Abstract
Background
Interplay between gut microbiota and heart, termed “gut-heart” axis, has a crucial role in the pathogenesis of atherosclerosis. Our previous study showed that lycopene possesses anti-inflammatory and anti-atherosclerotic effects, but its link to the gut microbiota is poorly understood. Herein, we surmised that lycopene could regulate the gut microbiota, exert anti-atherosclerotic effect by regulating the “gut-heart” axis.
Methods
Male ApoE−/− mice were fed a high-fat diet (HFD) with or without lycopene (0.1% w/w) for 19 weeks. Gut microbiota was analyzed by 16 S rRNA sequencing, the protein levels of zonula occludens-1 (ZO-1), occludin, toll-like receptor 4 (TLR4) and phospho-nuclear factor-κB (NF-κB) p65 were measured by Western blotting, the levels of serum inflammatory factors including monocyte chemotactic protein 1 (MCP-1), tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and IL-6 were assayed using ELISA kits. Also, the concentrations of serum lipopolysaccharide (LPS), D-lactic acid (D-LA) and diamine peroxidase (DAO) were measured through ELISA method.
Results
The aortic sinus sections revealed that lycopene supplementation significantly reduced the extent of atherosclerotic lesions and inhibited atherosclerosis development caused by HFD. The analysis of gut microbiota showed that lycopene reduced the ratio of Firmicutes/Bacteroides and increased the relative abundance of Verrucomicrobia, Akkermansia and Alloprevotella, which were related to elevated intestinal barrier function and reduced inflammation. Moreover, lycopene up-regulated the expression of intestinal ZO-1 and occludin and decreased serum LPS, D-LA and DAO levels. In addition, lycopene inhibited the expression of TLR4 and phospho-NF-κB p65 in aortic sinus plaque, serum MCP-1, TNF-α, IL-1β, and IL-6 levels were also lowered by lycopene treatment.
Conclusions
Our results indicated the protective effect of lycopene against atherosclerosis induced by HFD and further revealed that its mechanism might be its prebiotic effect on maintaining gut microbiota homeostasis and improving intestinal barrier function, consequently reducing serum LPS-triggered inflammatory response in the heart.
Similar content being viewed by others
Introduction
Atherosclerosis and its related cardiovascular diseases still have an extremely high morbidity and mortality rate globally, despite remarkable advances in their prevention, diagnosis and treatment [1]. The pathological signs of atherosclerosis consist of lipid infiltration and plaque formation in arteries. However, the molecular mechanism of atherosclerotic plaque formation has not yet been determined, although studies have linked it to genetics, diet, environmental factors, inflammation and autoimmune disorders [2, 3].
Emerging evidence has reported a strong association between the gut microbiota dysbiosis and the development of atherosclerosis, thus the concept of the “gut-heart” axis has been proposed [4, 5]. A high-fat diet (HFD) is a well-established risk factor for atherosclerosis. Recent research has shown that alterations in gut microbial diversity caused by HFD are directly related to the pathogenesis of atherosclerosis, which is initiated through the activation of the gut-heart axis [6]. Gut microbiota dysbiosis can impair the intestinal barrier function by disrupting intestinal tight junction proteins including zonula occludens-1 (ZO-1) and occludin, thereby increasing intestinal permeability and allowing more lipopolysaccharide (LPS) to enter the circulation, which in turn stimulates the toll-like receptor 4 (TLR4)/nuclear factor-kappa B (NF-κB) signal cascades, thus activating various inflammatory factors and potentially resulting in atherosclerosis [5]. The atherosclerotic apolipoprotein E knockout (ApoE−/−) mice showed significantly increased intestinal permeability due to the disruption of intestinal tight junction proteins [7], suggesting that intestinal barrier function might also be crucial in the development of atherosclerosis.
Lycopene is a type of fat-soluble carotenoid found in red fruits such as tomatoes, apricots, papayas, and watermelons [8]. In Western diets, lycopene consumption is high, with a total intake of nearly 2~5 mg/d, and the physiological content of lycopene is approximately 1~2 µmol/L [8, 9]. According to multiple research, lycopene has antioxidant, anti-inflammatory and lipid-lowering properties [8,9,10,11]. Dietary lycopene supplementation has also been found to improve gut microbiota dysbiosis and intestinal barrier function in weaned piglets and alleviate stress from early weaning [12]. In addition, epidemiologic research has demonstrated a negative correlation between serum lycopene concentration and atherosclerosis [13]. Supplementation with lycopene has also been shown to protect against HFD-induced atherosclerosis in ApoE−/− mice [14]. The underlying anti-atherogenic mechanism of lycopene may be related to its antioxidant, anti-inflammatory and lipid-lowering effects [9]. Since the development of atherosclerosis is also influenced by the gut microbiota dysbiosis, we, herein, addressed a question whether lycopene can achieve this anti-atherosclerotic effect by inhibiting gut microbiota dysbiosis and consequently reducing serum LPS-triggered inflammatory response in the heart and suppressing atherosclerosis.
Therefore, we hypothesized that lycopene could effectively protect gut microbiota dysbiosis induced by the high-fat diet, reduce intestinal permeability and blood LPS levels, thereby inhibiting cardiovascular TLR4/NF-κB signal cascade and preventing the progression of atherosclerosis. To that end, we selected ApoE−/− mice that were given a long-term high-fat diet, which is the most commonly used animal model to simulate atherosclerosis.
Materials and methods
Animal model and treatment
Male ApoE−/− mice at the age of six weeks were provided by Vital River Laboratory Animal Technology Co. Ltd (Beijing, China). The animals were maintained in a SPF grade and regulated environment (temp. 22 ± 2℃ and a 12-hour day-night cycle). All mice were first given a standard chow diet for a week before being randomly allocated to one of three groups: low-fat diet (Control group, n = 8), high-fat diet (HFD group, n = 8), high-fat diet supplemented with 0.1% w/w lycopene (HFD + LY group, n = 8) for 19 weeks. Table 1 lists the diets’ constituents. As previously described [15], fecal samples were collected for each mouse separately and stored at -80 °C for extracting DNA profile of the gut microbiota. After an overnight fast, mice were anaesthetized with pentobarbital and sacrificed. Orbital blood was collected to obtain serum. Blood specimen, small intestine, aortas, and the entire heart were collected, separated and stored at -80℃ until needed. After that, 1mL of lysis buffer was used to dissolve the scraped intestinal mucosa.
The experimental animals procedures were conducted in accordance with the standards set by the Institutional Animal Care and Use Committee (IACUC) office of Sun Yat-sen University and was approved by them (No. 2019-035).
Biochemical analysis
Blood total cholesterol (TC), triglycerides (TG), low density lipoprotein cholesterol (LDL-C) and high density lipoprotein cholesterol (HDL-C) were assessed using the relevant enzymatic kits (Jiancheng, Nanjing, China) according to the kits instructions.
Enzyme-linked immunosorbent assay (ELISA)
Serum LPS, D-lactic acid (D-LA) and diamine peroxidase (DAO) concentrations were measured using ELISA kits (Mlbio, Shanghai, China). Also, the commercial ELISA kits were used to assay serum monocyte chemotactic protein 1 (MCP-1), tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and IL-6 levels (Neobioscience, Shenzhen, China). All of the experimental procedures were conducted according to the manuals’ guidelines.
Immunohistochemical analysis
Immunohistochemical assay was performed as previously described [15, 16], the intestinal tract and the aortic sinus were preserved in 4% paraformaldehyde, embedded in paraffin and dissected to 10 μm sections. Ileal and aortic sinus sections were treated with antigen retrieval (50×citrate sodium buffer, pH 6.0) and endogenous peroxidase inhibition (3% H2O2 for 15 min), followed by incubation with 1:200 diluted antibodies of ZO-1, occludin, TLR4 and phospho-NF-κB p65 (p-NF-κB p65) (Santa Cruz Biotechnology, Santa Cruz, CA, USA; Abcam, Cambridge, MA, USA) at 4℃ overnight. After washing, the sections were incubated with a peroxidase-conjugated secondary antibody at 37 ℃ for 30 min and labelled with diaminobenzidine, then counter-stained with Harris hematoxylin. The chromogenic slices were finally evaluated under a 200× or 40× magnification optical microscope for image analysis and acquisition.
Analysis of atherosclerotic lesions
As mentioned before [16], the heart was fixed with optimum cutting temperature compound (Tissue-Tek O.C.T. Compound, SAKURA, Japan) and frozen at -80℃. The heart was placed at -20℃ to make 10 μm thickness frozen sections when necessary. When three valves were present in the aortic sinus, sections were sliced until the valves disappeared. Oil red O staining of frozen sections was used to determine the atherosclerotic lesions in the aortic sinus. After being examined under a microscope with a magnification of 40 times, the size and area of atherosclerotic lesions were finally measured using Image J software (NIH, USA).
Western blotting
The expression levels of intestinal barrier proteins were quantitatively measured by Western blotting as described previously [15]. The supernatant of a lysed intestinal mucosal solution was taken for protein concentration determination and denaturation, the protein concentration was determined by the BCA Protein Assay Kit (Beyotime, Shanghai, China). The residual supernatant was electrophoresed on a 7.5% SDS-PAGE before being transferred to a nitrocellulose membrane. The transmembrane was then blocked and incubated with polyclonal primary ZO-1 and occludin antibodies (Abcam, Cambridge, MA, USA) with a dilution ratio of 1:1000 at 4 °C overnight. In addition, the protein in the aorta was extracted and subjected to electrophoresis and transmembrane, then the membranes were incubated with polyclonal primary TLR4, p-NF-κB P65 and NF-κB P65 antibodies (Affinity, USA) with a dilution ratio of 1:1000 at 4 °C overnight. Next, the membranes were incubated with 1:10000 diluted secondary antibodies at 25 °C for 2 h. GAPDH antibody was used as the loading control (Santa Cruz Biotechnology, Santa Cruz, CA, USA). Finally, Image J software (NIH, USA) was utilized to quantify and analyze the protein bands.
Fecal DNA extraction and gut microbiota analysis
As described previously [15], genomic DNA was extracted from feces using the QIAamp DNA Stool Mini Kit (Qiagen, Germany) according to the manufacturer’s recommendations. The V4 region of 16 S rRNA was amplified by PCR using primers 515 F and 806R, and then sequenced by Illumina Hiseq2500 platform. The R Programme Language was used to perform the analysis on the reads of the results. Calculations were done to determine the α-diversity, which included the Observed species, the Shannon index and the Simpson index. For the purpose of determining the level of β-diversity, a Principal Coordinates Analysis (PCoA) was carried out using Bray-Curtis as the distance metric. The test of Kruskal-Wallis and post hoc Dunn were performed to determine the relative abundance of gut microbiota at the phylum and genus levels. For alpha diversity analysis, the ggplot2, vegan, and ggpubr packages were installed firstly. The commands for calculating the Shannon index and Simpson index were in the vegan package, and the commands for calculating the significance of each group were in the ggpubr package; the ggplot2 command was used to draw a picture. To calculate the beta diversity analysis, we used the phyloseq package. For species difference analysis and abundance calculation, the reshape2, ggplot2, ggprism, and plyr packages were installed and used. The correlation coefficient between variables should be calculated by the built-in function cor () in R language and visualized by corrplot package. For data classification, based on the sequencing data obtained by Hiseq/Miseq platform, the Uclust method in QIIME software package was used for OTU cluster analysis according to the sequence similarity level of 97%. Then, based on the Silva reference database, the OTUs of each sample was annotated for species taxonomy. For quality control of sequencing data, we used cutadapt, a sequencing data quality control software, to remove the splice.
Spearman analysis was conducted to clarify the correlation between gut microbiota, lipids, inflammatory factors and intestinal permeability using R software. A corrplot package was used to perform the Spearman correlation.
Statistical analysis
SPSS statistical software(v19.0)was utilized for all analysis and the values were expressed as the means ± SD. Statistical analysis was conducted through one-way ANOVAs. The differences between groups were compared by SNK and LSD. LSD-t method has the highest sensitivity when comparing the mean of multiple groups, and can find the difference between groups to the maximum extent, which is suitable for preliminary verification trend. At the same time, in order to avoid the risk of Type I errors, a comprehensive pairwise comparison between all groups combined with SNK-q test was performed, so as to clarify the differences between groups and prevent false positives. The R Programming Language was used to analyze the gut microbiota. Pair-to-pair comparison for skewed data were performed using the Wilcox test, and three-group comparison were performed using the Kruskal-Wallis test. Statistical significance was assumed when p < 0.05.
Results
Basic parameters in ApoE−/− mice
As shown in Table 2, there was no statistically significant difference in body weight gain and daily food intake among the three groups (p > 0.05). Similarly, there was no significant difference in the weight of the heart or aorta among the three groups. According to the daily food intake of each mouse and 8 mice per group, the mean of daily calorie intake per group was about 150.4 kcal. In addition, lycopene was incorporated in the high-fat diet at a concentration of 1 g/kg of food (0.1% w/w), the mean of daily lycopene intake in HFD + lycopene group was about 32 mg.
Lycopene lowered blood lipids and serum atherogenic inflammatory factors
Serum TC, TG, LDL-C, TNF-α, MCP-1, IL-6 and IL-1β levels were the highest in the HFD group, while the HFD + lycopene group showed significantly reduced levels of serum TC, LDL-C, TNF-α, MCP-1, IL-6 and IL-1β (p < 0.05, p = 0.02, 0.03, 0.02, 0.01, 0.01, 0.02), but there was no difference in serum TG levels between HFD group and HFD + lycopene group. At the same time, the HFD group had the lowest serum HDL-C concentration, while the HDL-C concentration in HFD + lycopene group was markedly increased (p < 0.05, p = 0.03) (Table 2).
Lycopene suppressed Atherosclerosis progression in the aortic sinus
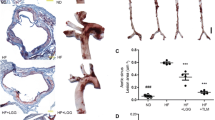
Oil Red O staining was applied to reveal the atherosclerotic lesions in the three groups. As shown in Fig. 1A, the percentage of atherosclerotic lesions was the lowest in the HFD + lycopene group, while the atherosclerotic lesions area in HFD group was larger than that in control group (p < 0.05, p = 0.01). However, the atherosclerotic lesions area was reduced by 84% in HFD + lycopene group compared to HFD group (Fig. 1B).
Lycopene supplementation inhibited the atherosclerosis of aortic sinus in HFD-fed ApoE−/− mice. ApoE−/− mice were fed a low-fat diet, a high-fat diet supplemented with or without 0.1% lycopene (w/w) for 19 weeks. (A) Oil red O staining of the aortic sinus is presented graphically (40×magnification), as described in Materials and Methods. The larger the area of red plaque, the deeper the atherosclerosis. (B) The ratio of the area of atherosclerotic plaque to the area of the aortic sinus was analyzed by Image J software. Data are presented as the mean ± SD (n = 8 per group); ap < 0.05 compared with the control group; bp < 0.05 compared with the high-fat diet group. HFD, high-fat diet; LY, lycopene
Lycopene modulated the diversity and composition of gut microbiota
In the α-diversity analysis, both the Observed species and the Shannon index were reduced in HFD group, while the Simpson index was increased in HFD group. However, lycopene administration improved the diversity of gut microbiota, the Observed species and Shannon index were elevated and the Simpson index was reduced after lycopene treatment (Fig. 2A-C). In the β-diversity analysis, the weighted PCoA diagrams of the three groups were shown in Fig. 2D, which reflected that the composition of gut microbiota in the HFD group was significantly different from the other two groups. Furthermore, a partial overlap was found between HFD + lycopene group and control group, indicating that lycopene supplementation could improve gut microbiota composition induced by HFD. In the heatmap of sample-sample distances, a similar effect was observed (Fig. 2E).
Lycopene supplementation improved the diversity and composition of gut microbiota in HFD-fed ApoE−/− mice. ApoE−/− mice were fed a low-fat diet, a high-fat diet supplemented with or without 0.1% lycopene (w/w) for 19 weeks. (A, B, C) The data of the alpha diversity was analyzed by the ggplot2, vegan, and ggpubr packages of R software. Data are presented as the mean ± SD (n = 6 per group); ap < 0.05 compared with the control group; bp < 0.05 compared with the high-fat diet group. (D, E) The data of the beta diversity was analyzed by the phyloseq package of R software. Figure D showed the specificity among three groups. Figure E showed the sample distance of three groups in heatmap; The redder the relationship was closer, while the bluer the relationship was more distant
Lycopene modulated the abundance of gut microbiota
To further analyze the alterations in gut microbiota composition, we analyzed the relative abundance of gut microbiota at the phylum and genus levels (Fig. 3A, B). At the phylum level, Firmicutes and Bacteroidetes occupied the largest proportion. Compared to the control group, the relative abundance of Firmicutes was significantly increased and the relative abundance of Bacteroidetes was reduced in HFD group, which was reversed by lycopene (Fig. 3C, D). The Firmicutes/Bacteroides ratio (F/B ratio) was considerably elevated than 1 in HFD group, whereas F/B ratio in the other two groups was significantly less than 1 (p < 0.05, p = 0.02) (Fig. 3E). Verrucomicrobia was less prevalent in HFD group, whereas its abundance was statistically enhanced in HFD-fed mice supplemented with lycopene (p < 0.05, p = 0.02) (Fig. 3F). At the genus level, Akkermansia and Alloprevotella were the lowest in the HFD group while being significantly more prevalent in the HFD + lycopene group (p < 0.05, p = 0.02) (Fig. 3G, H).
Lycopene supplementation modulated the relative abundance of gut microbiota in HFD-fed ApoE−/− mice. ApoE−/− mice were fed a low-fat diet, a high-fat diet supplemented with or without 0.1% lycopene (w/w) for 19 weeks. (A, C, D, F) Different expression of relative abundance of gut microbiota in three groups at phylum level. (E) The ratio of Firmicutes/Bacteroides in three groups. Data was analyzed by R software and presented as the mean ± SD (n = 6 per group); ap < 0.05 compared with the control group; bp < 0.05 compared with the high-fat diet group. (B, G, H) Different expressions of relative abundance of gut microbiota in three groups at genus level. Data was analyzed by R software and presented as the mean ± SD (n = 6 per group); ap < 0.05 compared with the control group; bp < 0.05 compared with the high-fat diet group. HFD, high-fat diet; LY, lycopene. For species difference analysis and abundance calculation, the reshape2, ggplot2, ggprism, and plyr packages of R software were used
Lycopene improved intestinal barrier function and reduced intestinal permeability
The intestinal barrier function can be reflected by the expression levels of epithelial tight junction protein ZO-1 and occludin. Immunohistochemical analysis and Western blotting revealed that the expression levels of intestinal ZO-1 and occludin in the HFD group were statistically decreased compared with the control group. In contrast, HFD + lycopene group showed higher levels of intestinal ZO-1 and occludin expression (Fig. 4A-F), reflecting an improved intestinal barrier function. Moreover, the concentrations of serum LPS, D-LA and DAO, which are the biomarkers of intestinal permeability, were noticeably increased in the HFD group, while the HFD + lycopene group showed decreased levels (p < 0.05, p = 0.02, 0.01, 0.03) (Fig. 4G-I), reflecting a reduced intestinal permeability.
Lycopene supplementation increased intestinal ZO-1 and occludin expression and reduced intestinal permeability in HFD-fed ApoE−/− mice. ApoE−/− mice were fed a low-fat diet, a high-fat diet supplemented with or without 0.1% lycopene (w/w) for 19 weeks. (A, B) Immunohistochemical results of ZO-1 and occludin protein expression in the ileum were presented in the form of pictures (200×magnification), as described in Materials and Methods. The browner the color, the higher the protein expression. (C, D, E, F) The protein expression of ZO-1 and occludin in the ileum was determined by Western blotting. Results are representative of three independent experiments. It was analyzed by Image J software. Expression values were normalized to housekeeping gene GAPDH, and expression in the control group was set to 1. Data are presented as the mean ± SD (n = 8 per group); ap < 0.05 compared with the control group; bp < 0.05 compared with the high-fat diet group. (G, H, I) Intestinal permeability index such as LPS, D-LA and DAO was analyzed by SPSS software. One-Way ANOVA statistical method was used for calculation, and LSD and SNK methods were used for inter-group comparison. Data are presented as the mean ± SD (n = 8 per group); ap < 0.05 compared with the control group; bp < 0.05 compared with the high-fat diet group. HFD, high-fat diet; LY, lycopene
Lycopene inhibited inflammation in the aortic sinus
Elevated LPS enters the circulation and binds to TLR4, which in turn stimulates the TLR4/NF-κB signaling pathway, thereby activating various inflammatory factors and potentially resulting in atherosclerosis. As illustrated in Fig. 5A, B, the
immunohistochemical analysis showed that the expression of inflammatory protein TLR4 and p-NF-κB p65 in the aortic sinus was markedly increased in the HFD group, which was significantly reduced in HFD + lycopene group compared to the HFD group. There was little difference between the control group and HFD + lycopene group. In Western blotting analysis,
the expression levels of aortic TLR4, p-NF-κB p65 and NF-κB p65 were considerably higher in HFD group. Surprisingly, the expression levels of the three proteins in the HFD + lycopene group were decreased, while the expression levels of the control group were the lowest (Fig. 5C, D). Accordingly, the serum levels of inflammatory cytokines, such as TNF-α, MCP-1, IL-6 and IL-1β, were significantly reduced in HFD + lycopene group compared with HFD group (p < 0.05, p = 0.01, 0.02, 0.02, 0.03) (Table 2).
Lycopene supplementation attenuated the inflammation of aortic sinus in HFD-fed ApoE−/− mice. ApoE−/− mice were fed a low-fat diet, a high-fat diet supplemented with or without 0.1% lycopene (w/w) for 19 weeks. (A, B) Immunohistochemical results of TLR4 and p-NF-κB P65 protein expression in the aortic sinus were presented graphically (40×magnification), as described in Materials and Methods. The browner the color, the higher the protein expression. (C, D) The protein expression levels of aortic TLR4, p-NF-ĸBp65 and NF-ĸBp65 were determined by Western blotting. Results are representative of three independent experiments. It was analyzed by Image J software. Expression values were normalized to housekeeping gene GAPDH, and expression in the control group was set to 1. Data are presented as the mean ± SD (n = 8 per group); ap < 0.05 compared with the control group; bp < 0.05 compared with the high-fat diet group. HFD, high-fat diet; LY, lycopene
Spearman’s correlations analysis between the gut microbiota and the biochemical markers
Spearman’s correlations analysis also showed that different gut microbiota was associated with serum lipid, inflammatory factors and intestinal permeability indexes. At the phylum level, Firmicutes were positively correlated with serum D-LA, DAO, MCP-1, IL-1β, IL-6, TC, TG and LDL-C levels, while Firmicutes were negatively correlated with serum HDL-C levels; Bacteroides were negatively correlated with serum LPS, DAO, D-LA, MCP-1, IL-6, TC and LDL-C levels, but Bacteroides were positively correlated with serum HDL-C levels. Similarly, Verrucomicrobia showed the same trend, but no negative correlation with TC was observed. The Firmicutes/Bacteroides ratio was positively correlated with serum LPS, D-LA, MCP-1, IL-6, TC, TG and LDL-C levels, while serum HDL-C levels were negatively correlated with the Firmicutes/Bacteroides ratio (Fig. 6A).
At the genus level, Akkermansia and Alloprevotella were negatively correlated with serum D-LA, DAO, MCP-1, IL-6, IL-1β, TNF-α, TC, TG and LDL-C levels, while the serum HDL-C levels showed a positive correlation (Fig. 6B).
Spearman’s correlations between the gut microbiota and the biochemical markers. (A) The correlations between the gut microbiota and the biochemical markers at the phylum level. (B) The correlations between the gut microbiota and the biochemical markers at the genus level. The colour intensity represents the degree of the associations between the gut microbiota and the biochemical parameters, red represents positive correlation and blue indicates negative correlations. n = 6 per group, significant correlations are marked by
*p < 0.05 and **p < 0.01. HFD, high-fat diet; LY, lycopene
Discussion
In this study, lycopene treatment significantly improved the gut microbiota dysbiosis, reduced the ratio of Firmicutes/Bacteroides and elevated the relative abundance of gut microbiota such as Verrucomicrobia, Akkermansia and Alloprevotella, which were associated with reduced inflammation and enhanced intestinal barrier function. Moreover, lycopene administration improved the intestinal barrier function and alleviated intestinal permeability, then reduced serum LPS levels and inhibited cardiovascular inflammation through inactivating the TLR4/NF-κB pathway, thereby inhibiting the progression of atherosclerosis. Our finding provides a novel insight into the role of lycopene in preventing atherosclerosis and the underlying mechanism associated with the gut-heart axis.
As a phytochemical, lycopene has been shown to regulate lipid metabolism and reduce lipid levels in vivo [17,18,19]. Higher lycopene intake has demonstrated a stronger protective effect on the heart [18]. As Chiva-Blanch et al. reported, an inverse correlation between the circulating levels of lycopene and the progression of atherosclerosis was observed in newly diagnosed patients with type 2 diabetes [20]. These results suggest that as a part of healthy diet, lycopene supplementation can inhibit atherosclerosis formation. In addition, lycopene supplementation has been shown to prevent the development of atherosclerosis in the carotid arteries of middle-aged and old people [13]. A recent study indicates that daily supplementation of lycopene for three months can inhibit the formation of atherosclerotic plaque in male rats [21]. Our previous study also showed that lycopene administration could lower serum lipid levels and suppress the formation of atherosclerotic plaque in the aortic sinus of HFD-fed ApoE−/− mice through inhibiting intestinal cholesterol absorption [19], which was consistent with our current study. Considering the poor absorption in the gut and low concentration of lycopene in the blood [22], its effect in the intestinal tract might be of more physiological implications than its systemic effects.
A number of evidence has reported that the gut microbiota has a crucial implication in the pathogenesis of atherosclerosis [4, 5]. Alterations in the gut microbiota caused either by the diet or other factors promote the progression of atherosclerosis via the gut-heart axis [23]. The high-fat diet has been shown to alter the composition and function of the gut microbiota in mice [6, 24]. A high-fat diet caused an increase in the ratio of Firmicutes/Bacteroides and a decrease in the relative abundance of Verrucomicrobia in the gut [24, 25], which are related to the gut microbiota disorder and the impairment of intestinal barrier function. Previous study showed that a mixture of lycopene and dark chocolate could regulate the gut microbiota in moderately obese people [26]. A recent research also reported that lycopene supplementation improved the gut microbiota dysbiosis and intestinal barrier function in weaned piglets [12]. Excitingly, our current study demonstrated that lycopene significantly improved the species diversity of gut microbiota, decreased the ratio of Firmicutes/Bacteroides and increased the relative abundance of Verrucomicrobia in HFD-fed ApoE−/− mice. It has been reported that the improvement in the ratio of Firmicutes/Bacteroides is associated with the restoration of intestinal barrier function and reduced serum LPS levels [25]. Similarly, increased Verrucomicrobia is thought to repair the intestinal barrier function and alleviate endotoxemia and systemic inflammation [27]. More importantly, at the genus level, lycopene administration was found to significantly increase the relative abundance of Akkermansia and Alloprevotella in the gut. Previous studies demonstrated that Akkermansia and Alloprevotella could restore the intestinal barrier function, eliciting a beneficial immune response [28, 29]. There is considerable evidence proving that intestinal Akkermansia prevalence is negatively correlated with inflammation-related disorders, including diabetes, obesity and atherosclerosis [28, 30, 31]. Intestinal Alloprevotella has also been shown to play a similar role in preventing these inflammatory diseases [15, 32, 33]. Furthermore, supplementation with Akkermansia could stimulate the expression of intestinal tight junction proteins such as ZO-1 and occludin and reduce intestinal permeability and serum endotoxin levels, thereby alleviating endotoxin-induced inflammation and preventing atherosclerosis in ApoE−/− mice fed western diet [28]. Likewise, our results also found that higher abundance of Akkermansia and Alloprevotella was associated with lower levels of serum LPS and inflammatory markers, such as IL-6, IL-1β, TNF-α and MCP-1. Additionally, Akkermansia and Alloprevotella were negatively correlated with serum lipid levels and intestinal permeability indicators. These results suggest that lycopene treatment restores the gut Akkermansia and Alloprevotella communities and improve the intestinal barrier function in HFD-fed ApoE−/− mice, leading to reduced intestinal permeability, endotoxin levels and inflammatory response.
The intestinal epithelial barrier serves as the primary defence line of body against the external environment. The gut microbiota dysbiosis may disrupt the tight junctions of intestinal epithelium cells, thus attenuating the intestinal barrier function. The main tight junction proteins such as ZO-1 and occludin play important roles in the regulation of intestinal permeability and in the maintenance of intestinal barrier integrity [34]. Reduced expression of intestinal tight junction proteins such as ZO-1 and occludin will disrupt the intestinal barrier by causing epithelial cell death, leading to increased intestinal permeability. A high-fat diet has been reported to cause gut microbiota dysbiosis and reduced the expression levels of intestinal tight junction proteins, resulting in increased intestinal permeability and serum endotoxin levels 6. Our current study also showed that the high-fat diet caused gut microbiota dysbiosis and significantly reduced the protein expression of ZO-1 and occludin, severely disrupting the tight junctions among intestinal epithelium cells. Meanwhile, serum LPS, D-lactate and DAO levels, which are the biomarkers of intestinal permeability [35], were also increased with the decreased expression of intestinal tight junction protein. However, lycopene supplementation could improve gut microbiota dysbiosis and increase ZO-1 and occludin protein expression, then effectively reduce intestinal permeability and serum LPS levels, as well as LPS entry into the heart.
LPS is also a natural ligand of TLR4 and can activate the TLR4-related inflammatory signaling pathway, resulting in the release of inflammatory cytokines [36]. TLR4 belongs to the TLR family and is considered as a linking molecule between the gut microbiota, endotoxemia and atherosclerosis [37]. TLR4 is highly expressed in atherosclerotic plaque and is associated with elevated serum LPS levels and impaired intestinal barrier [4]. Genetic deletion of TLR4 in ApoE−/− mice was related to the reduced serum LPS levels and macrophage inflammatory response and atherosclerotic lesions in the aorta [38]. The human studies also demonstrated that the elevated expression of TLR4 was associated with inflammatory activation in atherosclerosis, and promoted the development of atherosclerosis [39]. The gut microbiota dysbiosis can enhance the permeability of intestinal epithelial barrier and cause endotoxin LPS accumulation in the heart. The binding of LPS to TLR4 can activate its downstream pathway NF-κB. The activation of NF-κB leads to the release of several pro-inflammatory cytokines including IL-6, IL-1β, MCP-1 and TNF-α [5]. Pro-inflammatory cytokines, such as IL-6, IL-1β, MCP-1 and TNF-α, are involved in the pathogenesis of atherosclerosis through inducing inflammatory cascades in the heart [24, 40, 41]. A recent study has shown that tomato powder containing 2.39 mg lycopene can reduce the mRNA expression levels of IL-6, IL-1β, TNF-α and MCP-1 in high-fat diet-fed mice [42]. Our previous studies in RAW264.7 macrophages also found that lycopene treatment inhibited LPS-induced inflammatory response through suppressing the trafficking of TLR4 to lipid rafts and the subsequent activation of NF-κB pathway [10, 11], leading to reduced inflammatory cytokines production. Consistently, our current study also showed that lycopene supplementation improved gut microbiota dysbiosis and reduced serum endotoxin LPS levels, subsequently inhibited the activation of TLR4/NF-κB signaling pathway in the heart and decreased inflammatory cytokines release, ultimately achieving an anti-atherosclerotic effect in HFD-fed ApoE−/− mice.
Conclusion
In summary, our results indicated that the protective effect of lycopene against atherosclerosis induced by high-fat diet and further revealed that its mechanism might be its prebiotic effect on maintaining gut microbiota homeostasis and improving intestinal barrier function, consequently reducing serum lipopolysaccharide-triggered inflammatory response in the heart. Overall, this study provides a novel insight into the anti-atherosclerotic potential and mechanism of phytochemicals lycopene and the correlation between the gut microbiota and the heart, which lays a foundation for the clinic application of lycopene to protect against atherosclerosis and inflammation-related diseases.
Data Availability
The datasets used during the present study are available from the corresponding author upon reasonable request.
Abbreviations
- ApoE−/− :
-
Apolipoprotein E knockout
- ALT:
-
Alanine transferase
- AST:
-
Aspartic transaminase
- D-LA:
-
D-lactic acid
- DAO:
-
Diamine peroxidase
- F/B ratio:
-
Firmicutes / Bacteroides ratio
- HDL-C:
-
High density lipoprotein cholesterol
- HFD:
-
High-fat diet
- IL-1β:
-
interleukin-1β
- LDL-C:
-
Low density lipoprotein cholesterol
- LPS:
-
Lipopolysaccharide
- MCP-1:
-
Monocyte chemotactic protein-1
- NF-κB:
-
Nuclear factor-kappa B
- PCoA:
-
Principal coordinates analysis
- p-NF-κB p65:
-
Phospho-NF-κB p65
- TC:
-
Total cholesterol
- TG:
-
Triglyceride
- TLR4:
-
Toll-like receptor 4
- TNF-α:
-
Tumor necrosis factor-α
- ZO-1:
-
Zonula occludens-1
References
Libby P, Buring JE, Badimon L, Hansson GK, Deanfield J, Bittencourt MS, et al. Atherosclerosis Nat Rev Dis Primers. 2019;5(1):56.
Libby P. The changing landscape of Atherosclerosis. Nature. 2021;592:524–33.
Wolf D, Ley K. Immunity and inflammation in Atherosclerosis. Circ Res. 2019;124(2):315–27.
Jonsson AL, Bäckhed F. Role of gut microbiota in Atherosclerosis. Nat Rev Cardiol. 2017;14(2):79–87.
Ma J, Li HT. Role of gut microbiota in Atherosclerosis and Hypertension. Front Pharmacol. 2018;9:1082.
Anto L, Blesso CN. Interplay between diet, the gut microbiome, and Atherosclerosis: role of dysbiosis and microbial metabolites on inflammation and disordered lipid metabolism. J Nutr Biochem. 2022;105:108991.
Zhang L, Wang F, Wang J, Wang Y, Fang Y. Intestinal fatty acid-binding protein mediates atherosclerotic progress through increasing intestinal inflammation and permeability. J Cell Mol Med. 2020;24(9):5205–12.
Khan UM, Sevindik M, Zarrabi A, Nami M, Ozdemir B, Kaplan DN et al. Lycopene: Food Sources, Biological Activities, and Human Health Benefits. Oxid Med Cell Longev. 2021; 2021: 2713511.
Przybylska S, Tokarczyk G. Lycopene in the Prevention of Cardiovascular Diseases. Int J Mol Sci. 2022;23(4):1957.
Zou J, Feng D, Ling WH, Duan RD. Lycopene suppresses proinflammatory response in lipopolysaccharide-stimulated macrophages by inhibiting ROS-induced trafficking of TLR4 to lipid raft-like domains. J Nutr Biochem. 2013;24(6):1117–22.
Feng D, Ling WH, Duan RD. Lycopene suppresses LPS-induced NO and IL-6 production by inhibiting the activation of ERK, p38MAPK, and NF-kappaB in macrophages. Inflamm Res. 2010;59(2):115–21.
Meng Q, Zhang Y, Li J, Shi B, Ma Q, Shan A. Lycopene affects intestinal barrier function and the gut microbiota in weaned piglets via antioxidant signaling regulation. J Nutr. 2022;152(11):2396–408.
Wang C, Qiu R, Cao Y, Ouyang WF, Li HB, Ling WH, et al. Higher dietary and serum carotenoid levels are associated with lower carotid intima-media thickness in middle-aged and elderly people. Br J Nutr. 2018;119(5):590–8.
Mannino F, Pallio G, Altavilla D, Squadrito F, Vermiglio G, Bitto A, et al. Atherosclerosis plaque reduction by Lycopene is mediated by increased energy expenditure through AMPK and PPARα in ApoE KO Mice Fed with a high Fat Diet. Biomolecules. 2022;12(7):973.
Feng D, Zhang H, Jiang X, Zou J, Li Q, Mai H, et al. Bisphenol a exposure induces gut microbiota dysbiosis and consequent activation of gut-liver axis leading to hepatic steatosis in CD-1 mice. Environ Pollut. 2020;265(Pt A):114880.
Vujić N, Korbelius M, Sachdev V, Rainer S, Zimmer A, Huber A, et al. Intestine-specific DGAT1 deficiency improves Atherosclerosis in apolipoprotein E knockout mice by reducing systemic cholesterol burden. Atherosclerosis. 2020;310:26–36.
Fenni S, Hammou H, Astier J, Bonnet L, Karkeni E, Couturier C et al. Lycopene and tomato powder supplementation similarly inhibit high-fat diet induced obesity, inflammatory response, and associated metabolic disorders. Mol Nutr Food Res. 2017; 61(9).
Mazidi M, Katsiki N, George ES, Banach M. Tomato and lycopene consumption is inversely associated with total and cause-specific mortality: a population-based cohort study, on behalf of the International Lipid Expert Panel (ILEP). Br J Nutr. 2020;124(12):1303–10.
Liu H, Liu J, Liu Z, Wang Q, Liu J, Feng D, et al. Lycopene Reduces Cholesterol Absorption and prevents Atherosclerosis in ApoE–/– mice by downregulating HNF-1α and NPC1L1 expression. J Agric Food Chem. 2021;69(35):10114–20.
Chiva-Blanch G, Jiménez C, Pinyol M, Herreras Z, Catalán M, Martínez-Huélamo M, et al. 5-cis-, Trans- and total lycopene plasma concentrations inversely relate to atherosclerotic plaque burden in newly diagnosed type 2 Diabetes subjects. Nutrients. 2020;12(6):1696.
Albrahim T, Alonazi MA. Lycopene corrects metabolic syndrome and liver injury induced by high fat diet in obese rats through antioxidant, anti-inflammatory, antifibrotic pathways. Biomed Pharmacother. 2021;141:111831.
Perveen R, Suleria HA, Anjum FM, Butt MS, Pasha I, Ahmad S. Tomato (Solanum lycopersicum) carotenoids and lycopenes Chemistry; metabolism, Absorption, Nutrition, and Allied Health Claims–A Comprehensive Review. Crit Rev Food Sci Nutr. 2015;55(7):919–29.
Trøseid M, Andersen G, Broch K, Hov JR. The gut microbiome in coronary artery Disease and Heart Failure: current knowledge and future directions. EBioMedicine. 2020;52:102649.
Zhu L, Zhang D, Zhu H, Zhu J, Weng S, Dong L, et al. Berberine treatment increases Akkermansia in the gut and improves high-fat diet-induced Atherosclerosis in Apoe(-/-) mice. Atherosclerosis. 2018;268:117–26.
Bian Y, Lei J, Zhong J, Wang B, Wan Y, Li J, et al. Kaempferol Reduces Obesity, prevents intestinal inflammation, and modulates gut microbiota in high-fat Diet mice: Kaempferol reduce inflammation and dysbacteria. J Nutr Biochem. 2022;99:108840.
Wiese M, Bashmakov Y, Chalyk N, Nielsen DS, Krych Ł, Kot W, et al. Prebiotic Effect of Lycopene and Dark Chocolate on Gut Microbiome with systemic changes in liver metabolism, skeletal muscles and skin in moderately obese persons. Biomed Res Int. 2019;2019:4625279.
Yan X, Zhai Y, Zhou W, Qiao Y, Guan L, Liu H, et al. Intestinal Flora mediates Antiobesity Effect of Rutin in High-Fat-Diet mice. Mol Nutr Food Res. 2022;66(14):e2100948.
Li J, Lin S, Vanhoutte PM, Woo CW, Xu A. Akkermansia Muciniphila protects against Atherosclerosis by preventing Metabolic Endotoxemia-Induced inflammation in Apoe-/- mice. Circulation. 2016;133(24):2434–46.
Chen M, Hui S, Lang H, Zhou M, Zhang Y, Kang C, et al. SIRT3 Deficiency promotes high-Fat Diet-Induced nonalcoholic fatty Liver Disease in correlation with impaired intestinal permeability through Gut Microbial Dysbiosis. Mol Nutr Food Res. 2019;63(4):e1800612.
Schneeberger M, Everard A, Gómez-Valadés AG, Matamoros S, Ramírez S, Delzenne NM, et al. Akkermansia muciniphila inversely correlates with the onset of inflammation, altered adipose tissue metabolism and metabolic disorders during obesity in mice. Sci Rep. 2015;5:16643.
Depommier C, Everard A, Druart C, Plovier H, Van Hul M, Vieira-Silva S, et al. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: a proof-of-concept exploratory study. Nat Med. 2019;25(7):1096–103.
Cui H-X, Hu Y-N, Li J-W, Yuan K. Hypoglycemic Mechanism of the Berberine Organic Acid Salt under the Synergistic Effect of Intestinal Flora and Oxidative Stress. Oxid Med Cell Longev. 2018; 2018:8930374.
Liu J, Yue S, Yang Z, Feng W, Meng X, Wang A, et al. Oral hydroxysafflor yellow A reduces obesity in mice by modulating the gut microbiota and serum metabolism. Pharmacol Res. 2018;134:40–50.
Chelakkot C, Ghim J, Ryu SH. Mechanisms regulating intestinal barrier integrity and its pathological implications. Exp Mol Med. 2018;50(8):1–9.
Stevens BR, Goel R, Seungbum K, Richards EM, Holbert RC, Pepine CJ, et al. Increased human intestinal barrier permeability plasma biomarkers zonulin and FABP2 correlated with plasma LPS and altered gut microbiome in anxiety or depression. Gut. 2018;67(8):1555–7.
Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4(7):499–511.
Cani PD, Delzenne NM. The role of the gut microbiota in energy metabolism and metabolic Disease. Curr Pharm Des. 2009;15(13):1546–58.
Michelsen KS, Wong MH, Shah PK, Zhang W, Yano J, Doherty TM, et al. Lack of toll-like receptor 4 or myeloid differentiation factor 88 reduces Atherosclerosis and alters plaque phenotype in mice deficient in apolipoprotein E. Proc Natl Acad Sci U S A. 2004;101(29):10679–84.
Edfeldt K, Swedenborg J, Hansson GK, Yan ZQ. Expression of toll-like receptors in human atherosclerotic lesions: a possible pathway for plaque activation. Circulation. 2002;105(10):1158–61.
Hennig B, Meerarani P, Ramadass P, Toborek M, Malecki A, Slim R, et al. Zinc nutrition and apoptosis of vascular endothelial cells: implications in Atherosclerosis. Nutrition. 1999;15(10):744–8.
Zernecke A, Weber C. Inflammatory mediators in atherosclerotic vascular Disease. Basic Res Cardiol. 2005;100(2):93–101.
Xia H, Liu C, Li C-C, Fu M, Takahashi S, Hu K-Q, et al. Dietary tomato powder inhibits high-Fat Diet–Promoted Hepatocellular Carcinoma with Alteration of Gut Microbiota in mice lacking carotenoid cleavage enzymes. Cancer Prev Res. 2018;11(12):797–810.
Funding
This work was supported by grants from the Natural Science Foundation of Guangdong Province (2022A1515012268, 2018A030313782, 2022A1515011610, 2020A1515011167); the National Natural Science Foundation of China (81973019).
Author information
Authors and Affiliations
Contributions
DF and JZ conceived the study. TT, HL and ZL performed most of the experiments.TT, HL and ZL drafted the manuscript. All authors read, commented on and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Laboratory Animal Ethics Committee, School of Public Health, Sun Yat-sen University (No. 2019-035).
Competing interests
The authors declare no competing interests.
Conflict of interest
All authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Tu, T., Liu, H., Liu, Z. et al. Amelioration of Atherosclerosis by lycopene is linked to the modulation of gut microbiota dysbiosis and related gut-heart axis activation in high-fat diet-fed ApoE−/− mice. Nutr Metab (Lond) 20, 53 (2023). https://doi.org/10.1186/s12986-023-00772-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12986-023-00772-x