in preterm infants. We hypothesized that intraventricular hemorrhage (IVH), post-hemorrhagic hydrocephalus (PHH), and infection (meningitis) promote pro-inflammatory CSF conditions reflected in CSF parameters.
Methods
Biochemical and cytological profiles of lumbar CSF and peripheral blood samples were analyzed for 81 control, 29 IVH grade 1/2 (IVH1/2), 13 IVH grade 3/4 (IVH3/4), 15 PHH, 20 culture-confirmed bacterial meningitis (BM), and 27 viral meningitis (VM) infants at 36.5 ± 4 weeks estimated gestational age.
Results
PHH infants had higher (p < 0.02) CSF total cell and red blood cell (RBC) counts compared to control, IVH1/2, BM, and VM infants. No differences in white blood cell (WBC) count were found between IVH3/4, PHH, BM, and VM infants. CSF neutrophil counts increased (p ≤ 0.03) for all groups compared to controls except IVH1/2. CSF protein levels were higher (p ≤ 0.02) and CSF glucose levels were lower (p ≤ 0.003) for PHH infants compared to all other groups. In peripheral blood, PHH infants had higher (p ≤ 0.001) WBC counts and lower (p ≤ 0.03) hemoglobin and hematocrit than all groups except for IVH3/4.
Conclusions
Similarities in CSF parameters may reflect common pathological processes in the inflammatory response and show the complexity associated with interpreting CSF profiles, especially in PHH and meningitis/ventriculitis.
Similar content being viewed by others
Introduction
Analysis of cerebrospinal fluid (CSF) is an essential component of the evaluation and treatment of newborn neurological and infectious disorders. Classically, the CSF profile of infectious meningitis, for example, comprises pleocytosis with a preponderance of white blood cells (WBC), elevated protein levels, and low glucose levels [1, 2]. However, CSF differential cell count and biochemical profile may be influenced by a myriad of factors, all of which may occur on a systemic inflammatory background associated with preterm birth and/or comorbid conditions [3, 4].
Intraventricular hemorrhage (IVH), and resultant post-hemorrhagic hydrocephalus (PHH), are among the most common, severe neurological complications of preterm birth, and infants frequently require CSF sampling for diagnostic or therapeutic purposes [5, 6]. Growing evidence suggests that the antecedent IVH and PHH are associated with host-immune responses that can alter the CSF profile [7,8,9,10,11,12], potentially prompting empiric antimicrobial therapy on suspicion of infection or delaying time-sensitive surgical care [13, 14].
We hypothesized that IVH, PHH, and meningitis promote pro-inflammatory CSF states that are reflected in CSF/peripheral blood parameters and show common pathological inflammatory processes. We compared the CSF and peripheral blood of infants with viral (VM) and bacterial (BM) meningitis to infants with IVH or PHH (without infection) and control infants.
Methods
Patient selection and sample collection
CSF and peripheral blood profiles of 6 groups of infants at an estimated gestational age (EGA) of 36.5 ± 4 weeks were assessed: 1) Control: no known neurological disease who required CSF sampling for routine sepsis evaluation but were found to have sterile CSF; 2) IVH1/2: Papile Grade 1/2 IVH identified on cranial ultrasound but no hydrocephalus [15, 16] who had sterile CSF; 3) IVH3/4: Papile Grade 3/4 IVH without hydrocephalus [15, 16] who had sterile CSF; 4) PHH: hydrocephalic infants who required neurosurgical intervention and had sterile CSF, sample collected prior to surgery; 5) BM: culture-confirmed bacterial meningitis with growth of aerobic bacteria in CSF culture but with negative viral RNA on PCR and no IVH/PHH; and 6) VM: PCR-confirmed viral meningitis with negative bacterial cultures and no IVH/PHH. Well-established Hydrocephalus Clinical Research Network criteria were applied to identify the severity of the hemorrhage by ultrasound to determine IVH grade (1/2 or 3/4), the diagnosis of PHH, and neurosurgical intervention [5, 17]. Clinical signs and symptoms such as fever, poor feeding, vomiting, lethargy, and irritability were also evaluated [18,19,20,21], but lumbar puncture results were essential for establishing meningitis diagnosis [22].
All samples were acquired in the Neonatal Intensive Care Unit at St. Louis Children’s Hospital (October 2006-December 2016). CSF samples for all groups were obtained via lumbar puncture (LP) and analyzed at equivalent time points (Table 1). Peripheral blood samples were collected and analyzed within 0–72 h from the CSF sample. CSF microbiological cultures were monitored for 3.68 ± 0.13 days for bacterial growth [23]. Anaerobic CSF cultures were not routinely performed.
Data collection
CSF parameters were retrieved from the medical records: total cell counts, red blood cell (RBC) count, WBC counts (neutrophil, lymphocyte, monocyte, eosinophil, and macrophage counts), protein, glucose, microbial cultures, and PCR results. Control LP samples with RBC counts greater than 10,000 cells/µl but no xanthochromia were excluded to minimize the effects of traumatic sampling [24]. Peripheral blood sample profile included glucose, hemoglobin, hematocrit, WBC (including neutrophils), RBC, and platelets counts. The CSF/blood glucose ratio was calculated by dividing the CSF levels between the blood levels collected within 2 days of the CSF samples. Additional data recorded and analyzed included EGA at birth and sample collection, gender, postnatal steroids, premature rupture of the membrane (PROM), chorioamnionitis, lung disease [pneumothorax, pulmonary hemorrhage, pulmonary interstitial emphysema, persistent pulmonary hypertension of the newborn (PPHN), chronic lung disease, respiratory distress syndrome (RDS), and bronchopulmonary dysplasia (BPD)], sepsis, and clinical risk index for babies (CRIB) [25, 26] scores. Patients missing the data necessary to determine the study outcomes were not included.
Statistical analysis
Analyses were performed in STATA version 16.1 (StataCorp, College Station, TX) and GraphPad Software version 9.2.0 (San Diego, CA, USA). Groups were analyzed with Analysis of Variance (ANOVA) with Tukey–Kramer adjustment to correct for multiple pairwise comparisons. Variance components for each group were estimated to control for unequal variance when necessary. Fisher’s exact test was applied to compare categorical data. CRIB scores, PROM, lung disease, and steroid treatment were collected in preterm subjects and used for exploratory analyses through simple linear regressions [27, 28]. A two-sided p < 0.05 was considered statistical significant. Groups were blinded for all analyses.
Results
Patient Characteristics
CSF profile data from 185 infants were analyzed, 77 (41.6%) females and 108 (58.4%) males: 81 controls, 29 IVH1/2, 13 IVH3/4, 15 PHH, 20 culture-confirmed BM, and 27 PCR-confirmed VM. Post-menstrual age (PMA) at birth was 34.9 ± 5.3 weeks, and EGA at sampling was 36.5 ± 4 weeks (Table 1). Postnatal steroids were administered in 15 (8.1%) patients. PROM was more common in PHH vs. control and VM groups, but the percentage of patients with chorioamnionitis was similar among groups except for IVH3/4 vs. VM. Patients with lung disease were more frequent in PHH compared to all other groups. Sepsis was more frequent in all the other groups compared to control and VM groups (Table 1). In PHH, PROM, lung disease, and steroid treatment did not correlate with CSF and blood parameters except for steroid treatment and hemoglobin (R [2] = 0.3 p = 0.039). The exploratory analyses of CRIB scores across IVH1/2, IVH3/4, and PHH groups showed no consistent correlations with CSF/peripheral blood parameters (Additional file 1: Table S1). No correlations between CRIB scores and CSF and serum parameters were found except for CSF lymphocytes (R [2] = 0.3, p = 0.03), serum red blood cells (R [2] = 0.3, p = 0.039), hemoglobin (R [2] = 0.46, p = 0.0075), and hematocrit (R [2] = 0.38, p = 0.018) in PHH; CSF protein levels (R [2] = 0.43, p = 0.0001) in IVH1/2; serum platelets (R [2] = 0.71, p = 0.001) in IVH3/4; and estimated gestational age at birth in PHH (R [2] = 0.43, p = 0.0081) and both IVHs (R [2] = 0.76, p < 0.0001; R [2] = 0.81, p < 0.0001).
The most common organism present in the CSF of BM samples was coagulase-negative Staphylococcus in 7 (35%) patients. For VM samples, the most common virus was Enterovirus in 21 (78%) patients (Additional file 2: Table S2). 67 (38%) of total patients had received antibiotics within 0.5 ± 0.9 days prior to their CSF collection but subgroup analyses did not reveal any associations between antibiotics and CSF profile, except for peripheral blood platelets.
CSF Cell counts
PHH group had significantly higher (p < 0.02) total cell counts (7925 ± 16,016 cells/µl), including RBC counts (7829 ± 16,017 cells/µl), compared to all other groups except for IVH3/4. There were no differences in total cells, WBC, or RBC counts between the IVH1/2, IVH3/4, BM and VM groups (Fig. 1; Tables 2 and 3). There was no difference in total cells, RBCs, WBCs, when comparing BM and VM organism subcategories (Additional file 3: Table S3).
Cerebrospinal fluid total cell, white blood cell, and neutrophil counts profile. A Cerebrospinal fluid total cell count, B white blood cell (WBC) count, and C the percentage of neutrophils variations across groups. The # denotes the difference with the PHH group. The † denotes the difference with the control group. For specific p-values, see Table 3. BM, bacterial meningitis; IVH, intraventricular hemorrhage; PHH, post-hemorrhagic hydrocephalus; VM, viral meningitis
The PHH group had significantly higher mean WBC counts than the control (p = 0.012) and IVH1/2 (p = 0.039) groups (Fig. 1; Tables 2 and 3). On WBC differential analyses, neutrophil counts of all groups significantly differed from controls except for IVH1/2 (p ≤ 0.05). The PHH cohort had significantly higher neutrophil counts than every group save for the VM and IVH3/4 groups. WBC counts were not different between infants with CNS infections. The remaining nucleated cell lines did not demonstrate consistent differences between groups except for eosinophils in IVH1/2 compared to control (p < 0.001), BM (p = 0.01), and VM (p < 0.001; Fig. 1; Tables 2 and 3).
CSF protein levels
Protein levels in the PHH group were significantly higher than that of all groups (p ≤ 0.001; Fig. 2A; Tables 2 and 3). There was no difference between the BM and VM groups, although the BM group significantly differed from controls (p = 0.005). The IVH1/2 group had higher protein levels than the control group (p = 0.045) but did not differ from the BM group. However, the IVH3/4 group had significantly higher protein levels than the VM group (p < 0.001; Fig. 2A; Tables 2 and 3).
Cerebrospinal fluid protein and glucose concentrations. A Cerebrospinal fluid protein, and B glucose concentrations. The # denotes the difference with the PHH group. The † denotes the difference with the control group. For specific p-values, see Table 3. BM, bacterial meningitis; IVH, intraventricular hemorrhage; PHH, post-hemorrhagic hydrocephalus; VM, viral meningitis
CSF glucose levels
CSF glucose levels were significantly lower in the PHH group than all other groups (p ≤ 0.003; Fig. 2B; Tables 2 and 3). There were no differences between BM and VM groups, and these groups did not differ from the control group. Neither IVH group differed from any other group besides the PHH group (p ≤ 0.003) (Fig. 2B; Tables 2 and 3). Furthermore, CSF/blood glucose ratios were significantly lower (p < 0.004) in the PHH (0.34 ± 0.14) and IVH3/4 (0.38 ± 0.13) groups compared to the control group (0.65 ± 0.26), and the PHH glucose ratio was lower (p = 0.0406) than VM (0.63 ± 0.15).
Peripheral blood biochemical and cellular profiles
The PHH group had significantly higher peripheral blood WBC cell counts compared to control (p < 0.001), BM (p = 0.001), VM (p < 0.001), and IVH1/2 (p = 0.001) groups (Fig. 3; Tables 2 and 3). There were no differences between the non-PHH groups. In contrast, PHH infants had lower hemoglobin and hematocrit levels compared to IVH1/2 (p = 0.006), VM (p = 0.022 and p = 0.018), BM (p = 0.001) and control (p = 0.008 and p = 0.01) groups. There were no differences between the non-PHH groups with the exception of the IVH3/4 and BM groups (p = 0.024; Fig. 3; Tables 2 and 3).
Peripheral blood white blood cells, hemoglobin, hematocrit, and platelets profile. A Peripheral blood white blood cell (WBC) counts, B hemoglobin, C hematocrit, and D platelet counts across groups. The # denotes the difference with the PHH group. The † denotes the difference with the control group. For specific p-values, see Table 3. BM, bacterial meningitis; IVH, intraventricular hemorrhage; PHH, post-hemorrhagic hydrocephalus; VM, viral meningitis
Discussion
This study characterizes the spectrum of CSF biochemical and cellular profiles in PHH and non-PHH infants both with and without CNS infections. PHH CSF had the highest cell count and protein levels and the lowest glucose levels among all infants. PHH group had higher peripheral blood WBCs, lower hemoglobin and lower percent hematocrit than control, IVH1/2, BM, and VM groups, and higher peripheral blood WBCs compared to BM and VM groups. These similarities in CSF and peripheral blood counts suggest common pathological inflammatory processes among groups and reflex the complexity in interpretating CSF profiles.
PHH and BM demonstrated similar patterns of biochemical changes when compared to controls suggesting no set thresholds to distinguish infected from non-infected samples. While we assessed for the role of WBC as a complement to facilitate such decisions, we did not identify any reliable trends. We did identify a higher peripheral blood WBC counts in PHH infants compared to controls, IVH1/2, VM, and BM infants, but this may be an artifact of co-morbid conditions and/or prior infection and should be interpreted with caution. PHH infants may be more seriously ill than non-PHH infants, which also likely yields higher peripheral WBC counts.
Differentiating PHH and BM CSF findings is an important clinical challenge, as CSF profiles are often used as a basis for initiating empiric antimicrobial therapy or deferring intervention when CNS infection is suspected in a PHH infant [29]. While the consequences of untreated CNS infection can be devastating, preemptive therapies are not without risks [30]; therefore, caution should be exercised in the use of empiric antibiotic treatment in PHH based on their CSF profiles alone, as the CSF altered profile is likely simply reflective of PHH pathophysiology. Treatment recommendations would change, of course, if there were other clinical factors to suggest an infection such as sustained fevers, altered level of consciousness, identified extracranial source of infection, or sepsis. Uncovering new biomarkers for diagnosing meningitis and determining antibiotic prescription among preterm infants is needed and will help to differentiate infections versus other diseases and the timing of the treatment [31].
Classically, BM is characterized by elevated CSF protein, WBCs and decreased CSF glucose [32]. Elevated WBCs have been attributed to the effects of the inflammatory response to infection [33]. Cytokines and chemokines are produced and trigger an inflammatory cascade including interleukin-1 (IL-1), which increases the permeability of the blood–brain barrier (BBB) [34]. Greater BBB permeability permits proteins from systemic circulation to enter the subarachnoid space, raising CSF protein levels. Pro-inflammatory mediators such as tumor necrosis factor alpha (TNF-α) and IL-1 also correlate with the production of nitric oxide metabolites, which decrease oxygen uptake and yield increased CSF lactate and decreased CSF glucose levels through anaerobic glycolysis [33,34,35].
The VM group was comprised of subjects infected by four different viruses, the most common of which was enterovirus. The sequelae of VM are typically similar to that of BM, albeit less severe. Once the virus crosses the BBB it also triggers a response from lymphocytes and inflammatory cytokines [36]. Because the inflammatory response differs depending on the infecting pathogen, the CSF profile characteristic of a viral infection is variable, while the CSF usually demonstrates elevated WBC counts and protein levels [36]. Also, a compromised BBB may result in increased CSF protein levels as well as elevated total cell count [37].Elevated WBC counts and protein levels were seen in the VM cohort, which had increased WBC counts but only moderately higher protein levels from controls. However, unlike the BM cohort, glucose in the VM cohort remained around the normal level. Moving forward, insight into inflammatory processes may be provided through the addition of CSF markers such as cytokines and chemokines levels to CSF cell profiling.
Few previous studies have investigated CSF parameters in children with PHH [38, 39]. The protein and glucose levels identified in our PHH group were close to the range of the levels reported by both of the aforementioned studies. The mean CSF WBC count in our PHH group, exclusively obtained via LP, was more than they reported. Regarding these notable differences, the authors posited that large increases in CSF cell counts and proteins may be attributed to insertions of reservoirs, as significant decreases in CSF protein and neutrophils were observed over serial samples. Our study analyzed CSF samples prior to reservoir insertion suggesting that these alterations might not be related to ventricular diversion but from broader inflammatory process. Additionally, our study adds new comparisons to previous literature as it includes blood parameters and multiple comparison groups to assess if infection could be discerned in the absence of a positive culture. We also included CSF/blood glucose ratio as a helpful measurement for differential diagnosis [40, 41]. Thus, the increased CSF and peripheral blood cell counts detected in PHH infants might be associated with early inflammatory processes inherent to the disease and not related to device insertion.
Exacerbated alterations of CSF composition in PHH is suggestive of the activation of common inflammatory pathways in the PHH and BM groups. Recently, studies have demonstrated strong associations between CSF inflammatory markers and PHH including IL-1β, IL-6, and TNF-α [8,9,10,11,12]. Additionally, Karimy et al. [42] found that IVH caused CSF hypersecretion in an inflammatory-dependent manner involving TLR4 and NF-κB signaling. Similar inflammatory markers have been demonstrated in the pathophysiology of BM [33]. CSF IL-6 and IL-10 were found to be strong predictors of culture-proven BM [35]. Together, these data strengthen the argument that PHH and BM involve similar inflammatory processes [43]. Supporting this hypothesis, PHH together with IVH, showed the highest number of patients with PROM, lung disease, and sepsis. PROM is associated with several neonatal diseases, and it is one of the major risk factors for neonatal sepsis [44], and lung diseases [45]. The pro-inflammatory molecules IL-6, IL-8, and TNF-α, which are increased in PHH, have also been associated with neonatal sepsis with PROM [46]. However, whether inflammation drives development of PHH or vice-versa is unclear and requires additional investigation. It is important to note that the higher CSF total cells in the PHH group compared to the BM group was likely driven by far higher levels of RBCs in the PHH group.
Limitations of this study include differences between study groups: PMA at birth and EGA at sampling, use of antibiotics, comorbid illnesses, and timing of sample collection. Although many of our subjects received empirically administered antibiotics prior to sample procurement, our analysis on the effect of antibiotics demonstrated no major differences between those treated and not treated with antibiotics. Data in the literature on the effect of antibiotics in CSF parameters are inconclusive. Srinivasan et al. [47] found antibiotics had no effect on CSF parameters, while Nigrovic et al. [48] found significant effects on CSF protein and glucose. Additional limitations of the study include its retrospective nature and the small sample sizes in certain groups. Further studies with larger samples obtained prospectively, as well as a comparative cohort study of PHH infants with culture-proven infection, would further delineate the differences in CSF profiles. Finally, systematic data on antenatal steroid use and hypertension were missing, which could have altered inflammatory factors in CSF after birth.
In conclusion, CSF profiles of patients with PHH included elevated CSF protein, total cells, RBCs, and WBCs (neutrophils and monocytes) with decreased glucose levels compared to healthy values. The PHH CSF profile was similar to that of BM or VM when compared to controls, and may be more extreme in terms of protein, glucose, RBC, and total cell counts. There was variation in PHH blood parameters compared with control, VM, and BM groups, with higher peripheral blood WBC count and lower hemoglobin and percent hematocrit. Infants with IVH had similar CSF profiles to PHH but less exacerbated alterations. The similarities in CSF among these groups may reflect common pathological processes in the inflammatory response. Therefore, CSF profile alone should not dictate the administration of empiric antimicrobial therapy in preterm IVH/PHH neonates until CNS infection is culture-proven or supported by high clinical suspicion.
Availability of data and materials
Data are available upon request.
References
Majumdar A, Jana A, Jana A, Biswas S, Bhatacharyya S, Bannerjee S. Importance of normal values of CSF parameters in term versus preterm neonates. J Clin Neonatol. 2013;2:166–8.
Thomson J, Sucharew H, Cruz AT, et al. Cerebrospinal fluid reference values for young infants undergoing lumbar puncture. Pediatrics. 2018. https://doi.org/10.1542/peds.2017-3405.
Melville JM, Moss TJ. The immune consequences of preterm birth. Front Neurosci. 2013;7:79.
Cuenca AG, Wynn JL, Moldawer LL, Levy O. Role of innate immunity in neonatal infection. Am J Perinatol. 2013;30:105–12.
Wellons JC 3rd, Shannon CN, Holubkov R, et al. Shunting outcomes in posthemorrhagic hydrocephalus: results of a hydrocephalus clinical research network prospective cohort study. J Neurosurg Pediatr. 2017;20:19–29.
Mathews TJ, Miniño AM, Osterman MJ, Strobino DM, Guyer B. Annual summary of vital statistics: 2008. Pediatrics. 2011;127:146–57.
Strahle J, Garton HJ, Maher CO, Muraszko KM, Keep RF, Xi G. Mechanisms of hydrocephalus after neonatal and adult intraventricular hemorrhage. Transl Stroke Res. 2012;3:25–38.
Sävman K, Blennow M, Hagberg H, Tarkowski E, Thoresen M, Whitelaw A. Cytokine response in cerebrospinal fluid from preterm infants with posthaemorrhagic ventricular dilatation. Acta Paediatr. 2002;91:1357–63.
Baumeister FA, Pohl-Koppe A, Hofer M, Kim JO, Weiss M. IL-6 in CSF during ventriculitis in preterm infants with posthemorrhagic hydrocephalus. Infection. 2000;28:234–6.
Schmitz T, Heep A, Groenendaal F, et al. Interleukin-1beta, interleukin-18, and interferon-gamma expression in the cerebrospinal fluid of premature infants with posthemorrhagic hydrocephalus–markers of white matter damage? Pediatr Res. 2007;61:722–6.
Sival DA, Felderhoff-Müser U, Schmitz T, Hoving EW, Schaller C, Heep A. Neonatal high pressure hydrocephalus is associated with elevation of pro-inflammatory cytokines IL-18 and IFNgamma in cerebrospinal fluid. Cerebrospinal Fluid Res. 2008;5:21.
Habiyaremye G, Morales DM, Morgan CD, et al. Chemokine and cytokine levels in the lumbar cerebrospinal fluid of preterm infants with post-hemorrhagic hydrocephalus. Fluids Barriers CNS. 2017;14:35.
Cizmeci MN, Groenendaal F, Liem KD, et al. Randomized controlled early versus late ventricular intervention study in posthemorrhagic ventricular dilatation: outcome at 2 years. J Pediatr. 2020;226:28-35.e23.
de Vries LS, Groenendaal F, Liem KD, et al. Treatment thresholds for intervention in posthaemorrhagic ventricular dilation: a randomised controlled trial. Arch Dis Child Fetal Neonatal Ed. 2019;104:F70-f75.
Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1500 gm. J Pediatr. 1978;92:529–34.
Patra K, Wilson-Costello D, Taylor HG, Mercuri-Minich N, Hack M. Grades I-II intraventricular hemorrhage in extremely low birth weight infants: effects on neurodevelopment. J Pediatr. 2006;149:169–73.
Wellons JC 3rd, Holubkov R, Browd SR, et al. The assessment of bulging fontanel and splitting of sutures in premature infants: an interrater reliability study by the hydrocephalus clinical research network. J Neurosurg Pediatr. 2013;11:12–4.
El Bashir H, Laundy M, Booy R. Diagnosis and treatment of bacterial meningitis. Arch Dis Child. 2003;88:615–20.
Franco-Paredes C, Lammoglia L, Hernández I, Santos-Preciado JI. Epidemiology and outcomes of bacterial meningitis in Mexican children: 10-year experience (1993–2003). Int J Infect Dis. 2008;12:380–6.
Sáez-Llorens X, McCracken GH Jr. Bacterial meningitis in children. Lancet. 2003;361:2139–48.
McGill F, Griffiths MJ, Solomon T. Viral meningitis: current issues in diagnosis and treatment. Curr Opin Infect Dis. 2017;30:248–56.
Brouwer MC, Tunkel AR, van de Beek D. Epidemiology, diagnosis, and antimicrobial treatment of acute bacterial meningitis. Clin Microbiol Rev. 2010;23:467–92.
Morales DM, Silver SA, Morgan CD, et al. Lumbar cerebrospinal fluid biomarkers of posthemorrhagic hydrocephalus of prematurity: amyloid precursor protein, soluble amyloid precursor protein α, and L1 cell adhesion molecule. Neurosurgery. 2017;80:82–90.
Novak RW. Lack of validity of standard corrections for white blood cell counts of blood-contaminated cerebrospinal fluid in infants. Am J Clin Pathol. 1984;82:95–7.
The International Neonatal Network. The CRIB (clinical risk index for babies) score: a tool for assessing initial neonatal risk and comparing performance of neonatal intensive care units. Lancet. 1993;342:193–8.
Parry G, Tucker J, Tarnow-Mordi W. CRIB II: an update of the clinical risk index for babies score. Lancet. 2003;361:1789–91.
Kinoshita M, Hawkes CP, Ryan CA, Dempsey EM. Perfusion index in the very preterm infant. Acta Paediatr. 2013;102:e398–401.
Akima S, Kent A, Reynolds GJ, Gallagher M, Falk MC. Indomethacin and renal impairment in neonates. Pediatr Nephrol. 2004;19:490–3.
Tunkel AR, Hasbun R, Bhimraj A, et al. 2017 infectious diseases society of America’s clinical practice guidelines for healthcare-associated ventriculitis and meningitis. Clin Infect Dis. 2017;64:e34–65.
Esaiassen E, Fjalstad JW, Juvet LK, van den Anker JN, Klingenberg C. Antibiotic exposure in neonates and early adverse outcomes: a systematic review and meta-analysis. J Antimicrob Chemother. 2017;72:1858–70.
Mukhopadhyay S, Sengupta S, Puopolo KM. Challenges and opportunities for antibiotic stewardship among preterm infants. Arch Dis Child Fetal Neonatal Ed. 2019;104:F327-f332.
Ku LC, Boggess KA, Cohen-Wolkowiez M. Bacterial meningitis in infants. Clin Perinatol. 2015;42:29–45.
van Furth AM, Roord JJ, van Furth R. Roles of proinflammatory and anti-inflammatory cytokines in pathophysiology of bacterial meningitis and effect of adjunctive therapy. Infect Immun. 1996;64:4883–90.
Mustafa MM, Ramilo O, Sáez-Llorens X, Olsen KD, Magness RR, McCracken GH Jr. Cerebrospinal fluid prostaglandins, interleukin 1 beta, and tumor necrosis factor in bacterial meningitis. Clinical and laboratory correlations in placebo-treated and dexamethasone-treated patients. Am J Dis Child. 1990;144:883–7.
Srinivasan L, Kilpatrick L, Shah SS, Abbasi S, Harris MC. Cerebrospinal fluid cytokines in the diagnosis of bacterial meningitis in infants. Pediatr Res. 2016;80:566–72.
Chadwick DR. Viral meningitis. Br Med Bull. 2005;75–76:1–14.
Kadry H, Noorani B, Cucullo L. A blood–brain barrier overview on structure, function, impairment, and biomarkers of integrity. Fluids Barriers CNS. 2020;17:69.
Bajaj M, Lulic-Botica M, Natarajan G. Evaluation of cerebrospinal fluid parameters in preterm infants with intraventricular reservoirs. J Perinatol. 2012;32:786–90.
Lenfestey RW, Smith PB, Moody MA, et al. Predictive value of cerebrospinal fluid parameters in neonates with intraventricular drainage devices. J Neurosurg. 2007;107:209–12.
Verbeek MM, Leen WG, Willemsen MA, Slats D, Claassen JA. Hourly analysis of cerebrospinal fluid glucose shows large diurnal fluctuations. J Cereb Blood Flow Metab. 2016;36:899–902.
Tan QC, Xing XW, Zhang JT, et al. Correlation between blood glucose and cerebrospinal fluid glucose levels in patients with differences in glucose metabolism. Front Neurol. 2023;14:1103026.
Karimy JK, Zhang J, Kurland DB, et al. Inflammation-dependent cerebrospinal fluid hypersecretion by the choroid plexus epithelium in posthemorrhagic hydrocephalus. Nat Med. 2017;23:997–1003.
Karimy JK, Reeves BC, Damisah E, et al. Inflammation in acquired hydrocephalus: pathogenic mechanisms and therapeutic targets. Nat Rev Neurol. 2020;16:285–96.
Pasquier JC, Picaud JC, Rabilloud M, et al. Neonatal outcomes after elective delivery management of preterm premature rupture of the membranes before 34 weeks’ gestation (DOMINOS study). Eur J Obstet Gynecol Reprod Biol. 2009;143:18–23.
Ernest JM. Neonatal consequences of preterm PROM. Clin Obstet Gynecol. 1998;41:827–31.
Velemínský M Jr, Stránský P, Velemínský M Sr, Tosner J. Relationship of IL-6, IL-8, TNF and sICAM-1 levels to PROM, pPROM, and the risk of early-onset neonatal sepsis. Neuro Endocrinol Lett. 2008;29:303–11.
Srinivasan L, Shah SS, Padula MA, Abbasi S, McGowan KL, Harris MC. Cerebrospinal fluid reference ranges in term and preterm infants in the neonatal intensive care unit. J Pediatr. 2012;161:729–34.
Nigrovic LE, Malley R, Macias CG, et al. Effect of antibiotic pretreatment on cerebrospinal fluid profiles of children with bacterial meningitis. Pediatrics. 2008;122:726–30.
Acknowledgements
We would like to acknowledge the contributions of Alexis Elward, MD, MPH for her expert insights into the study design and newborn infectious and inflammatory disorders. We also thank Nicholas Reinhold for helping with the medical records.
Author information
Authors and Affiliations
Contributions
MGB, ATY, AMI, DMM, and BB performed data collection and analyses and wrote the manuscript. SHA and HB helped with data collection, and SHA and RHH with the statistical analysis. AMM, JPM, JMS, and CDS contributed to the study design and analyses, and all edited the manuscript. DDL conceived the study and its design, assisted data collection and analyses, and edited the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Approval from Washington University Human Research Protection Office was acquired prior to initiation of this study (IRB #201203126). Informed consent was waived per University’s Human Research Protection Office.
Consent for publication
Not applicable.
Competing interests
Dr. Limbrick has received research funding for unrelated projects from Microbot Medical, Inc., and research equipment through Medtronic, Inc. All the other authors declare no potential competing interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
Percentage of preterm neonates in the CRIB score categories.
Additional file 2: Table S2.
Bacterial and viral organisms identified in the cerebrospinal fluid of infants with meningitis.
Additional file 3: Table S3.
Cell counts by organism for bacterial and viral meningitis groups.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Garcia-Bonilla, M., Yahanda, A.T., Isaacs, A.M. et al. Pro-inflammatory cerebrospinal fluid profile of neonates with intraventricular hemorrhage: clinical relevance and contrast with CNS infection. Fluids Barriers CNS 21, 17 (2024). https://doi.org/10.1186/s12987-024-00512-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12987-024-00512-0